

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301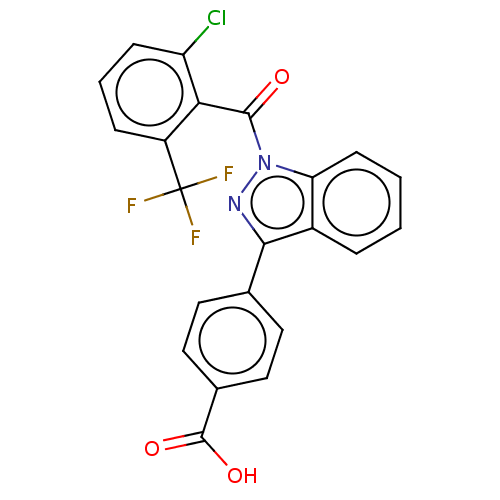 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Displacement of Alexa647-labeled MRL-87 from human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519883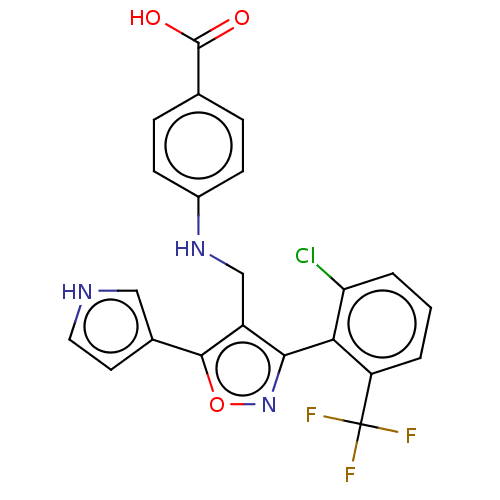 (CHEMBL4522217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519883 (CHEMBL4522217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Displacement of Alexa647-labeled MRL-87 from human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519883 (CHEMBL4522217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519881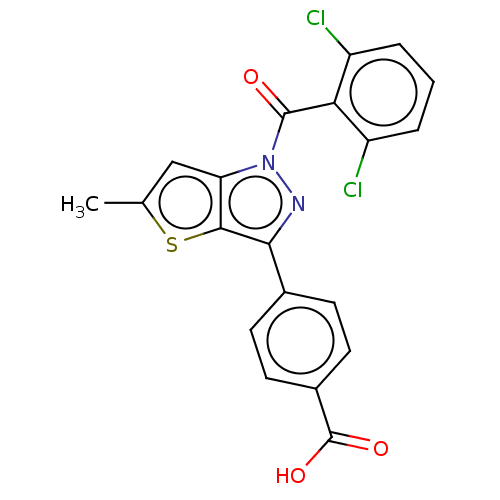 (CHEMBL4435145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Displacement of Alexa647-labeled MRL-87 from human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519883 (CHEMBL4522217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519883 (CHEMBL4522217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519881 (CHEMBL4435145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519881 (CHEMBL4435145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519881 (CHEMBL4435145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519881 (CHEMBL4435145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 547 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519885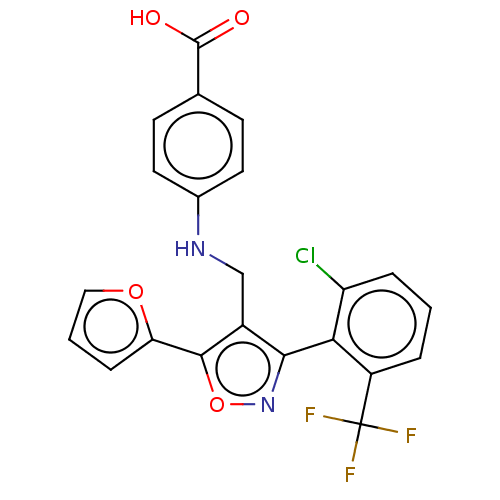 (CHEMBL4469931) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519884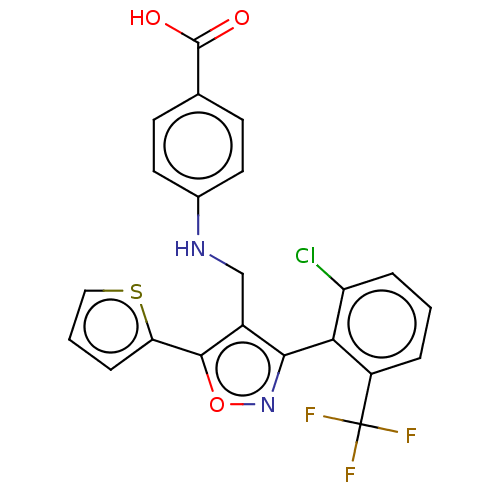 (CHEMBL4438380) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519882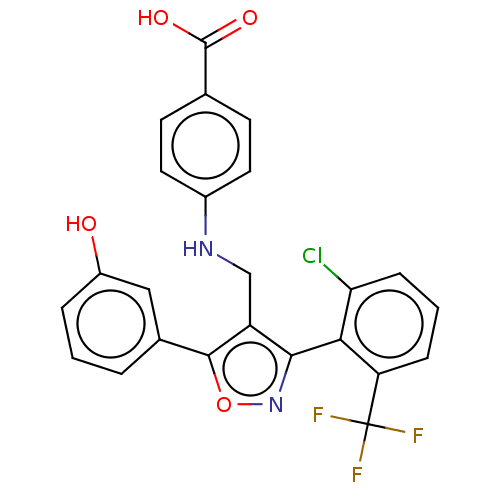 (CHEMBL4465726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM46355 (DIGOXIN | MLS000069819 | SMR000059217 | US10668094...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519891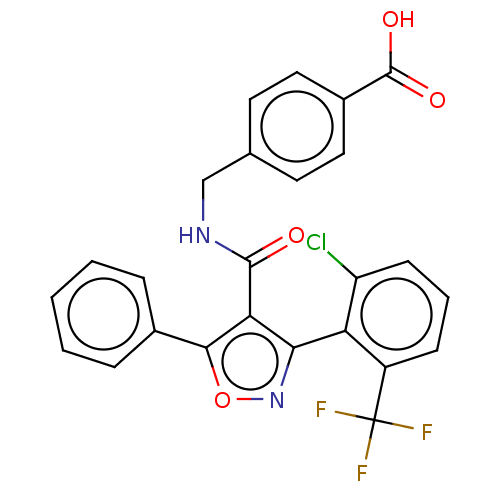 (CHEMBL4452679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519878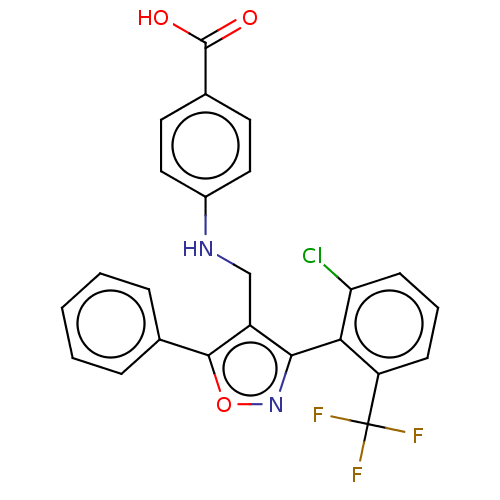 (CHEMBL4550831) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519881 (CHEMBL4435145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519886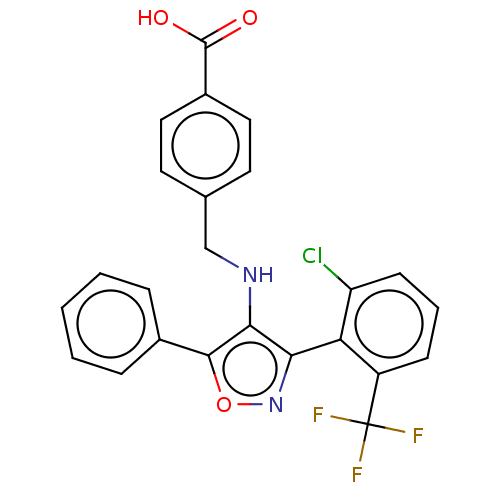 (CHEMBL4587804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM46355 (DIGOXIN | MLS000069819 | SMR000059217 | US10668094...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519880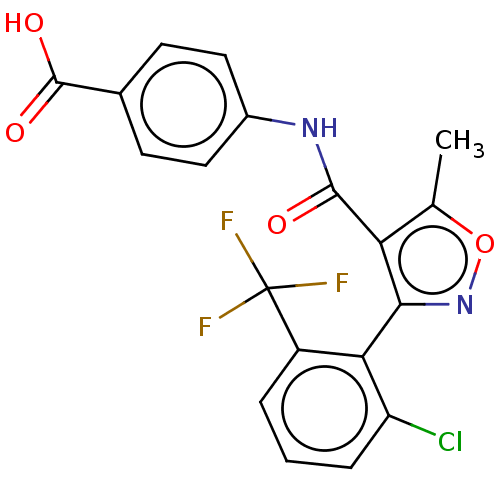 (CHEMBL4518713) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519894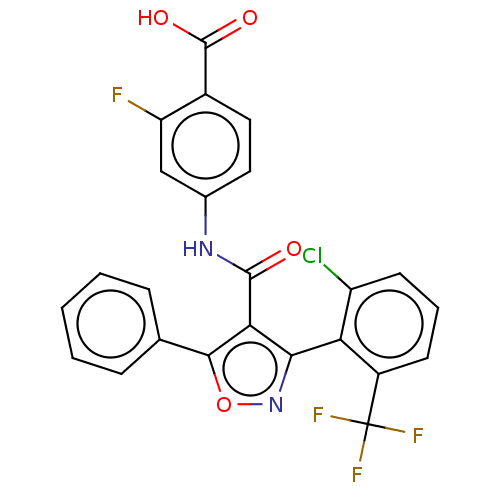 (CHEMBL4436630) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519893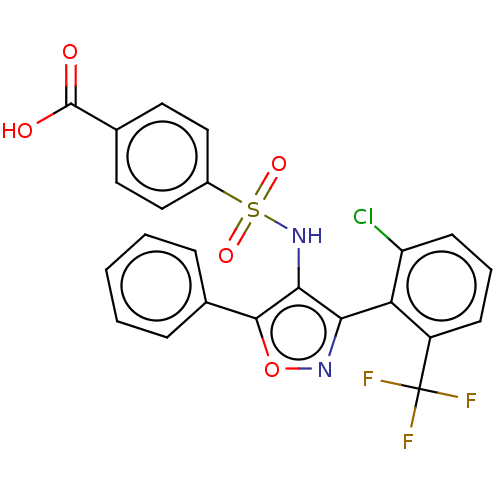 (CHEMBL4437814) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519890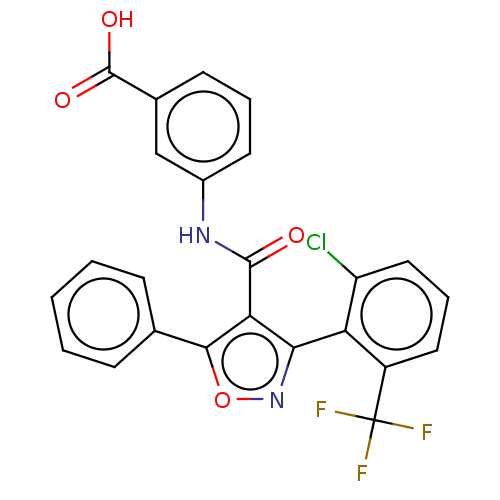 (CHEMBL4452409) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519895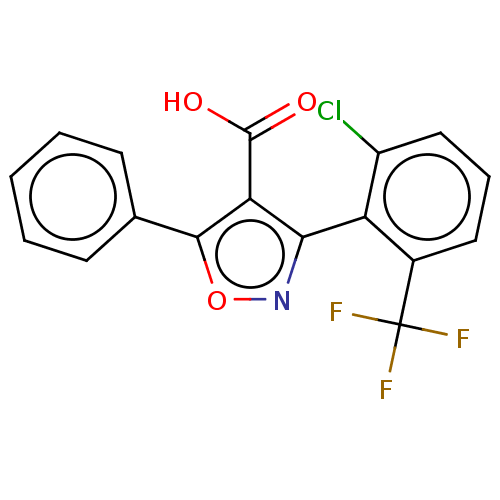 (CHEMBL4435027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519886 (CHEMBL4587804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519887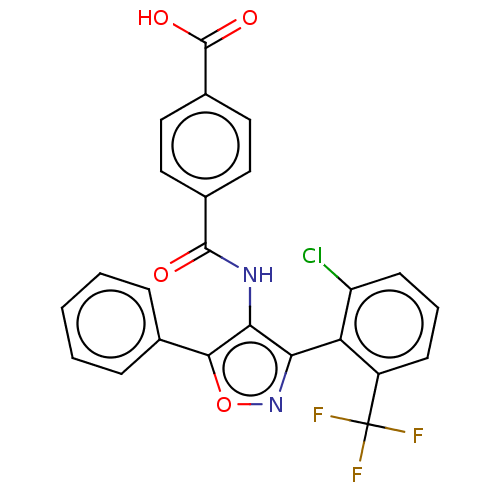 (CHEMBL4457177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519891 (CHEMBL4452679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519879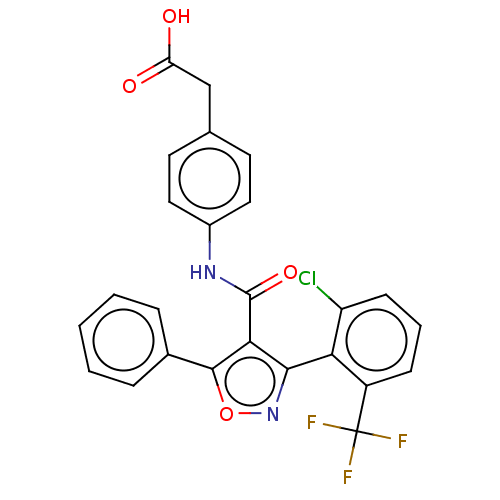 (CHEMBL4574983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519889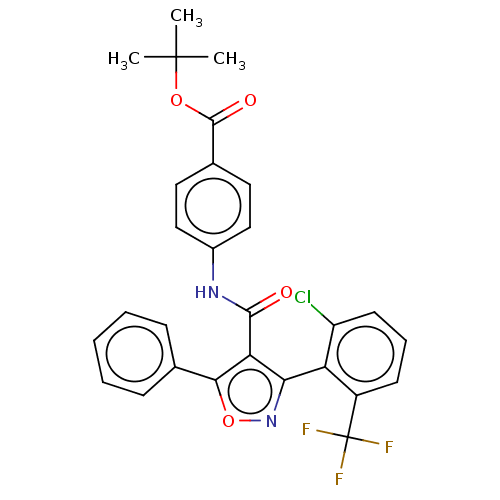 (CHEMBL4466846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519888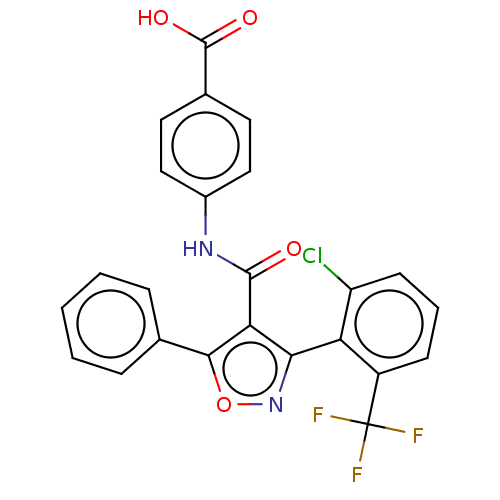 (CHEMBL4444484) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519888 (CHEMBL4444484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519893 (CHEMBL4437814) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519890 (CHEMBL4452409) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519878 (CHEMBL4550831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM46355 (DIGOXIN | MLS000069819 | SMR000059217 | US10668094...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Competitive inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) ass... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519894 (CHEMBL4436630) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519883 (CHEMBL4522217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519882 (CHEMBL4465726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519884 (CHEMBL4438380) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519885 (CHEMBL4469931) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50519892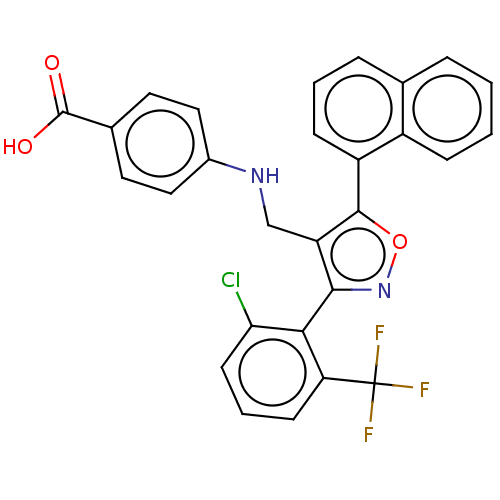 (CHEMBL4514441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at His6-tagged PPARgamma LBD (unknown origin) assessed as inhibition of rosiglitazone-induced N-terminal biotinylated co-act... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519892 (CHEMBL4514441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519889 (CHEMBL4466846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50519887 (CHEMBL4457177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universiteit Eindhoven Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal His6-tagged RORgammat LBD (265 to 518 residues) expressed in Escherichia coli BL21 (DE3) assessed as inh... | J Med Chem 63: 241-259 (2020) Article DOI: 10.1021/acs.jmedchem.9b01372 BindingDB Entry DOI: 10.7270/Q2XP78BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |