

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50346374 (3-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.000178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in Sprague-Dawley rat brain cortex homogenates after 30 mins by liquid scintillation counting | Eur J Med Chem 46: 2206-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.001 BindingDB Entry DOI: 10.7270/Q2M32W4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359960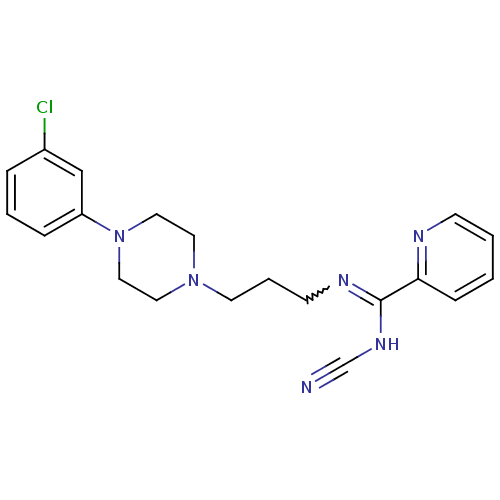 (CHEMBL1927094) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359961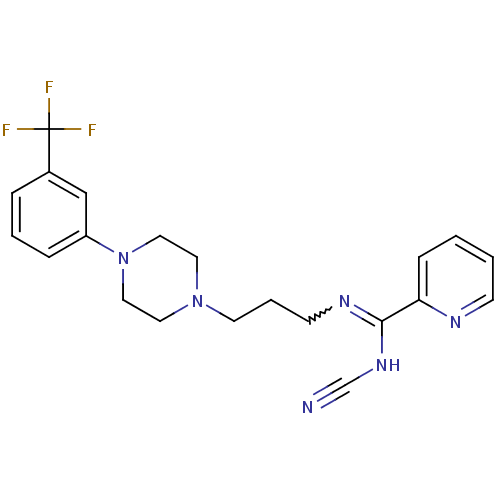 (CHEMBL1927095) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797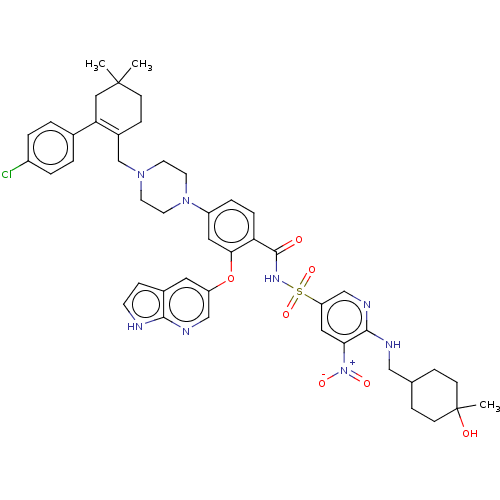 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189803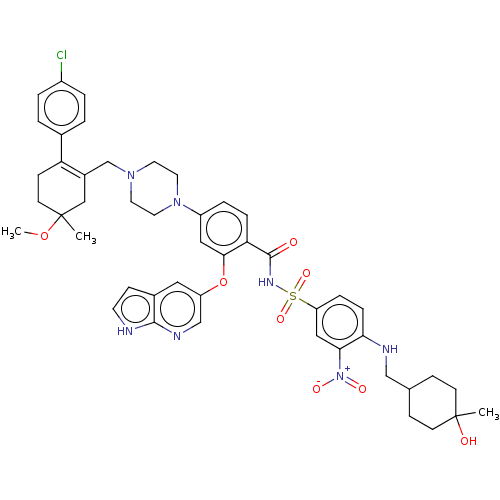 (US11369599, Compound 377 | US9174982, 377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799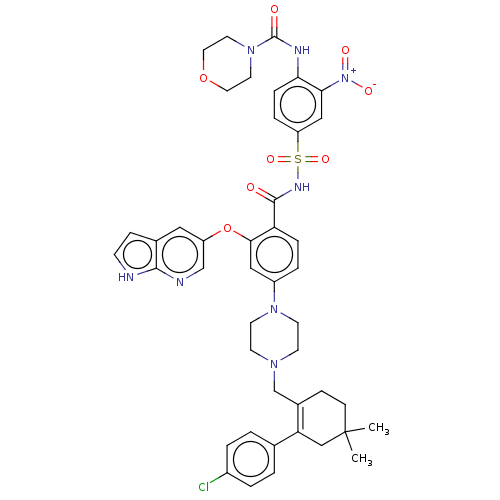 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804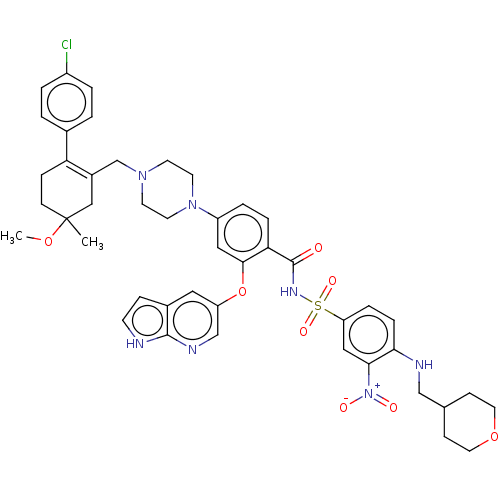 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147818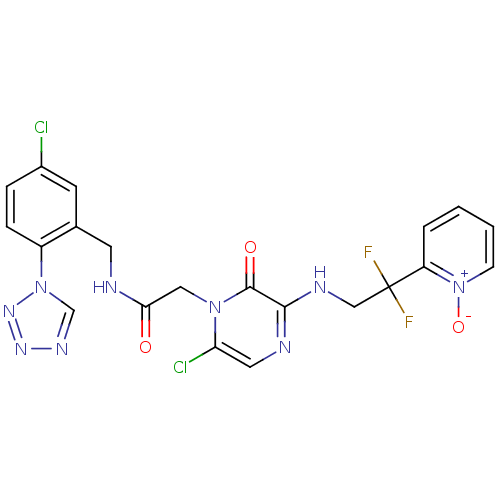 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824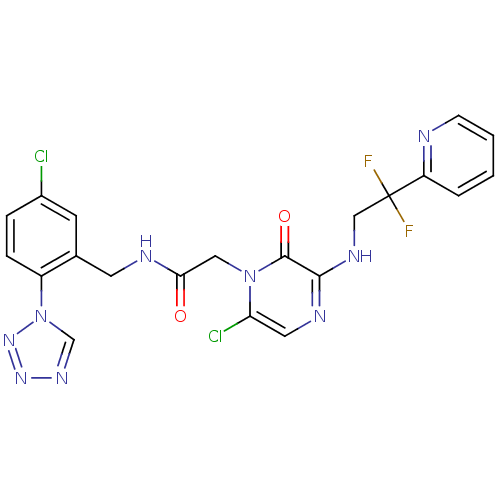 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359954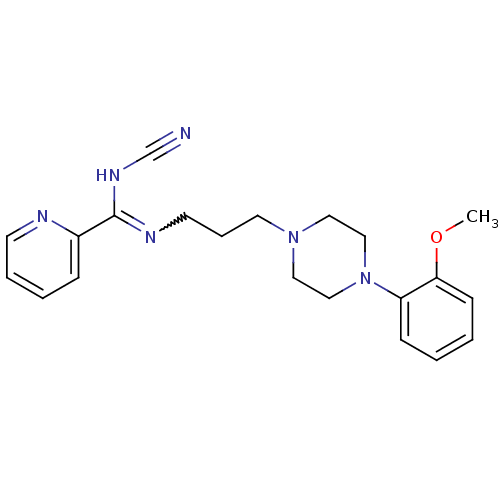 (CHEMBL1927088) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567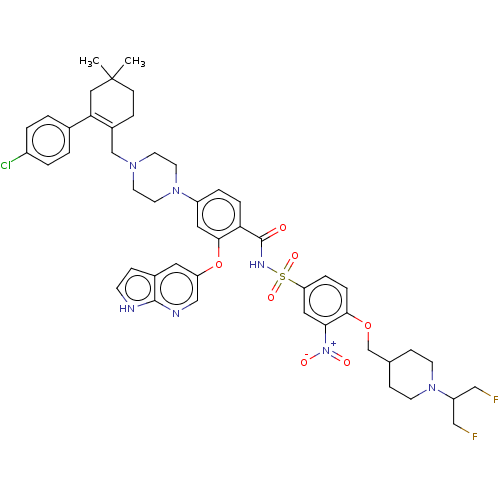 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50093352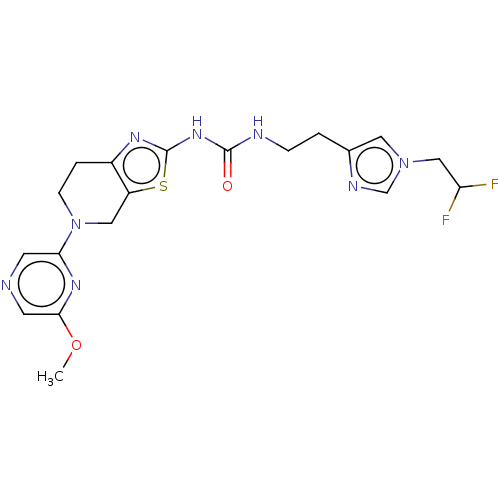 (CHEMBL3586678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method | J Med Chem 58: 5684-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00498 BindingDB Entry DOI: 10.7270/Q2Z89F53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50093355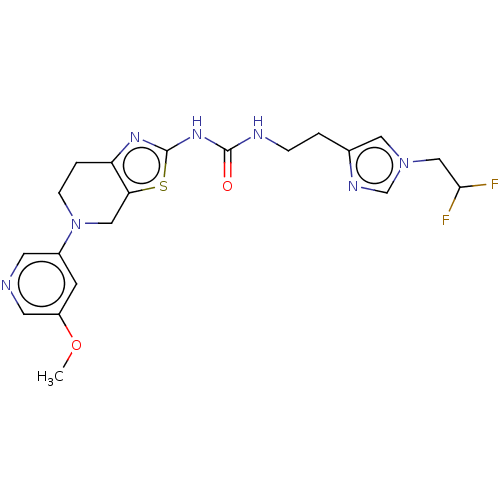 (CHEMBL3586677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method | J Med Chem 58: 5684-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00498 BindingDB Entry DOI: 10.7270/Q2Z89F53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50093351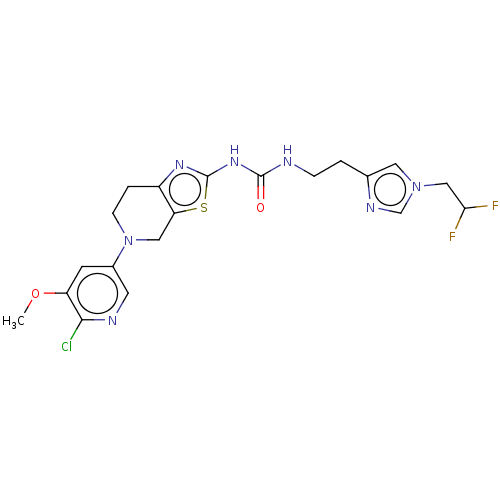 (CHEMBL3585362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method | J Med Chem 58: 5684-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00498 BindingDB Entry DOI: 10.7270/Q2Z89F53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM578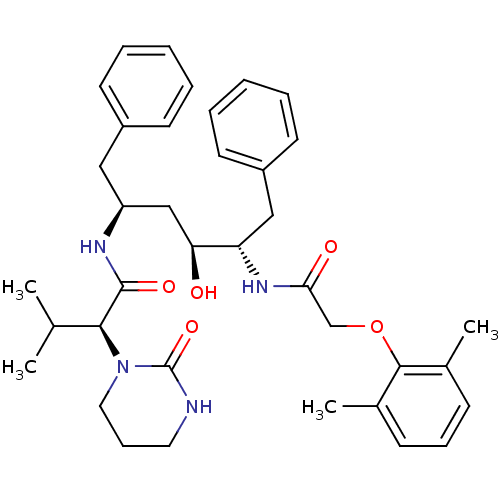 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Binding affinity against ritonavir-resistant strains. | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50537072 (CHEMBL440072) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics Curated by ChEMBL | Assay Description Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins | J Med Chem 62: 2708-2719 (2019) Article DOI: 10.1021/acs.jmedchem.8b02036 BindingDB Entry DOI: 10.7270/Q2NK3JJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50184069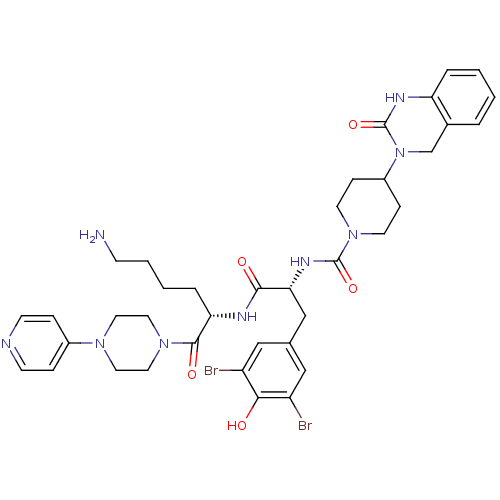 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry Merck & Co. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from human cloned CLR/RAMP1 receptor expressed in E10 cells | Bioorg Med Chem Lett 16: 2595-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.051 BindingDB Entry DOI: 10.7270/Q2HT2NX8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50093356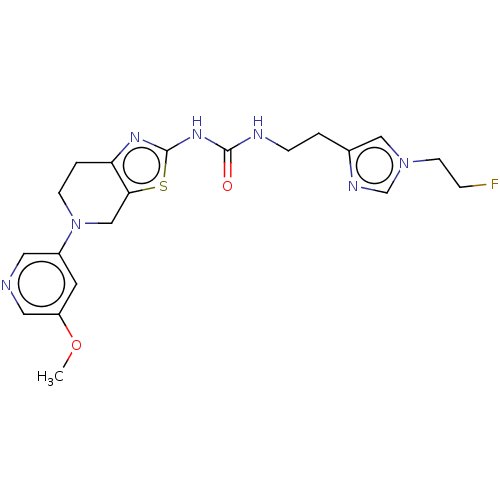 (CHEMBL3586676) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method | J Med Chem 58: 5684-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00498 BindingDB Entry DOI: 10.7270/Q2Z89F53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50093354 (CHEMBL3586679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method | J Med Chem 58: 5684-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00498 BindingDB Entry DOI: 10.7270/Q2Z89F53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50145591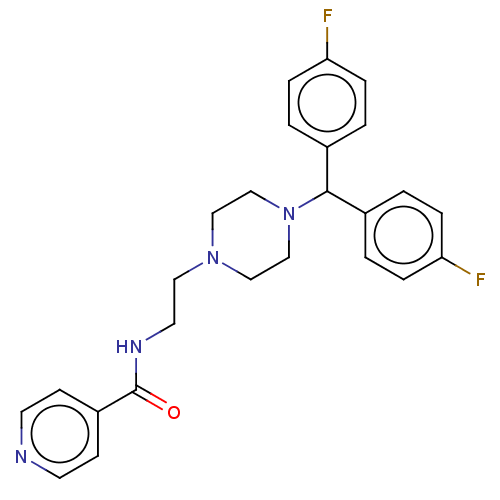 (CHEMBL3765471) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00956 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis | Eur J Med Chem 110: 133-50 (2016) Article DOI: 10.1016/j.ejmech.2016.01.021 BindingDB Entry DOI: 10.7270/Q24X59N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50145612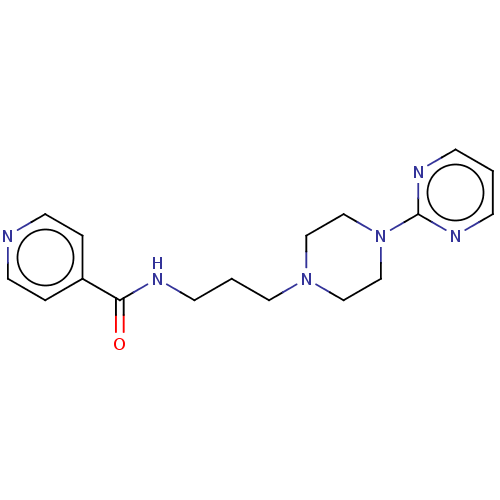 (CHEMBL3764177) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in Sprague-Dawley rat brain cortex incubated for 15 mins by liquid scintillation counting analysis | Eur J Med Chem 110: 133-50 (2016) Article DOI: 10.1016/j.ejmech.2016.01.021 BindingDB Entry DOI: 10.7270/Q24X59N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189751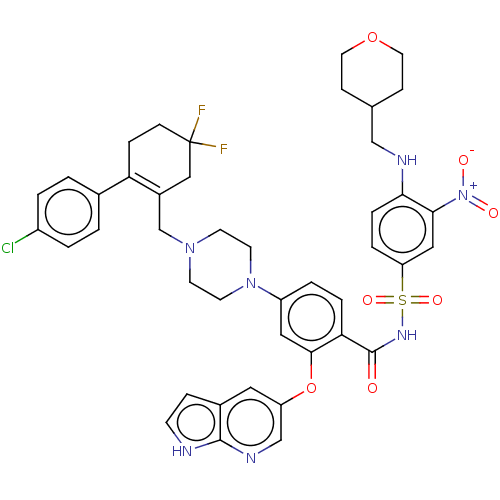 (US10213433, Compound 321 | US11369599, Compound 32...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189570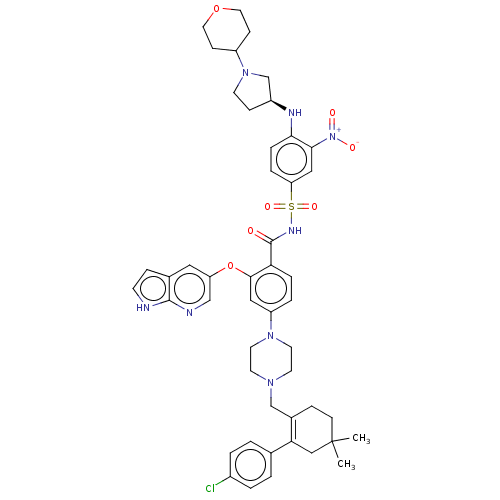 (US10213433, Compound 132 | US11369599, Compound 13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189752 (US10213433, Compound 322 | US11369599, Compound 32...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189571 (US10213433, Compound 133 | US11369599, Compound 13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189572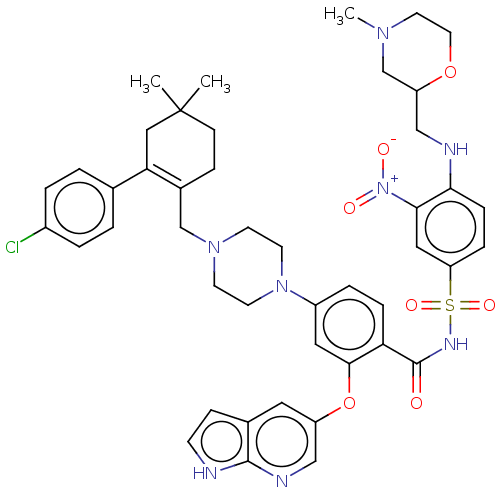 (US10213433, Compound 134 | US11369599, Compound 13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189754 (US10213433, Compound 324 | US11369599, Compound 32...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189573 (US10213433, Compound 135 | US11369599, Compound 13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189755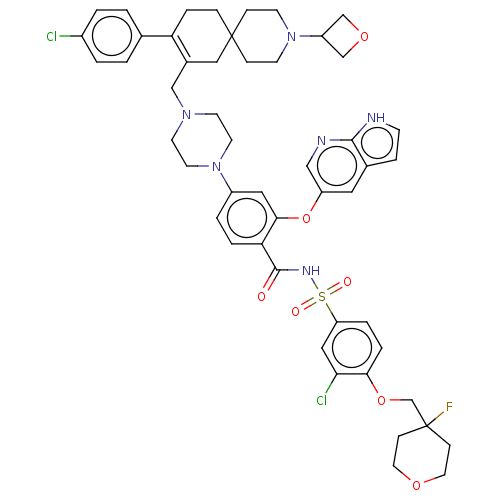 (US10213433, Compound 325 | US11369599, Compound 32...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189574 (US10213433, Compound 136 | US11369599, Compound 13...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356797 (US10213433, Compound 179 | US11369599, Compound 17...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM60828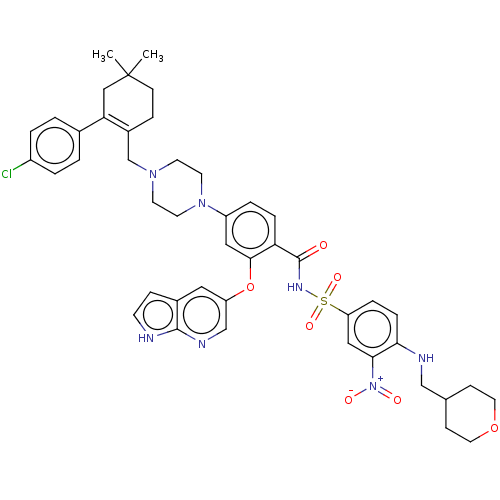 (ABT-199 | BDBM189459 | US10213433, Compound 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189618 (US10213433, Compound 180 | US11369599, Compound 18...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM559207 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356802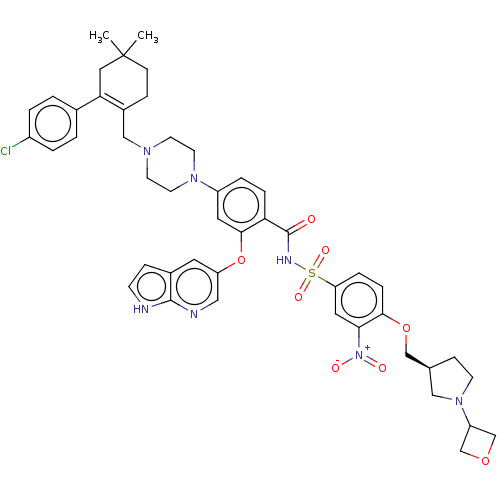 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM559217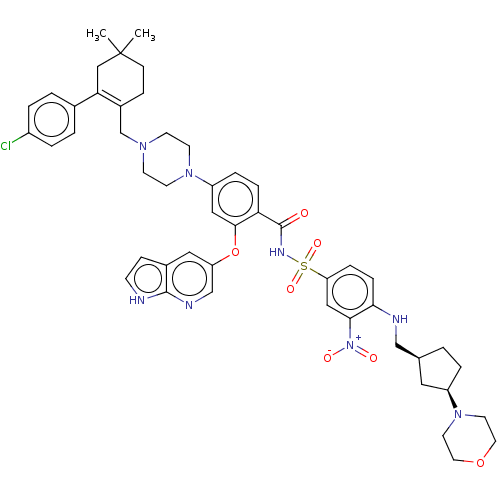 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189584 (US10213433, Compound 146 | US11369599, Compound 14...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189585 (US10213433, Compound 147 | US11369599, Compound 14...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189767 (US11369599, Compound 338 | US9174982, 337 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189586 (US10213433, Compound 148 | US11369599, Compound 14...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189587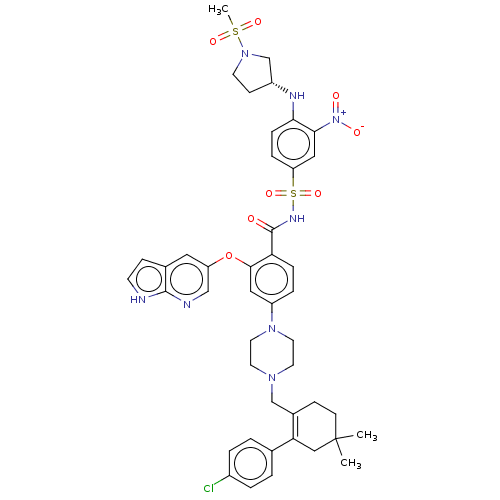 (US10213433, Compound 149 | US11369599, Compound 14...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189588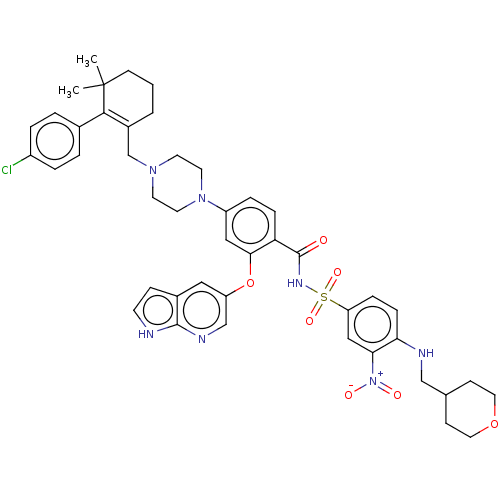 (US10213433, Compound 150 | US11369599, Compound 15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189769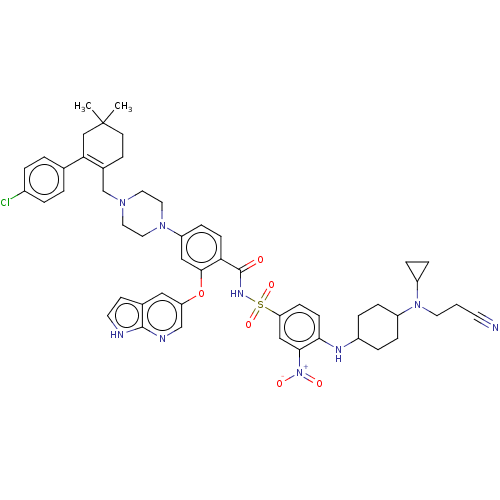 (US10213433, Compound 340 | US11369599, Compound 34...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189770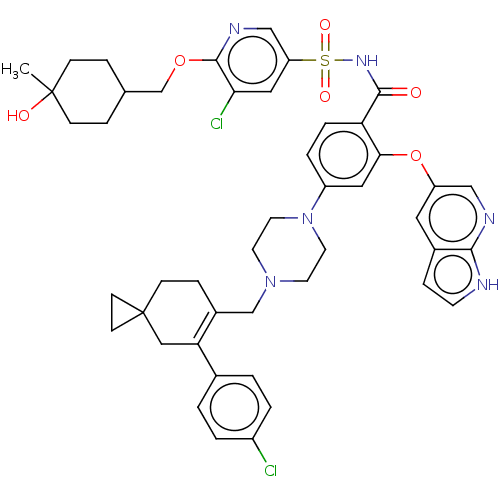 (US11369599, Compound 341 | US9174982, 341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189590 (US10213433, Compound 152 | US11369599, Compound 15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189771 (US10213433, Compound 342 | US11369599, Compound 34...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189591 (US10213433, Compound 153 | US11369599, Compound 15...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356959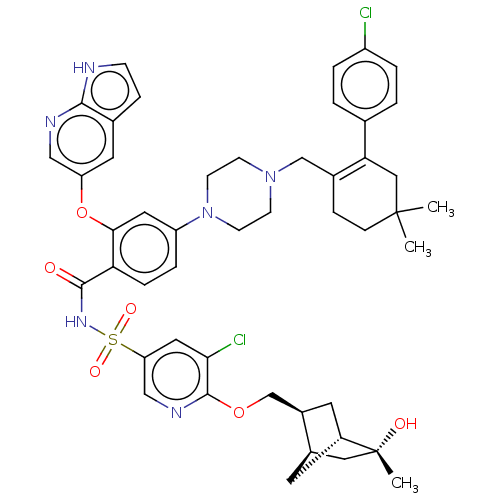 (N-[(5-chloro-6-{[(1R,2S,4R,5R)-5-hydroxy-5-methylb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87645 total ) | Next | Last >> |