 Found 18 hits with Last Name = 'leung-toung' and Initial = 'r'
Found 18 hits with Last Name = 'leung-toung' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164227
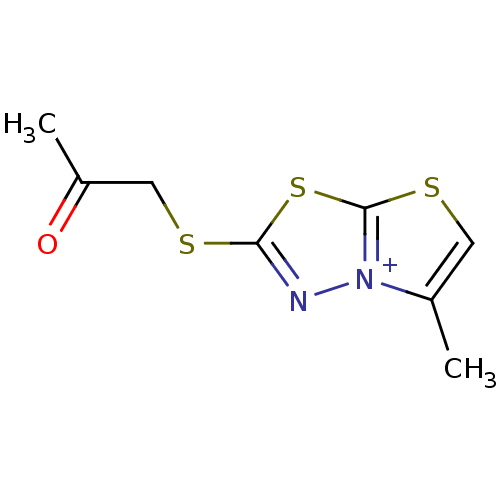
(5-Methyl-2-(2-oxo-propylsulfanyl)-thiazolo[2,3-b][...)Show InChI InChI=1S/C8H9N2OS3/c1-5-3-13-8-10(5)9-7(14-8)12-4-6(2)11/h3H,4H2,1-2H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164216

(CHEMBL190022 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C17H22N6O4S2/c1-21(16-20-28-17-18-11-13-22(16)17)12-7-3-2-6-10-19-29(26,27)15-9-5-4-8-14(15)23(24)25/h4-5,8-9,11,13,19H,2-3,6-7,10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164222

(CHEMBL193212 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES [O-][N+](=O)c1ccccc1S(=O)(=O)NCCCCCCNc1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C16H20N6O4S2/c23-22(24)13-7-3-4-8-14(13)28(25,26)19-10-6-2-1-5-9-17-15-20-27-16-18-11-12-21(15)16/h3-4,7-8,11-12,19H,1-2,5-6,9-10H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase K
(Homo sapiens (Human)) | BDBM50164227

(5-Methyl-2-(2-oxo-propylsulfanyl)-thiazolo[2,3-b][...)Show InChI InChI=1S/C8H9N2OS3/c1-5-3-13-8-10(5)9-7(14-8)12-4-6(2)11/h3H,4H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against guinea pig liver transglutaminase |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164223
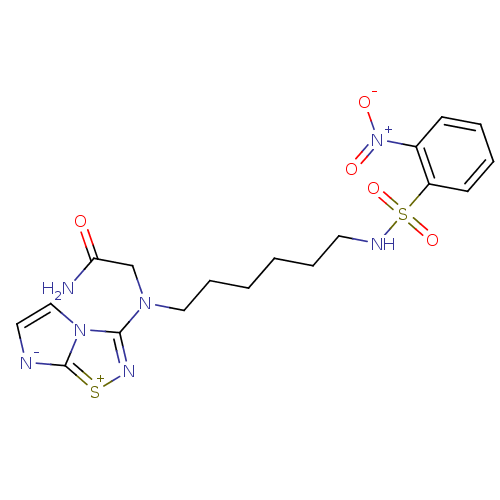
(2-{Imidazo[1,2-d][1,2,4]thiadiazol-3-yl-[6-(2-nitr...)Show SMILES NC(=O)CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C18H23N7O5S2/c19-16(26)13-23(17-22-31-18-20-10-12-24(17)18)11-6-2-1-5-9-21-32(29,30)15-8-4-3-7-14(15)25(27)28/h3-4,7-8,10,12,21H,1-2,5-6,9,11,13H2,(H2,19,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164224
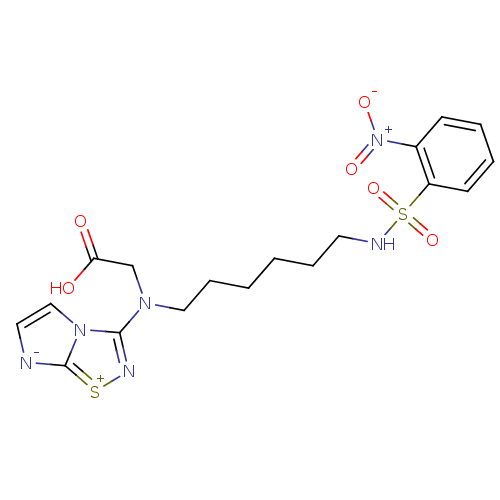
(CHEMBL192921 | {Imidazo[1,2-d][1,2,4]thiadiazol-3-...)Show SMILES OC(=O)CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C18H22N6O6S2/c25-16(26)13-22(17-21-31-18-19-10-12-23(17)18)11-6-2-1-5-9-20-32(29,30)15-8-4-3-7-14(15)24(27)28/h3-4,7-8,10,12,20H,1-2,5-6,9,11,13H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164225
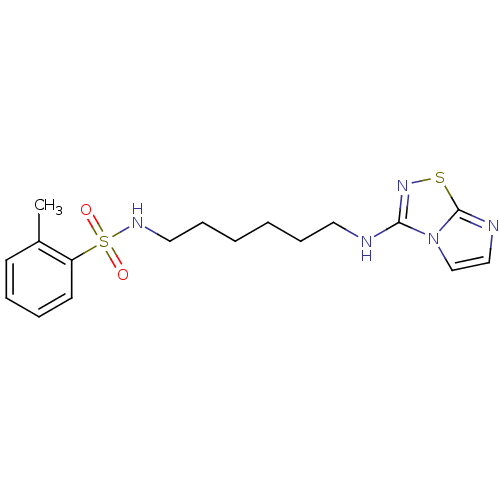
(CHEMBL365470 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show InChI InChI=1S/C17H23N5O2S2/c1-14-8-4-5-9-15(14)26(23,24)20-11-7-3-2-6-10-18-16-21-25-17-19-12-13-22(16)17/h4-5,8-9,12-13,20H,2-3,6-7,10-11H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164226
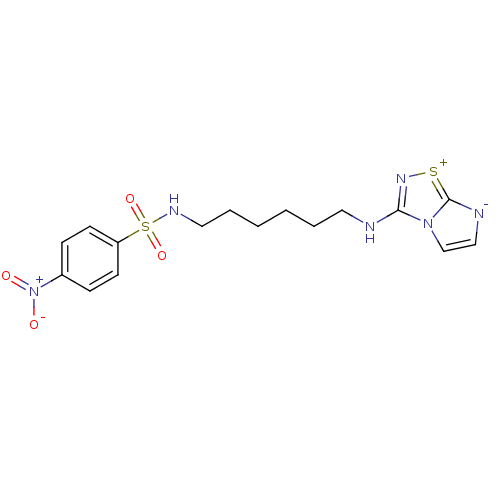
(CHEMBL193724 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES [O-][N+](=O)c1ccc(cc1)S(=O)(=O)NCCCCCCNc1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C16H20N6O4S2/c23-22(24)13-5-7-14(8-6-13)28(25,26)19-10-4-2-1-3-9-17-15-20-27-16-18-11-12-21(15)16/h5-8,11-12,19H,1-4,9-10H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164218
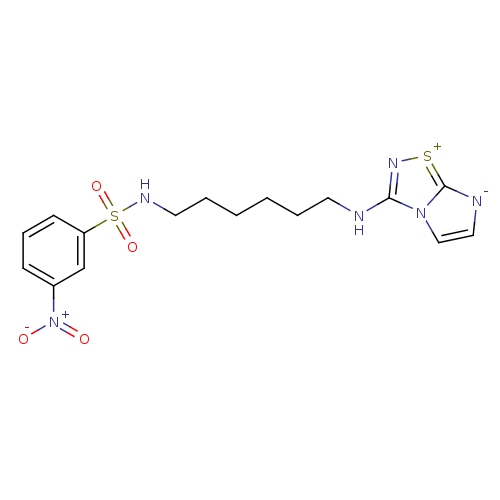
(CHEMBL192770 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES [O-][N+](=O)c1cccc(c1)S(=O)(=O)NCCCCCCNc1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C16H20N6O4S2/c23-22(24)13-6-5-7-14(12-13)28(25,26)19-9-4-2-1-3-8-17-15-20-27-16-18-10-11-21(15)16/h5-7,10-12,19H,1-4,8-9H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164221
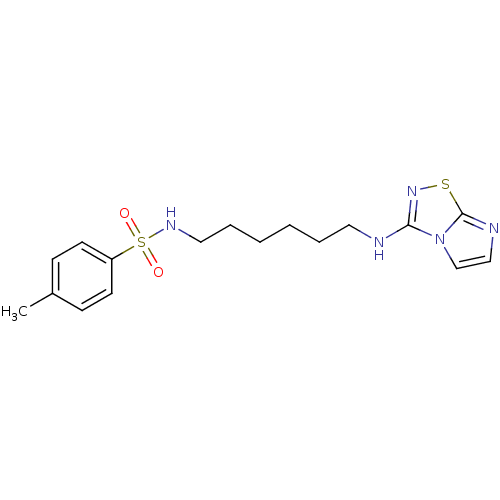
(CHEMBL189379 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show InChI InChI=1S/C17H23N5O2S2/c1-14-6-8-15(9-7-14)26(23,24)20-11-5-3-2-4-10-18-16-21-25-17-19-12-13-22(16)17/h6-9,12-13,20H,2-5,10-11H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164219

(CHEMBL190568 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show InChI InChI=1S/C16H21N5O2S2/c22-25(23,14-8-4-3-5-9-14)19-11-7-2-1-6-10-17-15-20-24-16-18-12-13-21(15)16/h3-5,8-9,12-13,19H,1-2,6-7,10-11H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase K
(Homo sapiens (Human)) | BDBM50164216

(CHEMBL190022 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C17H22N6O4S2/c1-21(16-20-28-17-18-11-13-22(16)17)12-7-3-2-6-10-19-29(26,27)15-9-5-4-8-14(15)23(24)25/h4-5,8-9,11,13,19H,2-3,6-7,10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against guinea pig liver transglutaminase |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase K
(Homo sapiens (Human)) | BDBM50164222

(CHEMBL193212 | N-[6-(Imidazo[1,2-d][1,2,4]thiadiaz...)Show SMILES [O-][N+](=O)c1ccccc1S(=O)(=O)NCCCCCCNc1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C16H20N6O4S2/c23-22(24)13-7-3-4-8-14(13)28(25,26)19-10-6-2-1-5-9-17-15-20-27-16-18-11-12-21(15)16/h3-4,7-8,11-12,19H,1-2,5-6,9-10H2,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against guinea pig liver transglutaminase |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164217
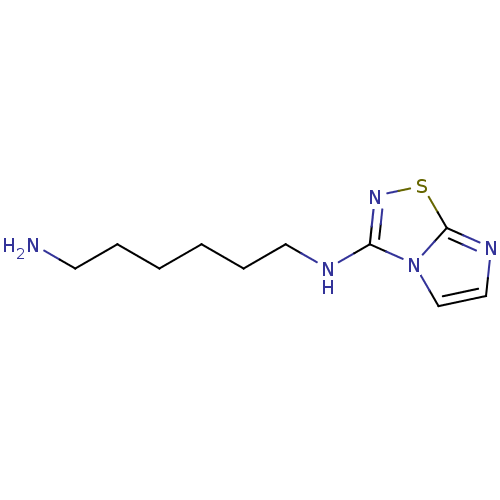
(CHEMBL192660 | N*1*-Imidazo[1,2-d][1,2,4]thiadiazo...)Show InChI InChI=1S/C10H17N5S/c11-5-3-1-2-4-6-12-9-14-16-10-13-7-8-15(9)10/h7-8H,1-6,11H2,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase K
(Homo sapiens (Human)) | BDBM50164224

(CHEMBL192921 | {Imidazo[1,2-d][1,2,4]thiadiazol-3-...)Show SMILES OC(=O)CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C18H22N6O6S2/c25-16(26)13-22(17-21-31-18-19-10-12-23(17)18)11-6-2-1-5-9-20-32(29,30)15-8-4-3-7-14(15)24(27)28/h3-4,7-8,10,12,20H,1-2,5-6,9,11,13H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against guinea pig liver transglutaminase |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase K
(Homo sapiens (Human)) | BDBM50164223

(2-{Imidazo[1,2-d][1,2,4]thiadiazol-3-yl-[6-(2-nitr...)Show SMILES NC(=O)CN(CCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O)c1n[s+]c2[n-]ccn12 Show InChI InChI=1S/C18H23N7O5S2/c19-16(26)13-23(17-22-31-18-20-10-12-24(17)18)11-6-2-1-5-9-21-32(29,30)15-8-4-3-7-14(15)25(27)28/h3-4,7-8,10,12,21H,1-2,5-6,9,11,13H2,(H2,19,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against guinea pig liver transglutaminase |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164220

(CHEMBL192376 | N-Hexyl-2-nitro-benzenesulfonamide)Show InChI InChI=1S/C12H18N2O4S/c1-2-3-4-7-10-13-19(17,18)12-9-6-5-8-11(12)14(15)16/h5-6,8-9,13H,2-4,7,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |
Coagulation factor XIII A chain
(Homo sapiens (Human)) | BDBM50164215
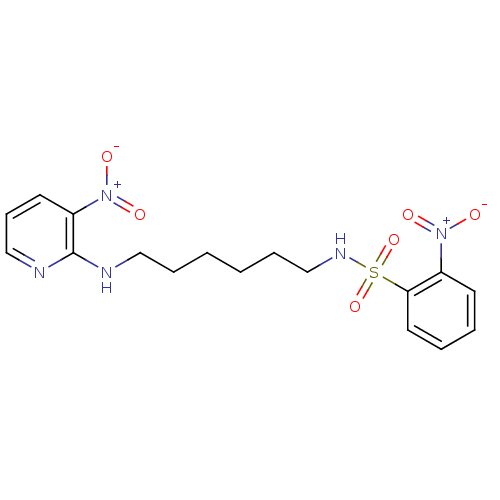
(2-Nitro-N-[6-(3-nitro-pyridin-2-ylamino)-hexyl]-be...)Show SMILES [O-][N+](=O)c1cccnc1NCCCCCCNS(=O)(=O)c1ccccc1[N+]([O-])=O Show InChI InChI=1S/C17H21N5O6S/c23-21(24)14-8-3-4-10-16(14)29(27,28)20-13-6-2-1-5-11-18-17-15(22(25)26)9-7-12-19-17/h3-4,7-10,12,20H,1-2,5-6,11,13H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Apotex Research Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of compound against factor XIIIa |
J Med Chem 48: 2266-9 (2005)
Article DOI: 10.1021/jm049221w
BindingDB Entry DOI: 10.7270/Q2R210XM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data