 Found 2927 hits with Last Name = 'li' and Initial = 'jj'
Found 2927 hits with Last Name = 'li' and Initial = 'jj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0510 | -58.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
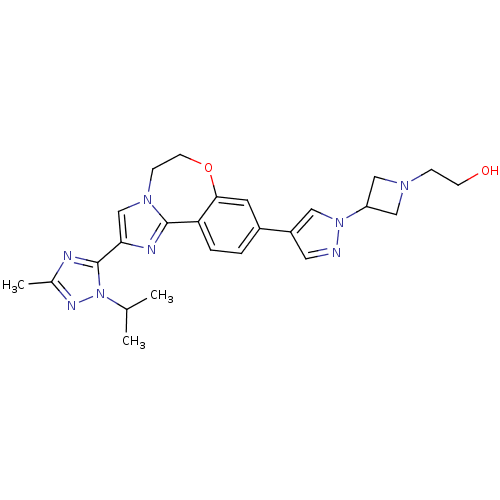
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434807

(CHEMBL2387079)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CC(C)(C)O)c1 Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-22(24-14-26-30)19-12-28-7-8-32-20-9-16(5-6-18(20)21(28)27-19)17-10-25-29(11-17)13-23(3,4)31/h5-6,9-12,14-15,31H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122970

(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-5-1-2-6-20(18)30-25-19(27)14-31(26(25)16-7-8-22-24(12-16)35-15-34-22)28(33)23-10-9-21(36-23)17-4-3-11-29-13-17/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434812

(CHEMBL2387086)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C21H23N7O2/c1-14(2)28-21(22-13-24-28)18-12-26-6-8-30-19-9-15(3-4-17(19)20(26)25-18)16-10-23-27(11-16)5-7-29/h3-4,9-14,29H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122969
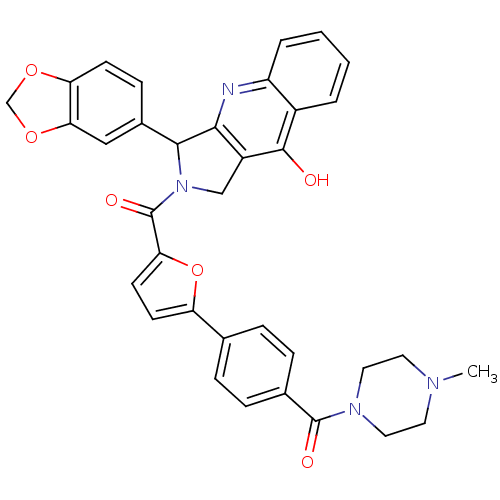
(3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C35H30N4O6/c1-37-14-16-38(17-15-37)34(41)22-8-6-21(7-9-22)27-12-13-29(45-27)35(42)39-19-25-31(36-26-5-3-2-4-24(26)33(25)40)32(39)23-10-11-28-30(18-23)44-20-43-28/h2-13,18,32H,14-17,19-20H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138930
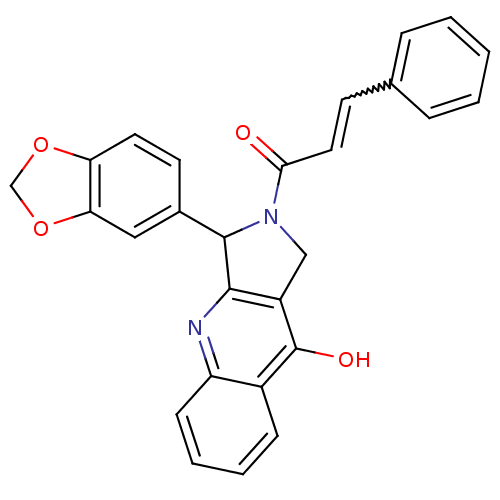
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C=Cc1ccccc1 |w:26.31| Show InChI InChI=1S/C27H20N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138939
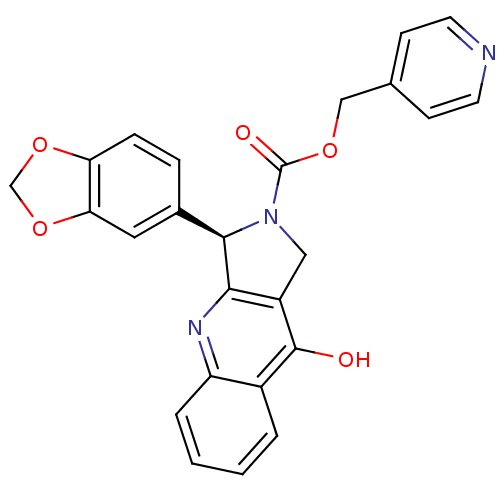
((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122990
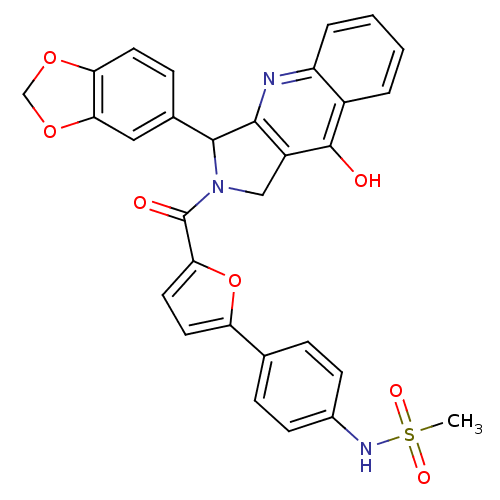
(CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H23N3O7S/c1-41(36,37)32-19-9-6-17(7-10-19)23-12-13-25(40-23)30(35)33-15-21-27(31-22-5-3-2-4-20(22)29(21)34)28(33)18-8-11-24-26(14-18)39-16-38-24/h2-14,28,32H,15-16H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138929

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434814

(CHEMBL2387082)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(C)c1 Show InChI InChI=1S/C21H23N7O/c1-13(2)28-21(23-14(3)25-28)18-12-27-7-8-29-19-9-15(16-10-22-26(4)11-16)5-6-17(19)20(27)24-18/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484029
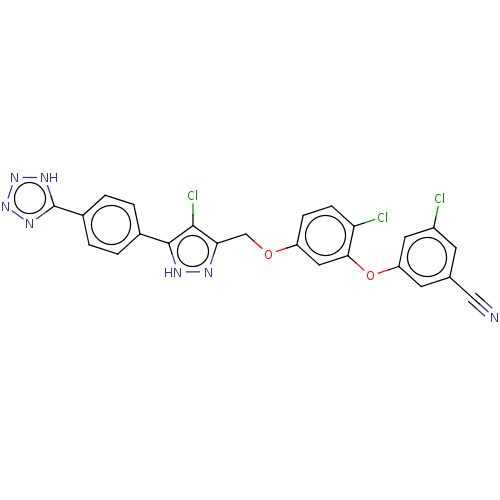
(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484039
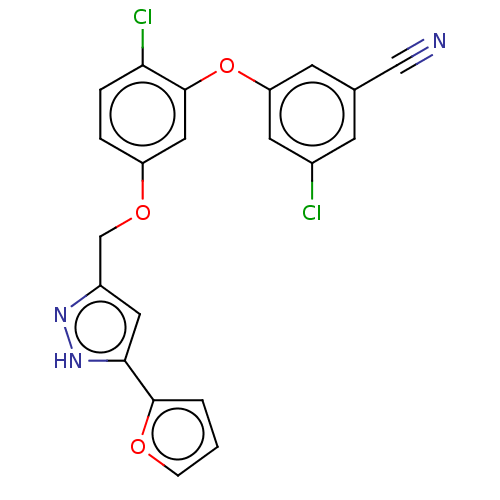
(CHEMBL1800087)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccco3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O3/c22-14-6-13(11-24)7-17(8-14)29-21-10-16(3-4-18(21)23)28-12-15-9-19(26-25-15)20-2-1-5-27-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434808
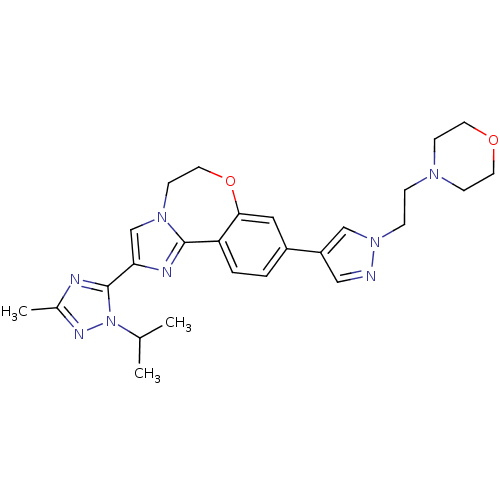
(CHEMBL2386972)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C26H32N8O2/c1-18(2)34-26(28-19(3)30-34)23-17-32-10-13-36-24-14-20(4-5-22(24)25(32)29-23)21-15-27-33(16-21)7-6-31-8-11-35-12-9-31/h4-5,14-18H,6-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434817

(CHEMBL2387081)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N7O/c1-12(2)26-19(20-11-23-26)16-10-25-5-6-27-17-7-13(14-8-21-22-9-14)3-4-15(17)18(25)24-16/h3-4,7-12H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122974
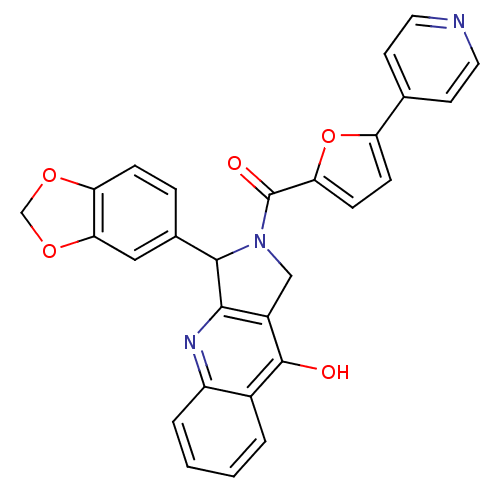
(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccncc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-3-1-2-4-20(18)30-25-19(27)14-31(26(25)17-5-6-22-24(13-17)35-15-34-22)28(33)23-8-7-21(36-23)16-9-11-29-12-10-16/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434809

(CHEMBL2386971)Show SMILES C[C@H](O)C(=O)N1CC(C1)n1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1nc(C)nn1C(C)C |r| Show InChI InChI=1S/C26H30N8O3/c1-15(2)34-25(28-17(4)30-34)22-14-31-7-8-37-23-9-18(5-6-21(23)24(31)29-22)19-10-27-33(11-19)20-12-32(13-20)26(36)16(3)35/h5-6,9-11,14-16,20,35H,7-8,12-13H2,1-4H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138936
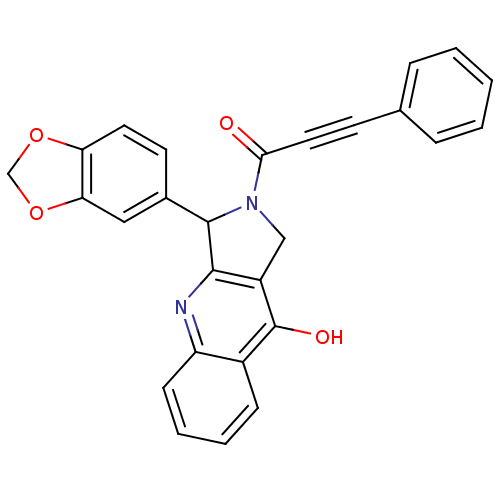
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propynoyl)-1,2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C#Cc1ccccc1 Show InChI InChI=1S/C27H18N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-9,11-12,14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122964

(3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...)Show SMILES Oc1ccc2cc(oc2c1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C27H18N2O6/c30-16-7-5-14-9-23(35-21(14)11-16)27(32)29-12-18-24(28-19-4-2-1-3-17(19)26(18)31)25(29)15-6-8-20-22(10-15)34-13-33-20/h1-11,25,30H,12-13H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304781
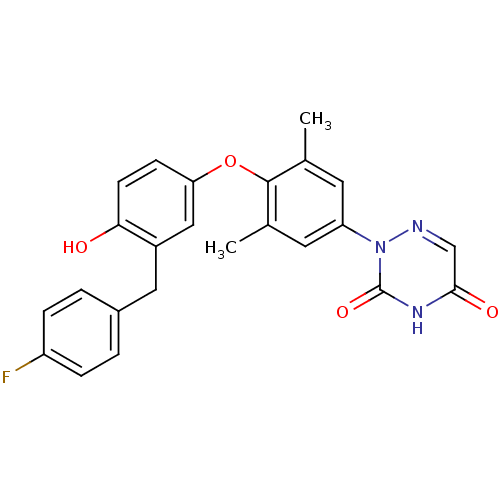
(2-(4-(3-(4-fluorobenzyl)-4-hydroxyphenoxy)-3,5-dim...)Show SMILES Cc1cc(cc(C)c1Oc1ccc(O)c(Cc2ccc(F)cc2)c1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C24H20FN3O4/c1-14-9-19(28-24(31)27-22(30)13-26-28)10-15(2)23(14)32-20-7-8-21(29)17(12-20)11-16-3-5-18(25)6-4-16/h3-10,12-13,29H,11H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434813

(CHEMBL2387083)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C22H25N7O2/c1-14(2)29-22(24-15(3)26-29)19-13-27-7-9-31-20-10-16(4-5-18(20)21(27)25-19)17-11-23-28(12-17)6-8-30/h4-5,10-14,30H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36543
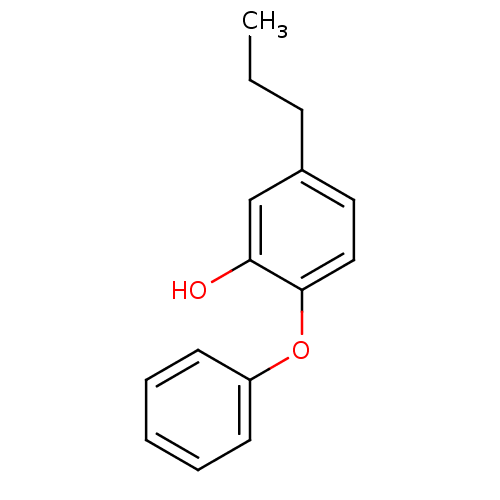
(5-propyl-2-phenoxyphenol | PT02)Show InChI InChI=1S/C15H16O2/c1-2-6-12-9-10-15(14(16)11-12)17-13-7-4-3-5-8-13/h3-5,7-11,16H,2,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122966
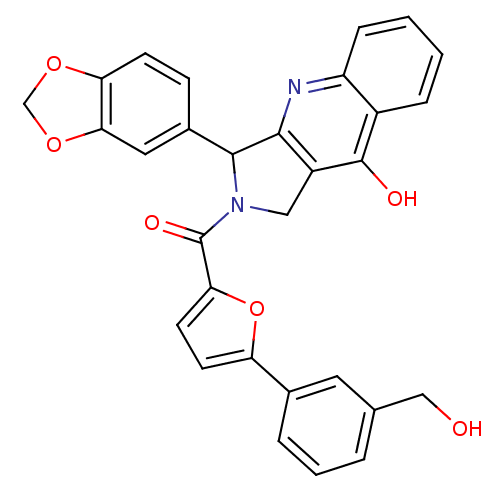
(3-Benzo[1,3]dioxol-5-yl-2-[5-(3-hydroxymethyl-phen...)Show SMILES OCc1cccc(c1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O6/c33-15-17-4-3-5-18(12-17)23-10-11-25(38-23)30(35)32-14-21-27(31-22-7-2-1-6-20(22)29(21)34)28(32)19-8-9-24-26(13-19)37-16-36-24/h1-13,28,33H,14-16H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484045
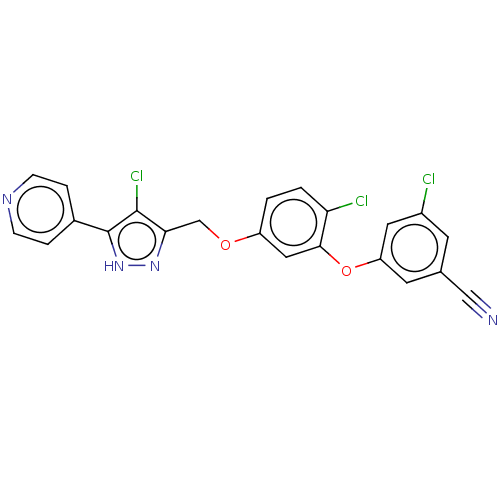
(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484045

(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304778
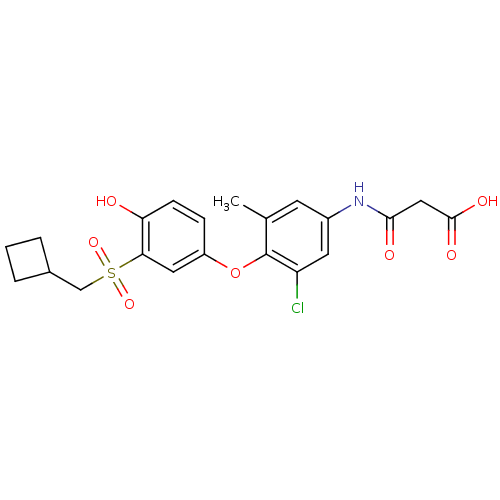
(3-(3-chloro-4-(3-(cyclobutylmethylsulfonyl)-4-hydr...)Show SMILES Cc1cc(NC(=O)CC(O)=O)cc(Cl)c1Oc1ccc(O)c(c1)S(=O)(=O)CC1CCC1 Show InChI InChI=1S/C21H22ClNO7S/c1-12-7-14(23-19(25)10-20(26)27)8-16(22)21(12)30-15-5-6-17(24)18(9-15)31(28,29)11-13-3-2-4-13/h5-9,13,24H,2-4,10-11H2,1H3,(H,23,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304777
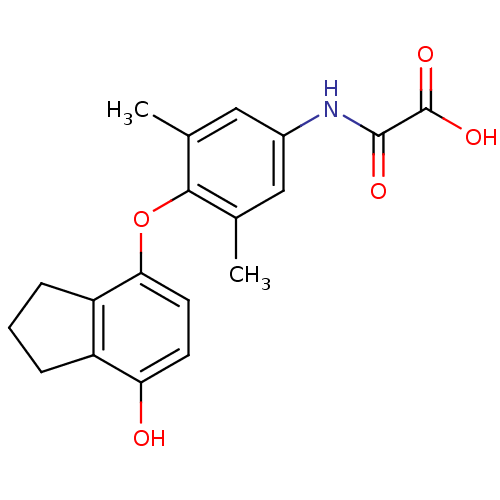
(2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...)Show SMILES Cc1cc(NC(=O)C(O)=O)cc(C)c1Oc1ccc(O)c2CCCc12 Show InChI InChI=1S/C19H19NO5/c1-10-8-12(20-18(22)19(23)24)9-11(2)17(10)25-16-7-6-15(21)13-4-3-5-14(13)16/h6-9,21H,3-5H2,1-2H3,(H,20,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18188
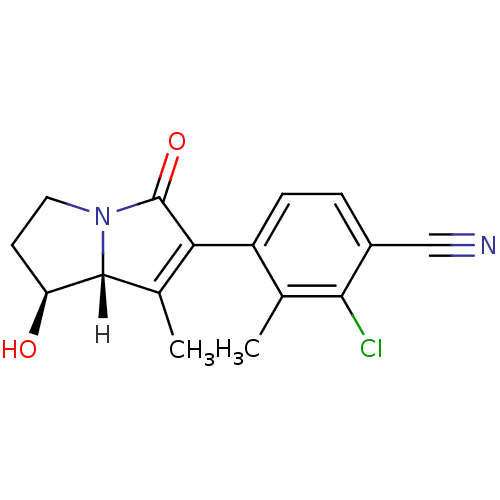
(4-[(1S,7aR)-1-hydroxy-7-methyl-5-oxo-2,3,5,7a-tetr...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)C(=C2C)c1ccc(C#N)c(Cl)c1C |r,c:10| Show InChI InChI=1S/C16H15ClN2O2/c1-8-11(4-3-10(7-18)14(8)17)13-9(2)15-12(20)5-6-19(15)16(13)21/h3-4,12,15,20H,5-6H2,1-2H3/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | 2 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50304780
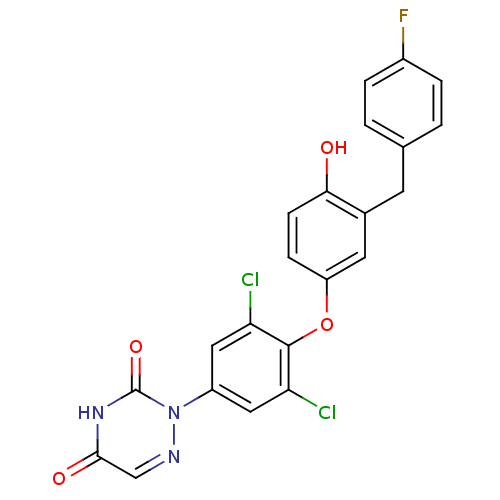
(2-(3,5-dichloro-4-(3-(4-fluorobenzyl)-4-hydroxyphe...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1Cc1ccc(F)cc1 Show InChI InChI=1S/C22H14Cl2FN3O4/c23-17-9-15(28-22(31)27-20(30)11-26-28)10-18(24)21(17)32-16-5-6-19(29)13(8-16)7-12-1-3-14(25)4-2-12/h1-6,8-11,29H,7H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor beta |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122971
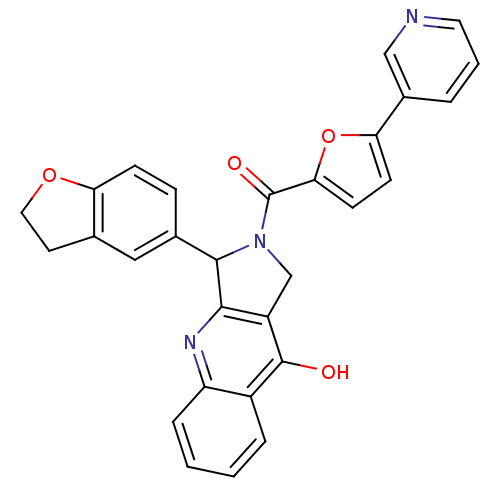
(3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C29H21N3O4/c33-28-20-5-1-2-6-22(20)31-26-21(28)16-32(27(26)18-7-8-23-17(14-18)11-13-35-23)29(34)25-10-9-24(36-25)19-4-3-12-30-15-19/h1-10,12,14-15,27H,11,13,16H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138927
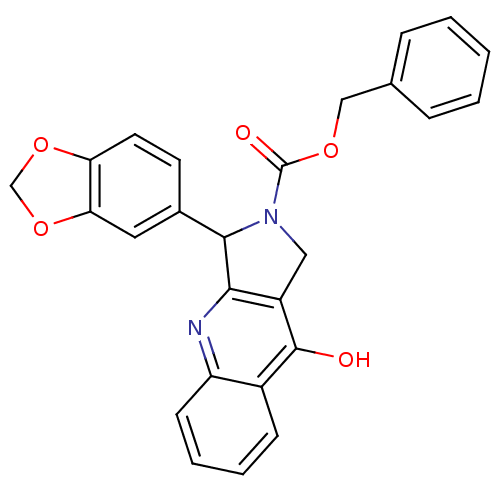
(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H20N2O5/c29-25-18-8-4-5-9-20(18)27-23-19(25)13-28(26(30)31-14-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)33-15-32-21/h1-12,24H,13-15H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484030

(CHEMBL1801256)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccccn1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-14-7-13(11-26)8-16(9-14)31-20-10-15(4-5-17(20)24)30-12-19-21(25)22(29-28-19)18-3-1-2-6-27-18/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484029

(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant by electrochemiluminescent assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122973

(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxymethyl-phen...)Show SMILES OCc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O6/c33-15-17-5-7-18(8-6-17)23-11-12-25(38-23)30(35)32-14-21-27(31-22-4-2-1-3-20(22)29(21)34)28(32)19-9-10-24-26(13-19)37-16-36-24/h1-13,28,33H,14-16H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122983

(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-nitro-phenyl)-fura...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C29H19N3O7/c33-28-19-3-1-2-4-21(19)30-26-20(28)14-31(27(26)17-7-10-23-25(13-17)38-15-37-23)29(34)24-12-11-22(39-24)16-5-8-18(9-6-16)32(35)36/h1-13,27H,14-15H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484029

(CHEMBL1801258)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccc(cc1)-c1nnn[nH]1 Show InChI InChI=1S/C24H14Cl3N7O2/c25-16-7-13(11-28)8-18(9-16)36-21-10-17(5-6-19(21)26)35-12-20-22(27)23(30-29-20)14-1-3-15(4-2-14)24-31-33-34-32-24/h1-10H,12H2,(H,29,30)(H,31,32,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484031
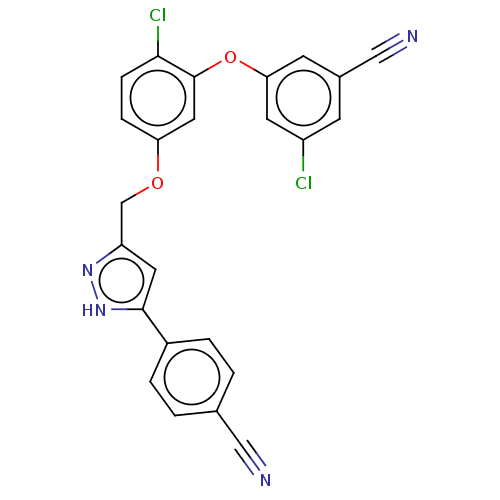
(CHEMBL1801255)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccc(cc3)C#N)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C24H14Cl2N4O2/c25-18-7-16(13-28)8-21(9-18)32-24-11-20(5-6-22(24)26)31-14-19-10-23(30-29-19)17-3-1-15(12-27)2-4-17/h1-11H,14H2,(H,29,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484032
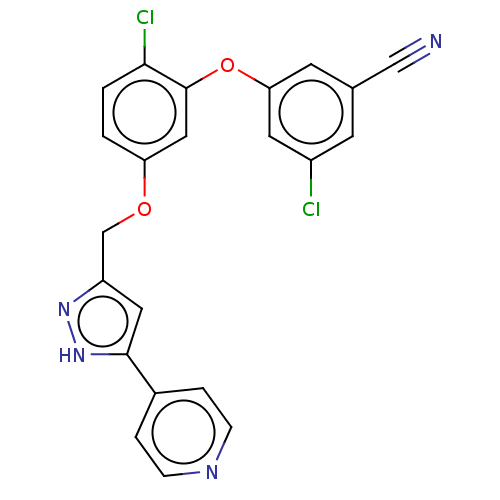
(CHEMBL1801231)Show SMILES Clc1cc(Oc2cc(OCc3cc([nH]n3)-c3ccncc3)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C22H14Cl2N4O2/c23-16-7-14(12-25)8-19(9-16)30-22-11-18(1-2-20(22)24)29-13-17-10-21(28-27-17)15-3-5-26-6-4-15/h1-11H,13H2,(H,27,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50304777

(2-(4-(7-hydroxy-2,3-dihydro-1H-inden-4-yloxy)-3,5-...)Show SMILES Cc1cc(NC(=O)C(O)=O)cc(C)c1Oc1ccc(O)c2CCCc12 Show InChI InChI=1S/C19H19NO5/c1-10-8-12(20-18(22)19(23)24)9-11(2)17(10)25-16-7-6-15(21)13-4-3-5-14(13)16/h6-9,21H,3-5H2,1-2H3,(H,20,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thyroid receptor alpha |
Bioorg Med Chem Lett 20: 306-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.109
BindingDB Entry DOI: 10.7270/Q2J38SNP |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50484040
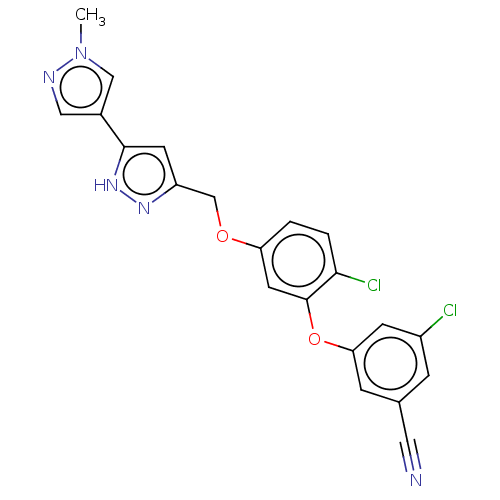
(CHEMBL1801228)Show SMILES Cn1cc(cn1)-c1cc(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]1 Show InChI InChI=1S/C21H15Cl2N5O2/c1-28-11-14(10-25-28)20-7-16(26-27-20)12-29-17-2-3-19(23)21(8-17)30-18-5-13(9-24)4-15(22)6-18/h2-8,10-11H,12H2,1H3,(H,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
Motilin receptor
(Homo sapiens (Human)) | BDBM50143028

(8'N-[1-[1-carbamoyl-3-phenyl-(1S)-propylcarbamoyl]...)Show SMILES NC(=O)[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)C1CCC2(CCNCC2)n2n1c(=O)n(Cc1ccc3OCOc3c1)c2=O Show InChI InChI=1S/C39H45N7O7/c40-34(47)29(14-11-26-7-3-1-4-8-26)42-35(48)30(15-12-27-9-5-2-6-10-27)43-36(49)31-17-18-39(19-21-41-22-20-39)46-38(51)44(37(50)45(31)46)24-28-13-16-32-33(23-28)53-25-52-32/h1-10,13,16,23,29-31,41H,11-12,14-15,17-22,24-25H2,(H2,40,47)(H,42,48)(H,43,49)/t29-,30-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human motilin receptor |
J Med Chem 47: 1704-8 (2004)
Article DOI: 10.1021/jm0304865
BindingDB Entry DOI: 10.7270/Q2571CRC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18183
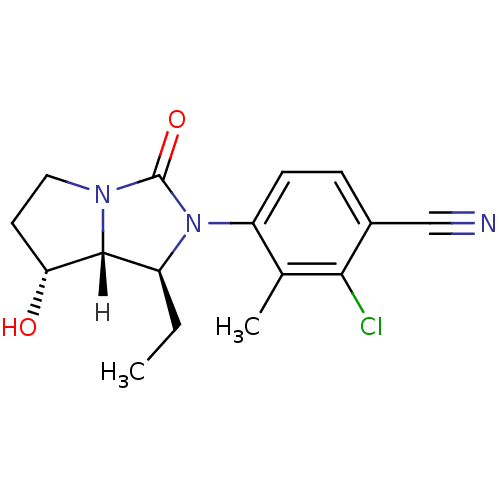
(4-[(1S,7R,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13+,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 2.60 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50484045

(CHEMBL1801257)Show SMILES Clc1c(COc2ccc(Cl)c(Oc3cc(Cl)cc(c3)C#N)c2)n[nH]c1-c1ccncc1 Show InChI InChI=1S/C22H13Cl3N4O2/c23-15-7-13(11-26)8-17(9-15)31-20-10-16(1-2-18(20)24)30-12-19-21(25)22(29-28-19)14-3-5-27-6-4-14/h1-10H,12H2,(H,28,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant by SPA assay |
Bioorg Med Chem Lett 20: 4328-32 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.083
BindingDB Entry DOI: 10.7270/Q2TH8QJ4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122980
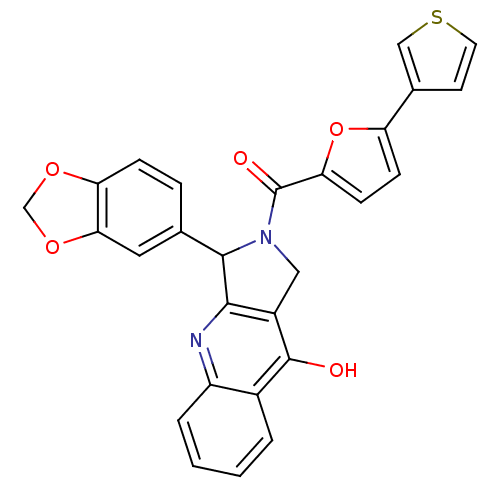
(3-Benzo[1,3]dioxol-5-yl-2-(5-thiophen-3-yl-furan-2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccsc1 Show InChI InChI=1S/C27H18N2O5S/c30-26-17-3-1-2-4-19(17)28-24-18(26)12-29(25(24)15-5-6-21-23(11-15)33-14-32-21)27(31)22-8-7-20(34-22)16-9-10-35-13-16/h1-11,13,25H,12,14H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data