 Found 839 hits with Last Name = 'leuthner' and Initial = 'b'
Found 839 hits with Last Name = 'leuthner' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862
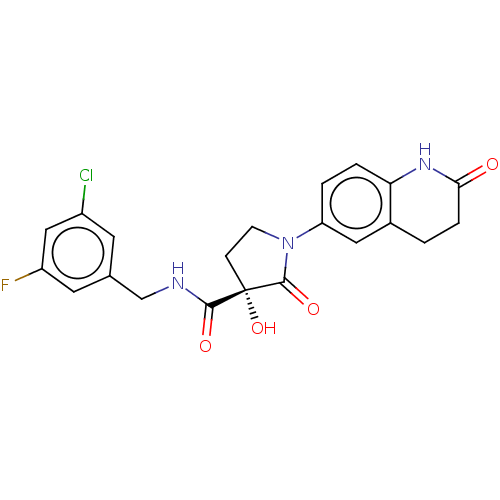
(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080960
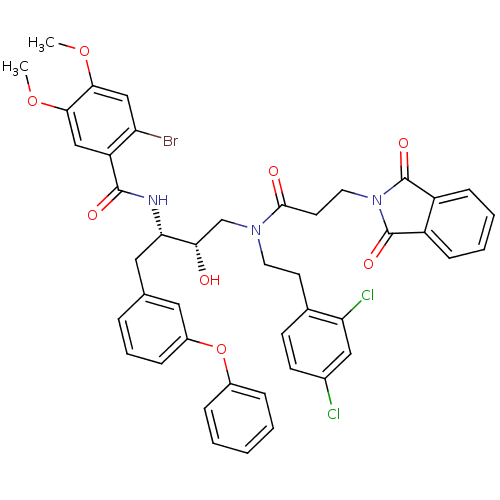
(2-Bromo-N-[(1S,2S)-3-{[2-(2,4-dichloro-phenyl)-eth...)Show SMILES COc1cc(Br)c(cc1OC)C(=O)N[C@@H](Cc1cccc(Oc2ccccc2)c1)[C@@H](O)CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C44H40BrCl2N3O8/c1-56-39-24-34(35(45)25-40(39)57-2)42(53)48-37(22-27-9-8-12-31(21-27)58-30-10-4-3-5-11-30)38(51)26-49(19-17-28-15-16-29(46)23-36(28)47)41(52)18-20-50-43(54)32-13-6-7-14-33(32)44(50)55/h3-16,21,23-25,37-38,51H,17-20,22,26H2,1-2H3,(H,48,53)/t37-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D (unknown origin) |
Bioorg Med Chem Lett 24: 4141-50 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.054
BindingDB Entry DOI: 10.7270/Q2W95BV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163
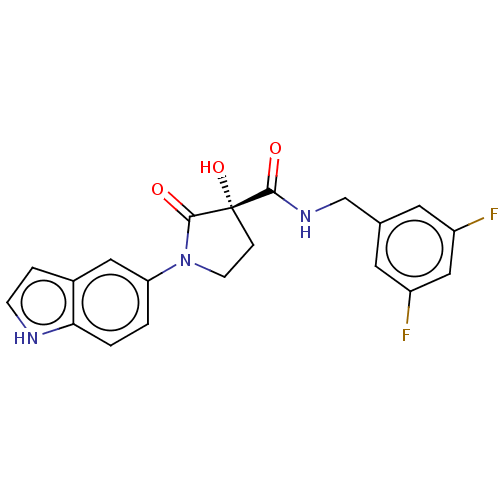
(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163

(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50505332
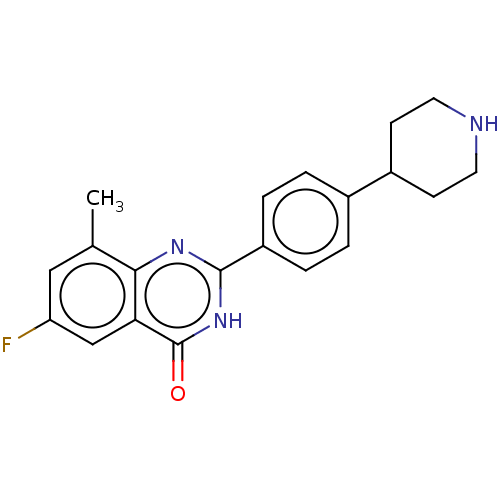
(CHEMBL4567515)Show SMILES Cl.Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C20H20FN3O/c1-12-10-16(21)11-17-18(12)23-19(24-20(17)25)15-4-2-13(3-5-15)14-6-8-22-9-7-14/h2-5,10-11,14,22H,6-9H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells at -80 mV holding potential by whole cell patch clamp method |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505331
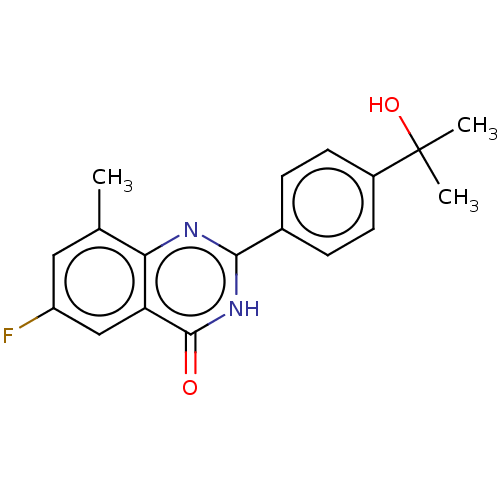
(CHEMBL4458129)Show SMILES Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C(C)(C)O Show InChI InChI=1S/C18H17FN2O2/c1-10-8-13(19)9-14-15(10)20-16(21-17(14)22)11-4-6-12(7-5-11)18(2,3)23/h4-9,23H,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50593627
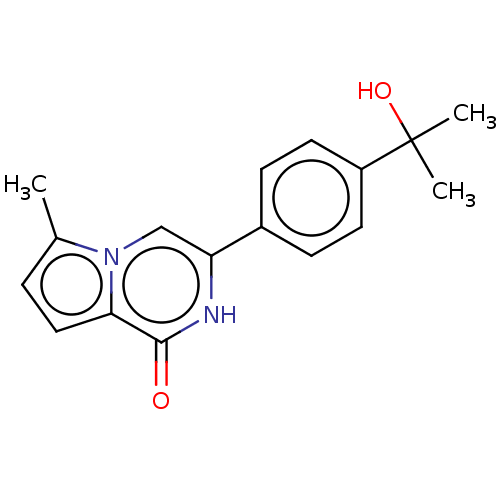
(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505332

(CHEMBL4567515)Show SMILES Cl.Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C20H20FN3O/c1-12-10-16(21)11-17-18(12)23-19(24-20(17)25)15-4-2-13(3-5-15)14-6-8-22-9-7-14/h2-5,10-11,14,22H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5274873

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CC2)C1 Show InChI InChI=1S/C24H33Cl2N3O2/c25-21-4-3-19(13-22(21)26)24(6-5-23(30)29(17-24)14-18-1-2-18)7-8-27-15-20(16-27)28-9-11-31-12-10-28/h3-4,13,18,20H,1-2,5-12,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5272750

Show SMILES Cc1cccc(CN2CC(CCN3CC(C3)N3CCOCC3)(CCC2=O)c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C28H35Cl2N3O2/c1-21-3-2-4-22(15-21)17-33-20-28(8-7-27(33)34,23-5-6-25(29)26(30)16-23)9-10-31-18-24(19-31)32-11-13-35-14-12-32/h2-6,15-16,24H,7-14,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505331

(CHEMBL4458129)Show SMILES Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C(C)(C)O Show InChI InChI=1S/C18H17FN2O2/c1-10-8-13(19)9-14-15(10)20-16(21-17(14)22)11-4-6-12(7-5-11)18(2,3)23/h4-9,23H,1-3H3,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5274873

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(CC2CC2)C1 Show InChI InChI=1S/C24H33Cl2N3O2/c25-21-4-3-19(13-22(21)26)24(6-5-23(30)29(17-24)14-18-1-2-18)7-8-27-15-20(16-27)28-9-11-31-12-10-28/h3-4,13,18,20H,1-2,5-12,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5272750

Show SMILES Cc1cccc(CN2CC(CCN3CC(C3)N3CCOCC3)(CCC2=O)c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C28H35Cl2N3O2/c1-21-3-2-4-22(15-21)17-33-20-28(8-7-27(33)34,23-5-6-25(29)26(30)16-23)9-10-31-18-24(19-31)32-11-13-35-14-12-32/h2-6,15-16,24H,7-14,17-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232262

(US9345742, 20)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C42H64N6O8/c1-11-27(6)36(39(52)48-35(26(4)5)40(53)55-10)46-33(49)24-30(43)31(22-28-18-14-12-15-19-28)44-38(51)34(25(2)3)47-37(50)32(23-29-20-16-13-17-21-29)45-41(54)56-42(7,8)9/h12-21,25-27,30-32,34-36H,11,22-24,43H2,1-10H3,(H,44,51)(H,45,54)(H,46,49)(H,47,50)(H,48,52)/t27-,30-,31-,32-,34-,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5271127

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2=O)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H31Cl2N3O3/c28-23-7-6-21(14-24(23)29)27(9-8-25(33)31(19-27)15-20-4-2-1-3-5-20)10-11-30-16-22(17-30)32-12-13-35-18-26(32)34/h1-7,14,22H,8-13,15-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D (unknown origin) |
Bioorg Med Chem Lett 24: 4141-50 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.054
BindingDB Entry DOI: 10.7270/Q2W95BV1 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5271127

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2=O)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H31Cl2N3O3/c28-23-7-6-21(14-24(23)29)27(9-8-25(33)31(19-27)15-20-4-2-1-3-5-20)10-11-30-16-22(17-30)32-12-13-35-18-26(32)34/h1-7,14,22H,8-13,15-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505324

(CHEMBL4588170)Show InChI InChI=1S/C18H18N2O2/c1-11-5-4-6-14-15(11)19-16(20-17(14)21)12-7-9-13(10-8-12)18(2,3)22/h4-10,22H,1-3H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5284314

Show SMILES OC1CCCN(C1)C1CN(CCC2(CCC(=O)N(Cc3ccccc3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C28H35Cl2N3O2/c29-25-9-8-22(15-26(25)30)28(11-10-27(35)33(20-28)16-21-5-2-1-3-6-21)12-14-31-17-23(18-31)32-13-4-7-24(34)19-32/h1-3,5-6,8-9,15,23-24,34H,4,7,10-14,16-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505332

(CHEMBL4567515)Show SMILES Cl.Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C20H20FN3O/c1-12-10-16(21)11-17-18(12)23-19(24-20(17)25)15-4-2-13(3-5-15)14-6-8-22-9-7-14/h2-5,10-11,14,22H,6-9H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505324

(CHEMBL4588170)Show InChI InChI=1S/C18H18N2O2/c1-11-5-4-6-14-15(11)19-16(20-17(14)21)12-7-9-13(10-8-12)18(2,3)22/h4-10,22H,1-3H3,(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50593627

(CHEMBL5199558) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505334
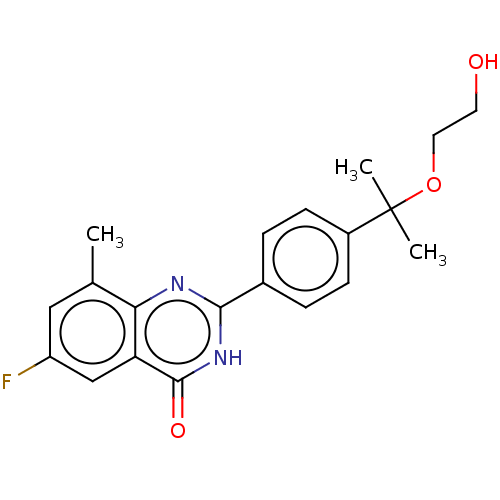
(CHEMBL4551200)Show SMILES Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C(C)(C)OCCO Show InChI InChI=1S/C20H21FN2O3/c1-12-10-15(21)11-16-17(12)22-18(23-19(16)25)13-4-6-14(7-5-13)20(2,3)26-9-8-24/h4-7,10-11,24H,8-9H2,1-3H3,(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5279709

Show SMILES NS(=O)(=O)N1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C24H35Cl2N5O3S/c25-21-4-3-19(13-22(21)26)24(6-5-23(32)30(17-24)14-18-1-2-18)7-8-28-15-20(16-28)29-9-11-31(12-10-29)35(27,33)34/h3-4,13,18,20H,1-2,5-12,14-17H2,(H2,27,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5281076

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCNCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H34Cl2N4O/c28-24-7-6-22(16-25(24)29)27(10-13-31-18-23(19-31)32-14-11-30-12-15-32)9-8-26(34)33(20-27)17-21-4-2-1-3-5-21/h1-7,16,23,30H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505335

(CHEMBL4584689)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1nc2c(C)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C20H21FN4O/c1-13-11-15(21)12-17-18(13)22-19(23-20(17)26)14-3-5-16(6-4-14)25-9-7-24(2)8-10-25/h3-6,11-12H,7-10H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5280682

Show SMILES CCOC1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C27H39Cl2N3O2/c1-2-34-23-8-12-31(13-9-23)22-17-30(18-22)14-11-27(21-5-6-24(28)25(29)15-21)10-7-26(33)32(19-27)16-20-3-4-20/h5-6,15,20,22-23H,2-4,7-14,16-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505334

(CHEMBL4551200)Show SMILES Cc1cc(F)cc2c1nc([nH]c2=O)-c1ccc(cc1)C(C)(C)OCCO Show InChI InChI=1S/C20H21FN2O3/c1-12-10-15(21)11-16-17(12)22-18(23-19(16)25)13-4-6-14(7-5-13)20(2,3)26-9-8-24/h4-7,10-11,24H,8-9H2,1-3H3,(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5269584

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1CCCCC1 Show InChI InChI=1S/C26H37Cl2N3O2/c27-23-7-6-20(16-24(23)28)26(9-8-25(32)31(19-26)21-4-2-1-3-5-21)10-11-29-17-22(18-29)30-12-14-33-15-13-30/h6-7,16,21-22H,1-5,8-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from rat liver |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5275576

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCS(=O)CC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3O2S/c28-24-7-6-22(16-25(24)29)27(9-8-26(33)32(20-27)17-21-4-2-1-3-5-21)10-11-30-18-23(19-30)31-12-14-35(34)15-13-31/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505335

(CHEMBL4584689)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1nc2c(C)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C20H21FN4O/c1-13-11-15(21)12-17-18(13)22-19(23-20(17)26)14-3-5-16(6-4-14)25-9-7-24(2)8-10-25/h3-6,11-12H,7-10H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5269897

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3O2/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5269584

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)N(C1)C1CCCCC1 Show InChI InChI=1S/C26H37Cl2N3O2/c27-23-7-6-20(16-24(23)28)26(9-8-25(32)31(19-26)21-4-2-1-3-5-21)10-11-29-17-22(18-29)30-12-14-33-15-13-30/h6-7,16,21-22H,1-5,8-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5279709

Show SMILES NS(=O)(=O)N1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C24H35Cl2N5O3S/c25-21-4-3-19(13-22(21)26)24(6-5-23(32)30(17-24)14-18-1-2-18)7-8-28-15-20(16-28)29-9-11-31(12-10-29)35(27,33)34/h3-4,13,18,20H,1-2,5-12,14-17H2,(H2,27,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | CHEMBL5278616

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCSCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3OS/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5280682

Show SMILES CCOC1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C27H39Cl2N3O2/c1-2-34-23-8-12-31(13-9-23)22-17-30(18-22)14-11-27(21-5-6-24(28)25(29)15-21)10-7-26(33)32(19-27)16-20-3-4-20/h5-6,15,20,22-23H,2-4,7-14,16-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-2
(Homo sapiens (Human)) | BDBM50505325
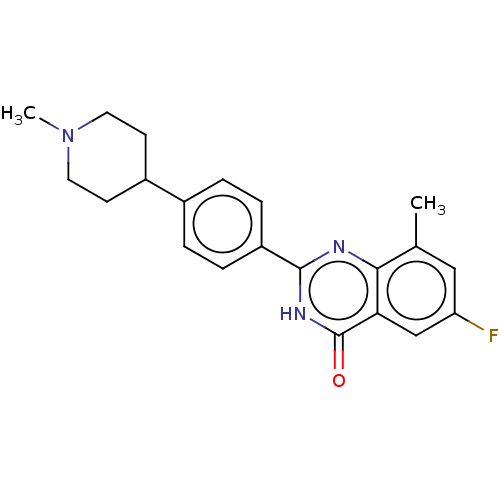
(CHEMBL4450725)Show SMILES CN1CCC(CC1)c1ccc(cc1)-c1nc2c(C)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C21H22FN3O/c1-13-11-17(22)12-18-19(13)23-20(24-21(18)26)16-5-3-14(4-6-16)15-7-9-25(2)10-8-15/h3-6,11-12,15H,7-10H2,1-2H3,(H,23,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS2 (873 to 1166 residues) expressed in baculovirus infected sf9 cells assessed ... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | BDBM50505325

(CHEMBL4450725)Show SMILES CN1CCC(CC1)c1ccc(cc1)-c1nc2c(C)cc(F)cc2c(=O)[nH]1 Show InChI InChI=1S/C21H22FN3O/c1-13-11-17(22)12-18-19(13)23-20(24-21(18)26)16-5-3-14(4-6-16)15-7-9-25(2)10-8-15/h3-6,11-12,15H,7-10H2,1-2H3,(H,23,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare KGaA
Curated by ChEMBL
| Assay Description
Inhibition of GST tagged-TEV cleavage site-fused human recombinant TNKS1 (1023 to 1327 residues) expressed in baculovirus infected sf9 cells assessed... |
J Med Chem 62: 7897-7909 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00656
BindingDB Entry DOI: 10.7270/Q2FJ2M28 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
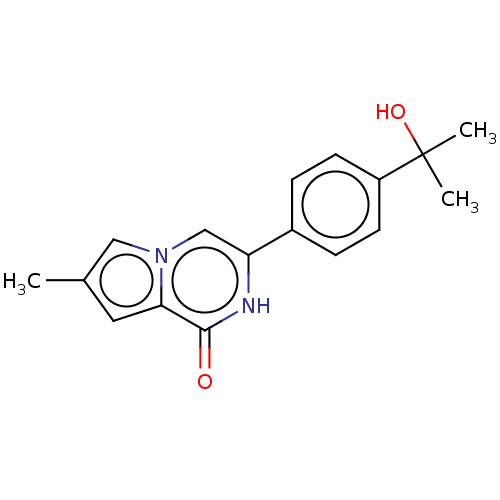
(Homo sapiens (Human)) | CHEMBL5280345
Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCOCC2)CCC(=O)NC1 Show InChI InChI=1S/C20H27Cl2N3O2/c21-17-2-1-15(11-18(17)22)20(4-3-19(26)23-14-20)5-6-24-12-16(13-24)25-7-9-27-10-8-25/h1-2,11,16H,3-10,12-14H2,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5281076

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCNCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H34Cl2N4O/c28-24-7-6-22(16-25(24)29)27(10-13-31-18-23(19-31)32-14-11-30-12-15-32)9-8-26(34)33(20-27)17-21-4-2-1-3-5-21/h1-7,16,23,30H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Plasmodium berghei |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5275576

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCS(=O)CC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3O2S/c28-24-7-6-22(16-25(24)29)27(9-8-26(33)32(20-27)17-21-4-2-1-3-5-21)10-11-30-18-23(19-30)31-12-14-35(34)15-13-31/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5280167

Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)C1CN(CCC2(CCC(=O)N(CC3CC3)C2)c2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C26H39Cl2N5O3S/c1-29(2)37(35,36)33-13-11-31(12-14-33)22-17-30(18-22)10-9-26(21-5-6-23(27)24(28)15-21)8-7-25(34)32(19-26)16-20-3-4-20/h5-6,15,20,22H,3-4,7-14,16-19H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of Neisseria gonorrhoea |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase tankyrase-1
(Homo sapiens (Human)) | CHEMBL5278616

Show SMILES Clc1ccc(cc1Cl)C1(CCN2CC(C2)N2CCSCC2)CCC(=O)N(Cc2ccccc2)C1 Show InChI InChI=1S/C27H33Cl2N3OS/c28-24-7-6-22(16-25(24)29)27(10-11-30-18-23(19-30)31-12-14-34-15-13-31)9-8-26(33)32(20-27)17-21-4-2-1-3-5-21/h1-7,16,23H,8-15,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data