

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415362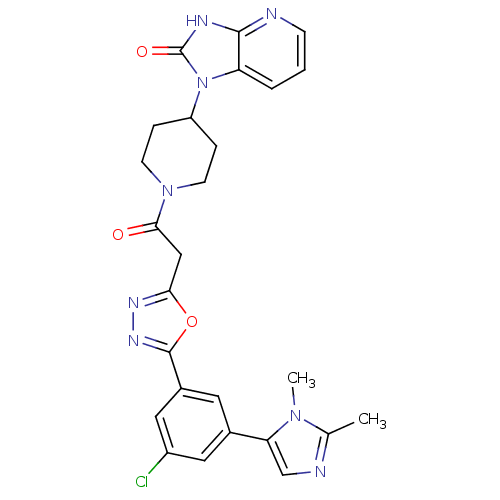 (CHEMBL601857) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415365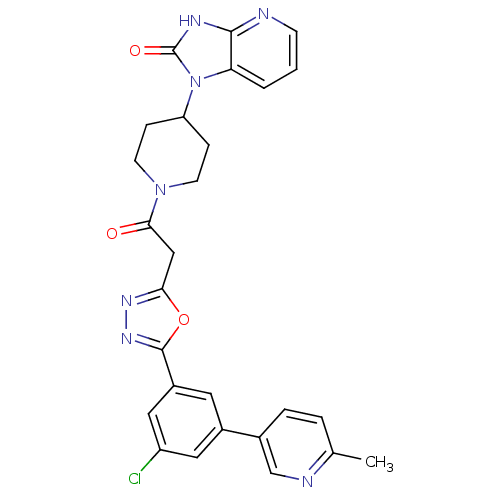 (CHEMBL609757) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415354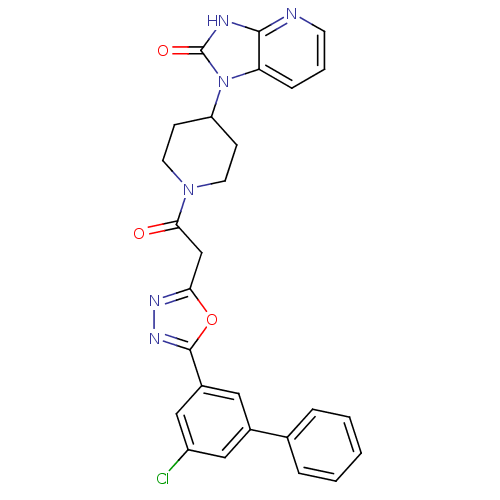 (CHEMBL600836) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415360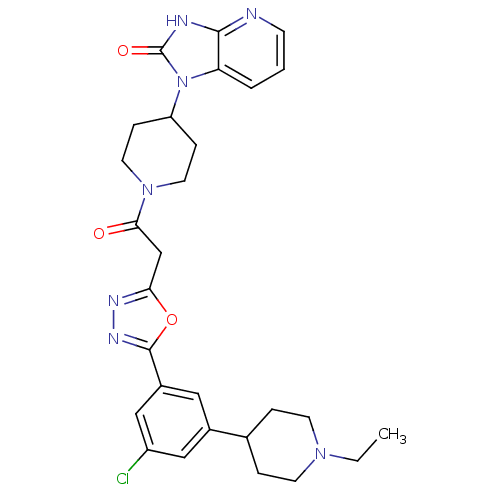 (CHEMBL610062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415361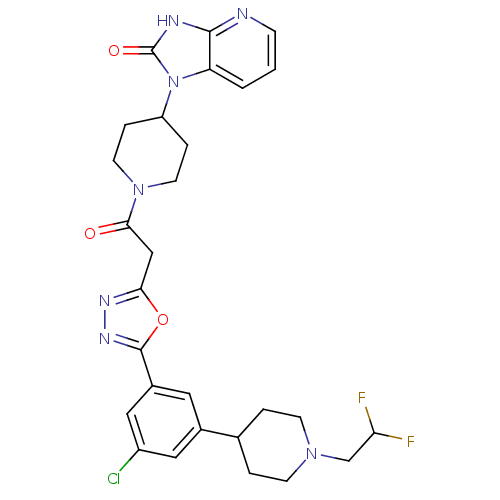 (CHEMBL592649) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415352 (CHEMBL600636) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415359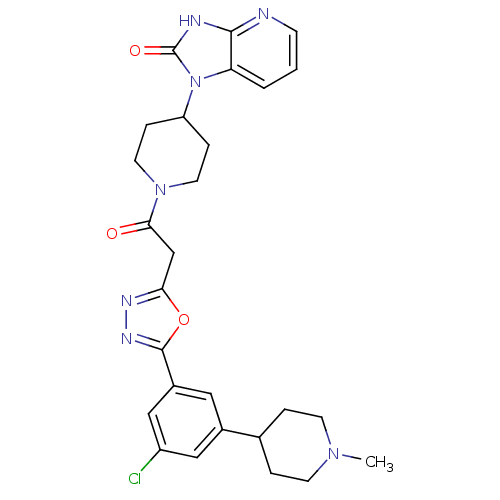 (CHEMBL601854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415363 (CHEMBL605579) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415358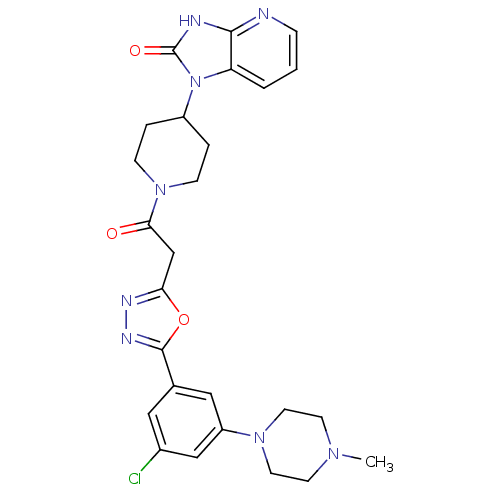 (CHEMBL601639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415355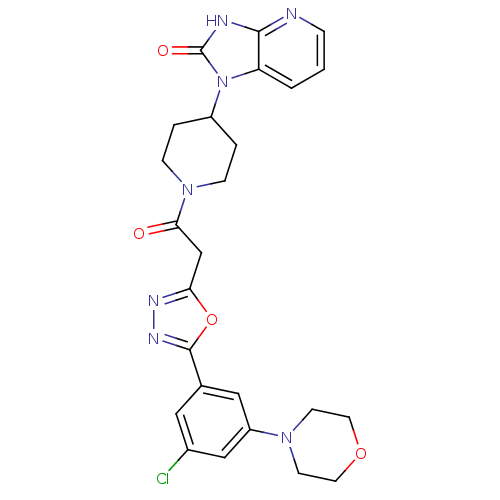 (CHEMBL600837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415356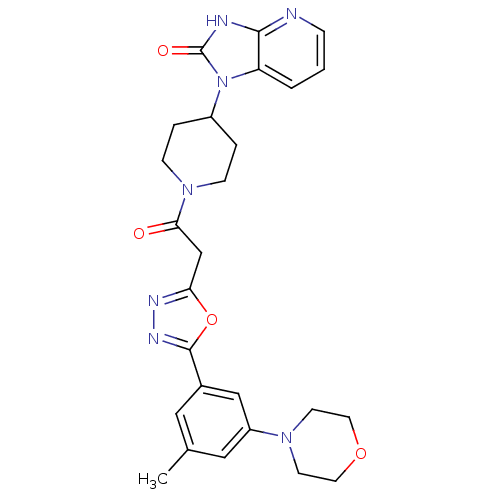 (CHEMBL609472) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394447 (CHEMBL2159662) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate | Bioorg Med Chem 20: 6739-50 (2012) Article DOI: 10.1016/j.bmc.2012.09.016 BindingDB Entry DOI: 10.7270/Q2ZW1N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415353 (CHEMBL604949) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50516038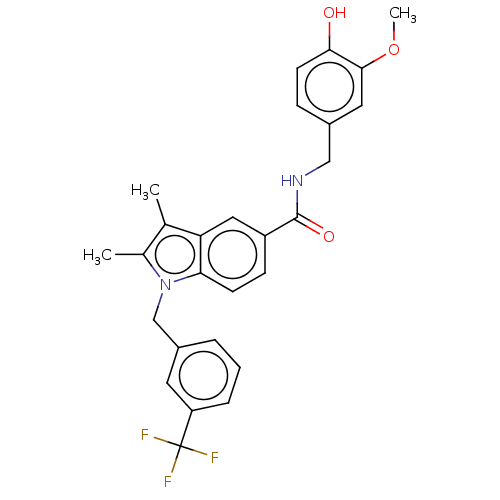 (CHEMBL4558373) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Oklahoma Health Science Center Curated by ChEMBL | Assay Description Displacement of fluormone PPAR green tracer ligand from recombinant GST-tagged PPARgamma ligand binding domain (unknown origin) incubated for 4 hrs b... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126664 BindingDB Entry DOI: 10.7270/Q2XW4P50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using acetylthiocholine as substrate | Bioorg Med Chem 20: 6739-50 (2012) Article DOI: 10.1016/j.bmc.2012.09.016 BindingDB Entry DOI: 10.7270/Q2ZW1N1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50394448 (CHEMBL2159661) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate | Bioorg Med Chem 20: 6739-50 (2012) Article DOI: 10.1016/j.bmc.2012.09.016 BindingDB Entry DOI: 10.7270/Q2ZW1N1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024721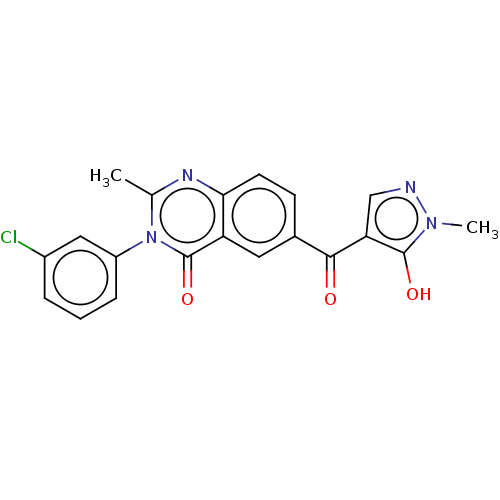 (CHEMBL3342603) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024752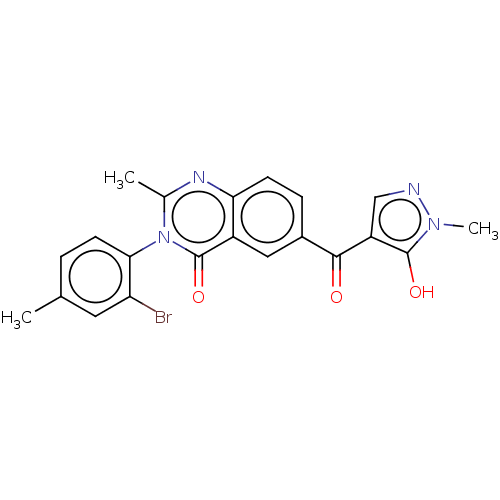 (CHEMBL3343183) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Escherichia coli (Enterobacteria)) | BDBM50511223 (CHEMBL4589187) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 | J Med Chem 63: 4617-4627 (2020) Article DOI: 10.1021/acs.jmedchem.9b01918 BindingDB Entry DOI: 10.7270/Q2CJ8HTJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-glucuronidase (Bos taurus (Bovine)) | BDBM50511223 (CHEMBL4589187) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica Curated by ChEMBL | Assay Description Inhibition of bovine GUS pre-incubated for 5 to 30 mins before 4-methylumbelliferone beta-D-glucuronide substrate addition at pH 6.7 | J Med Chem 63: 4617-4627 (2020) Article DOI: 10.1021/acs.jmedchem.9b01918 BindingDB Entry DOI: 10.7270/Q2CJ8HTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM147258 (US8957093, 78) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Oklahoma Health Science Center Curated by ChEMBL | Assay Description Displacement of fluormone PPAR green tracer ligand from recombinant GST-tagged PPARgamma ligand binding domain (unknown origin) incubated for 4 hrs b... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126664 BindingDB Entry DOI: 10.7270/Q2XW4P50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415351 (CHEMBL601246) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415364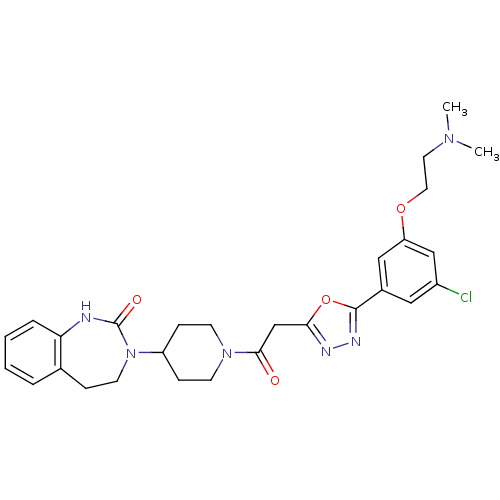 (CHEMBL602475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50516035 (CHEMBL4434731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Oklahoma Health Science Center Curated by ChEMBL | Assay Description Displacement of fluormone PPAR green tracer ligand from recombinant GST-tagged PPARgamma ligand binding domain (unknown origin) incubated for 4 hrs b... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126664 BindingDB Entry DOI: 10.7270/Q2XW4P50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024719 (CHEMBL3342605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50516043 (CHEMBL4591564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Oklahoma Health Science Center Curated by ChEMBL | Assay Description Displacement of fluormone PPAR green tracer ligand from recombinant GST-tagged PPARgamma ligand binding domain (unknown origin) incubated for 4 hrs b... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126664 BindingDB Entry DOI: 10.7270/Q2XW4P50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024720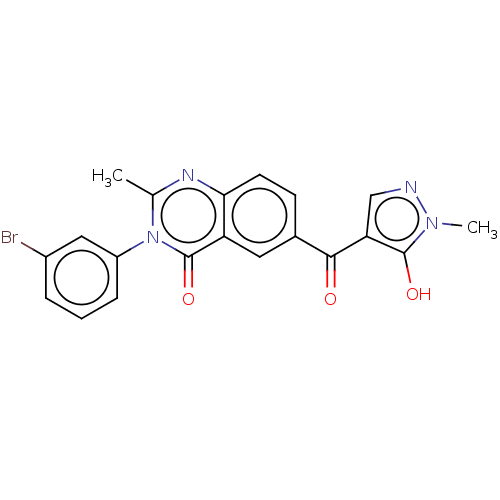 (CHEMBL3342604) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024727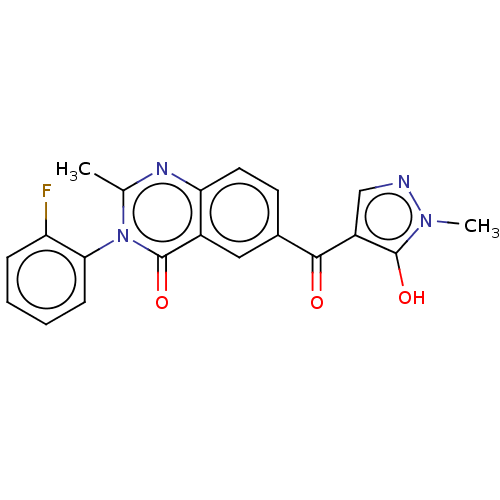 (CHEMBL3342432) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415364 (CHEMBL602475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415346 (CHEMBL602085) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415360 (CHEMBL610062) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024758 (CHEMBL3342610) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024728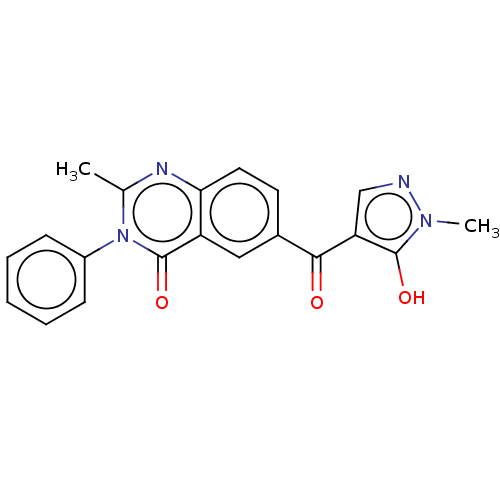 (CHEMBL3342431) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415359 (CHEMBL601854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024722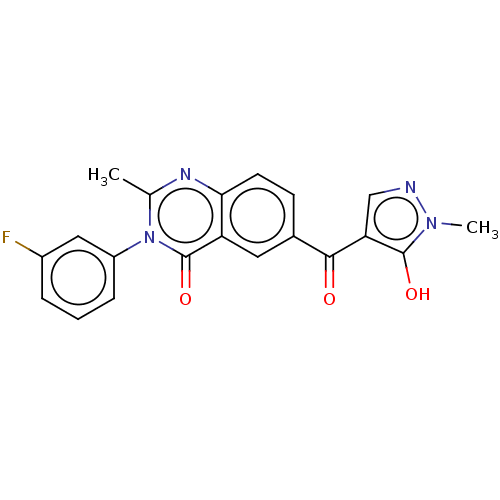 (CHEMBL3342602) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024726 (CHEMBL3342598) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024759 (CHEMBL3342609) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024725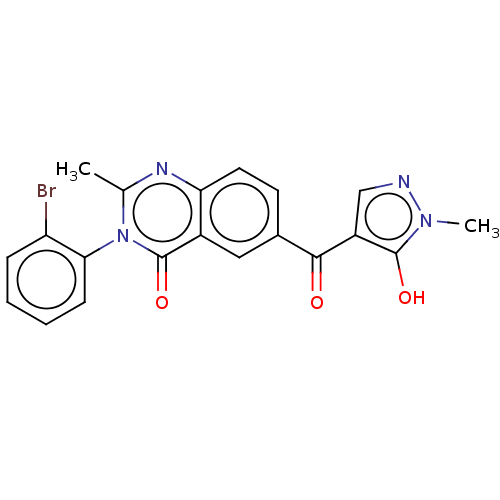 (CHEMBL3342599) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024751 (CHEMBL3343184) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024757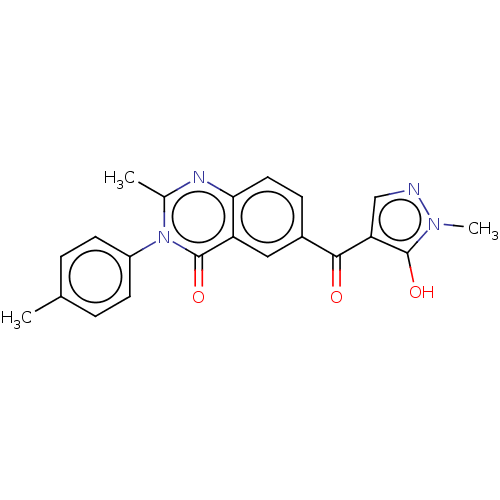 (CHEMBL3342611) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415358 (CHEMBL601639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415350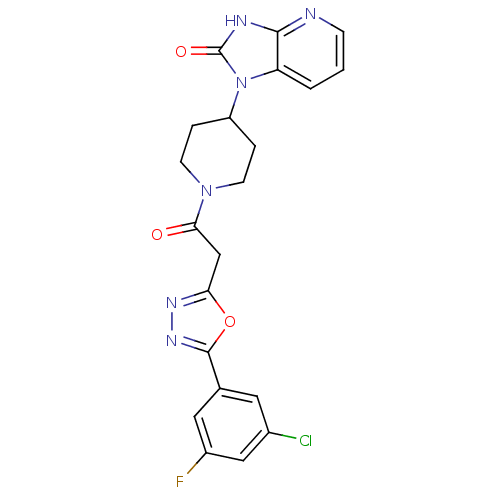 (CHEMBL601034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415366 (CHEMBL601455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50415348 (CHEMBL602086) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... | Bioorg Med Chem Lett 20: 1368-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.012 BindingDB Entry DOI: 10.7270/Q23T9JG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50516034 (CHEMBL4568299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Oklahoma Health Science Center Curated by ChEMBL | Assay Description Displacement of fluormone PPAR green tracer ligand from recombinant GST-tagged PPARgamma ligand binding domain (unknown origin) incubated for 4 hrs b... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126664 BindingDB Entry DOI: 10.7270/Q2XW4P50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024716 (CHEMBL3342607) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate | Bioorg Med Chem 20: 6739-50 (2012) Article DOI: 10.1016/j.bmc.2012.09.016 BindingDB Entry DOI: 10.7270/Q2ZW1N1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024754 (CHEMBL3342614) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024760 (CHEMBL3342608) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxyphenylpyruvate dioxygenase (Homo sapiens (Human)) | BDBM50024718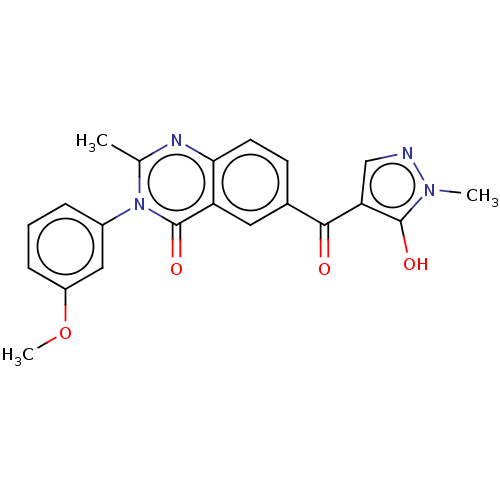 (CHEMBL3342606) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of purified His6-tagged recombinant human HPPD assessed as inhibition of maleylacetoacetate formation after 30 mins by UV/visible spectrop... | Bioorg Med Chem 22: 5194-211 (2014) Article DOI: 10.1016/j.bmc.2014.08.011 BindingDB Entry DOI: 10.7270/Q2DF6SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1248 total ) | Next | Last >> |