

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349524 (CHEMBL1808849) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349522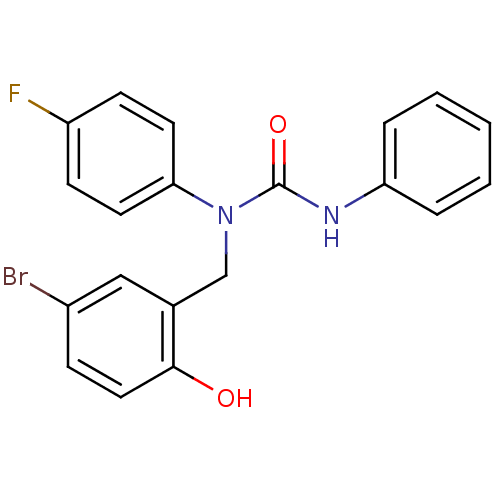 (CHEMBL1808847) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349526 (CHEMBL1808851) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349525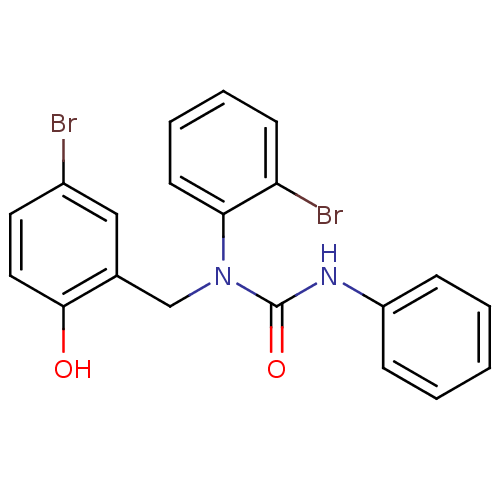 (CHEMBL1808850) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000706 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349530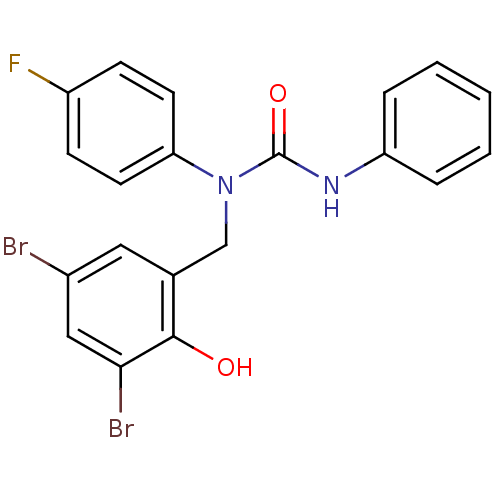 (CHEMBL1808855) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000713 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349529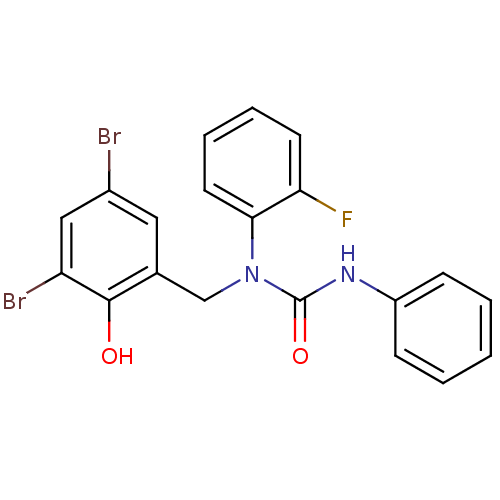 (CHEMBL1808854) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349531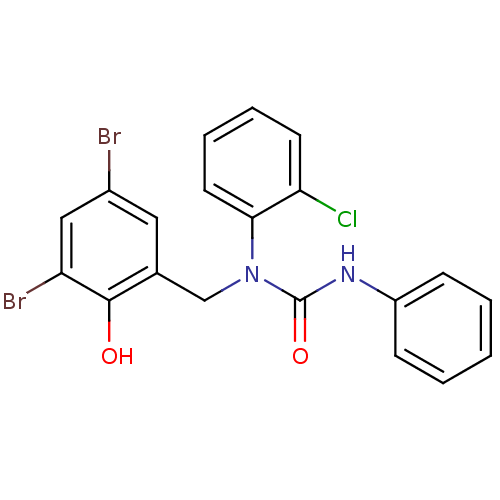 (CHEMBL1808856) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000843 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349532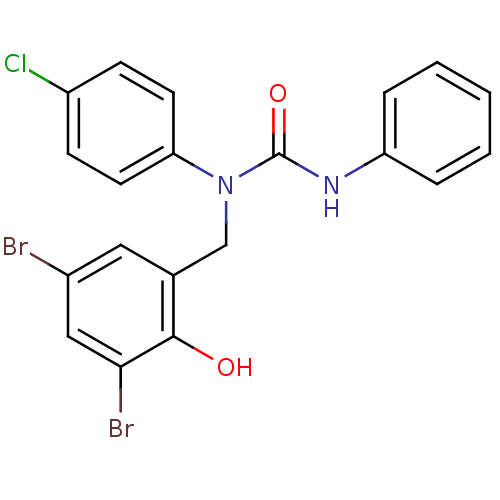 (CHEMBL1808857) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00000911 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349523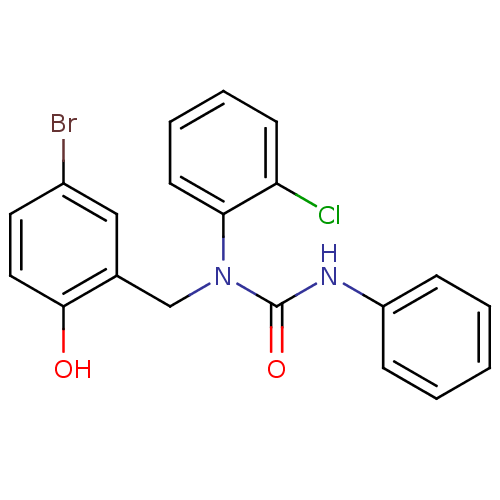 (CHEMBL1808848) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349534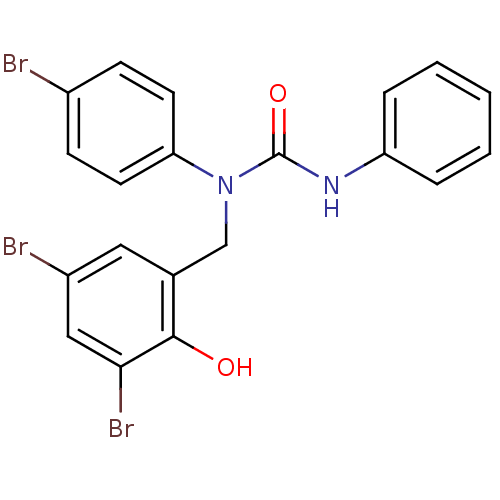 (CHEMBL1808859) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349535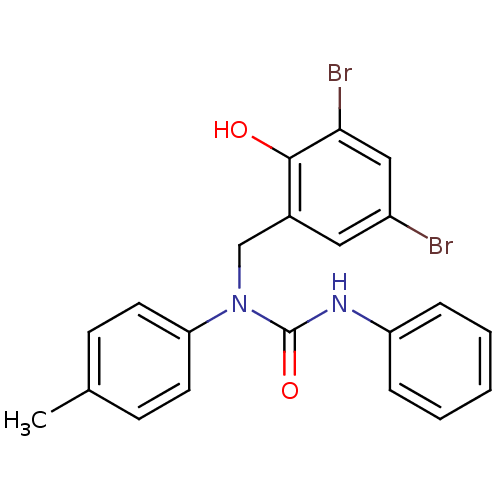 (CHEMBL1808860) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349528 (CHEMBL1808853) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349521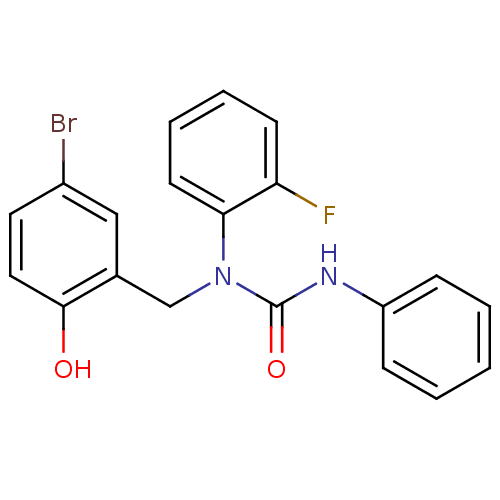 (CHEMBL1808846) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349527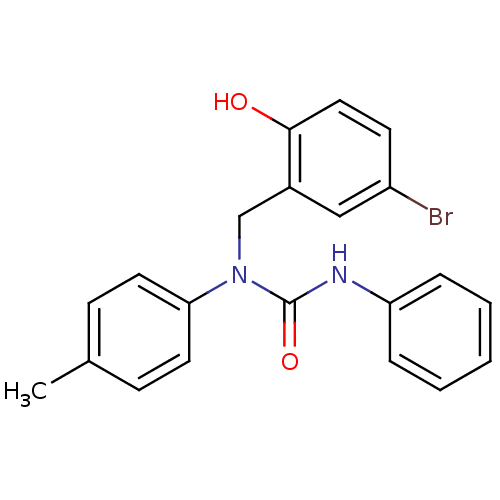 (CHEMBL1808852) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0000578 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349536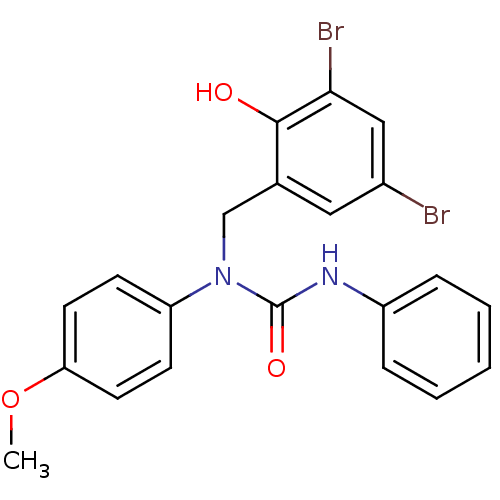 (CHEMBL1808861) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 3 (Escherichia coli) | BDBM50349533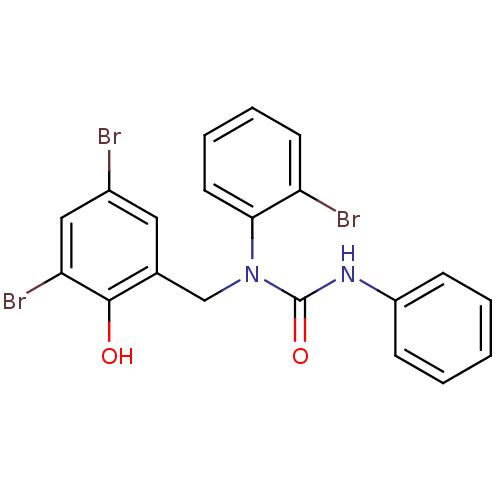 (CHEMBL1808858) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli FabH expressed in Escherichia coli DH10B cells assessed as incorporation of 3H signal in the product after 25 mins by ... | Bioorg Med Chem 19: 4413-20 (2011) Article DOI: 10.1016/j.bmc.2011.06.049 BindingDB Entry DOI: 10.7270/Q2HD7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50085683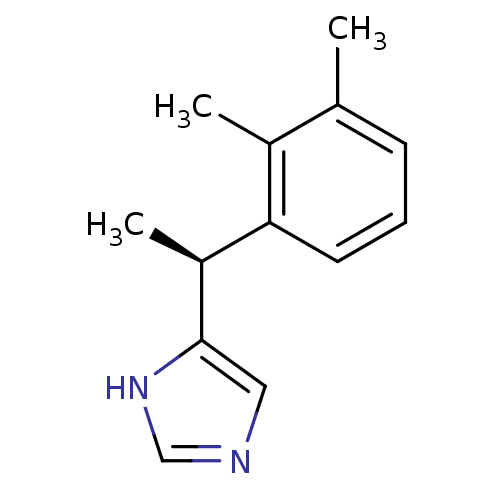 ((+)-4-((S)-alpha,2,3-trimethylbenzyl)imidazole | 4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207116 (CHEMBL3905247 | US9550741, I-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207162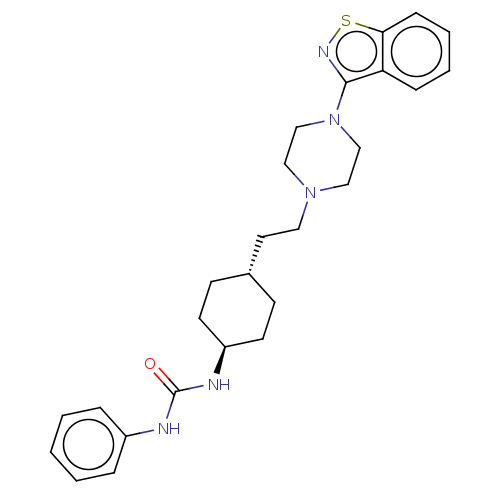 (CHEMBL3918755 | US9550741, IV-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207143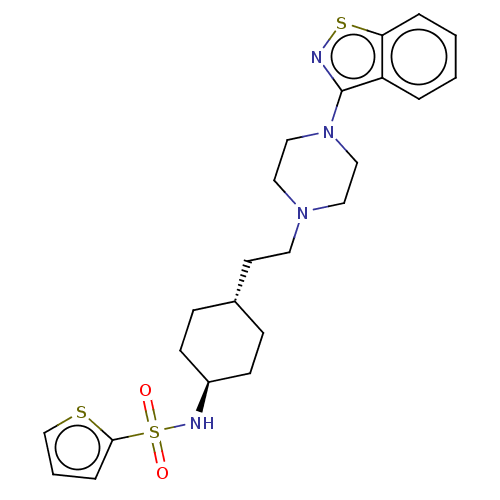 (CHEMBL3966842 | US9550741, II-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207155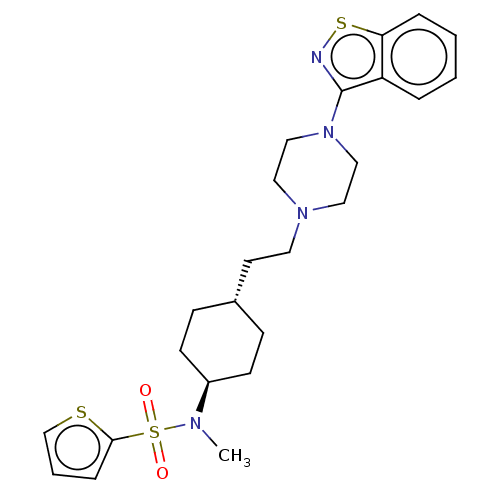 (CHEMBL3895540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207141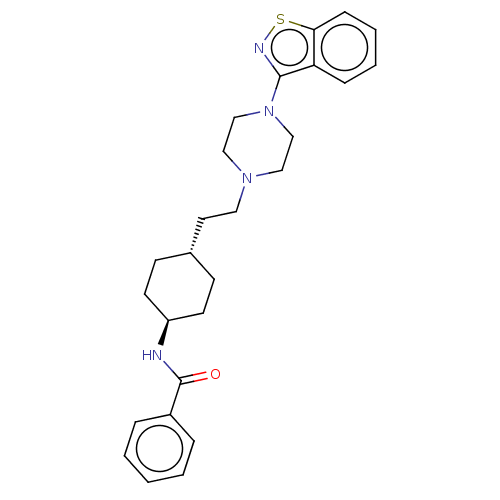 (CHEMBL3920252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207148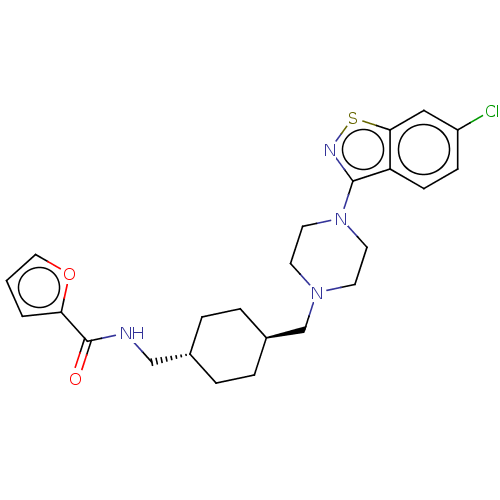 (CHEMBL3932186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207154 (CHEMBL3902496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50199865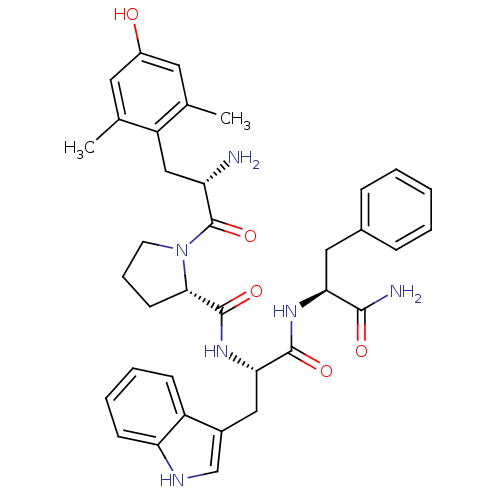 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain P2 synaptosome | Bioorg Med Chem 15: 1237-51 (2007) Article DOI: 10.1016/j.bmc.2006.11.019 BindingDB Entry DOI: 10.7270/Q2348K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207113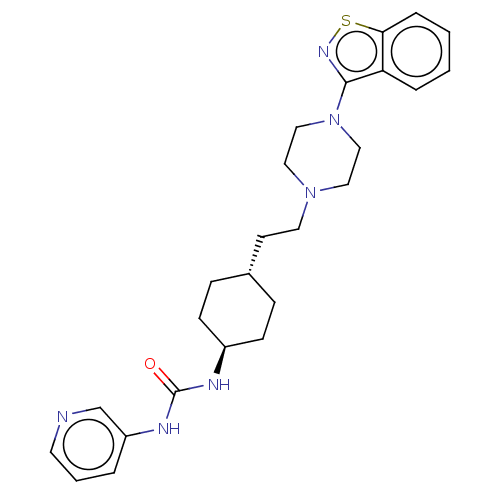 (CHEMBL3896937 | US9550741, IV-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207094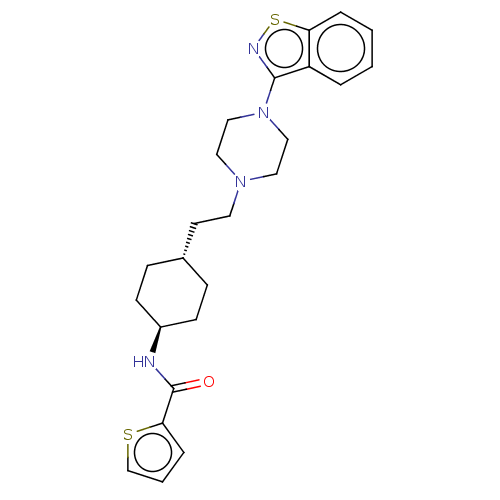 (CHEMBL3976282 | US9550741, I-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207150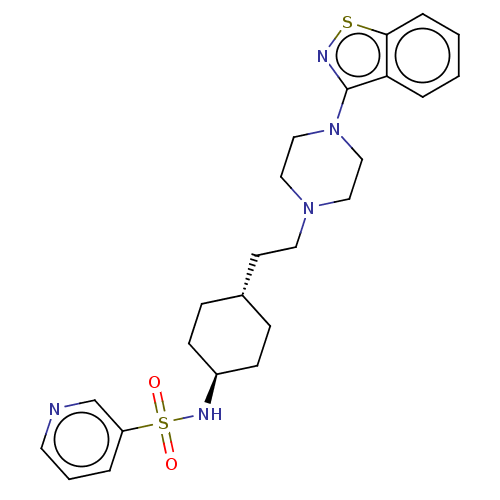 (CHEMBL3933256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207158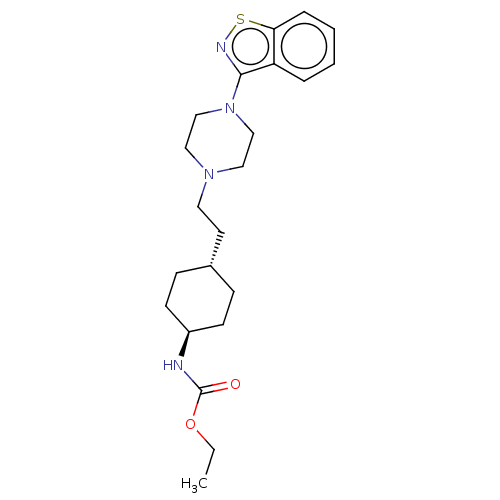 (CHEMBL3982486 | US9550741, III-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207115 (CHEMBL3948056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50607169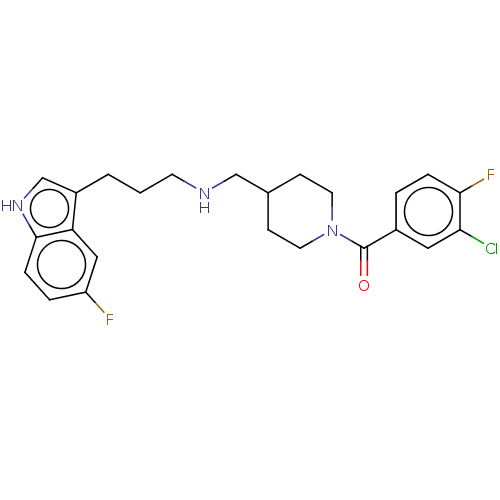 (CHEMBL5220371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.129006 BindingDB Entry DOI: 10.7270/Q2ZC8701 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370036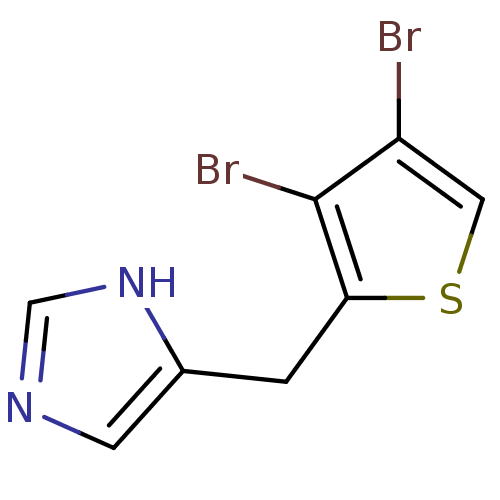 (CHEMBL1203855) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370037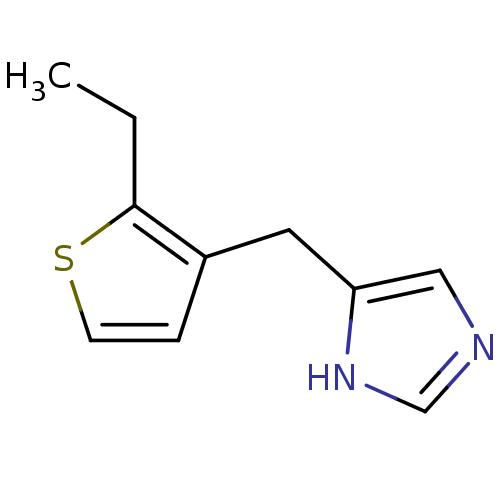 (CHEMBL1744288) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370035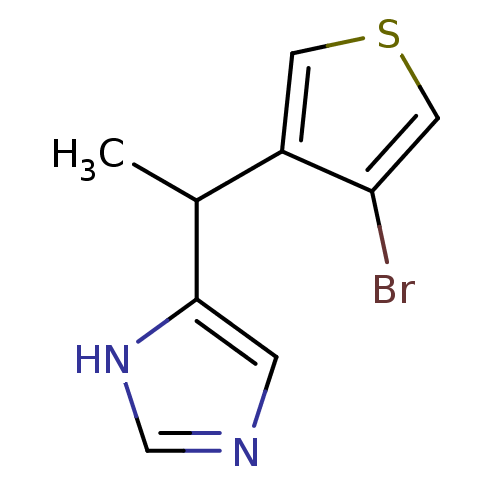 (CHEMBL1788145) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127681 BindingDB Entry DOI: 10.7270/Q2BZ69PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370026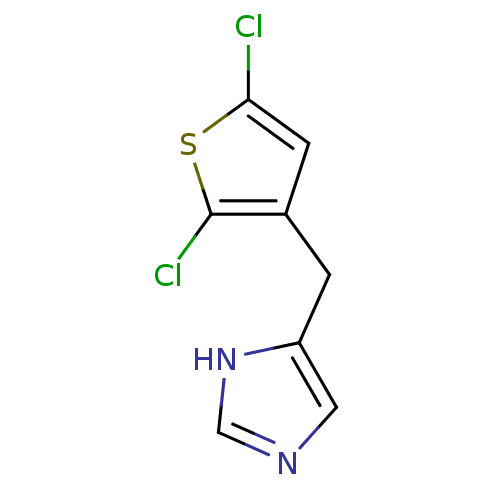 (CHEMBL1744273) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370020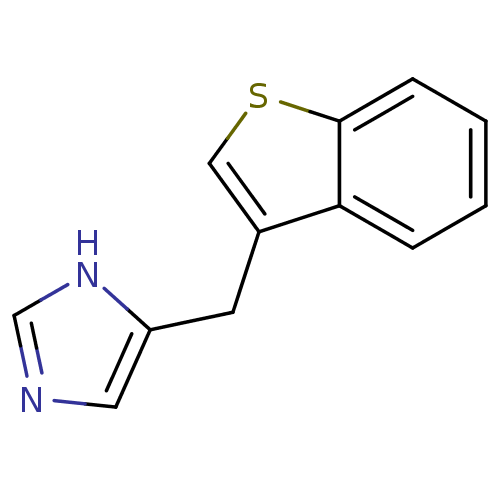 (CHEMBL1744270) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207112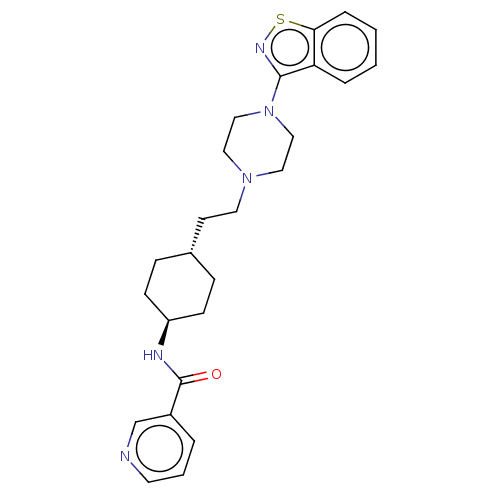 (CHEMBL3967779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50599186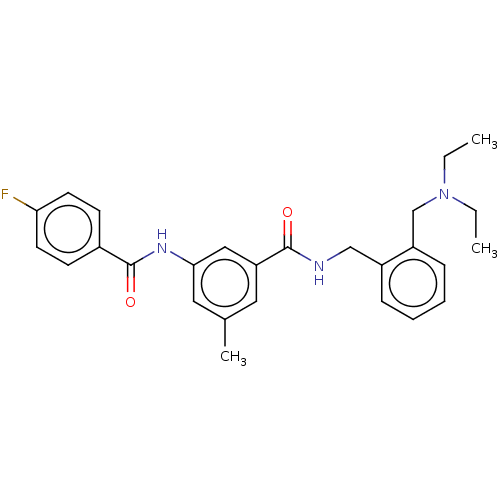 (CHEMBL5201089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00944 BindingDB Entry DOI: 10.7270/Q2HX1HP4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207114 (CHEMBL3892440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50607170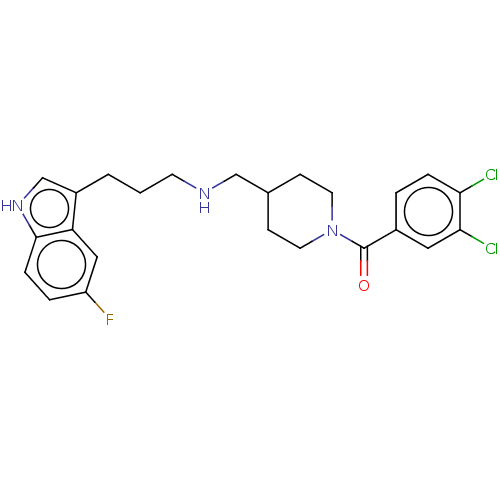 (CHEMBL5220872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.129006 BindingDB Entry DOI: 10.7270/Q2ZC8701 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50191633 ((3S)-3-{(cyclopropylmethyl)[3-(5-fluoro-1H-indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 49: 4785-9 (2006) Article DOI: 10.1021/jm060218h BindingDB Entry DOI: 10.7270/Q261114D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50370021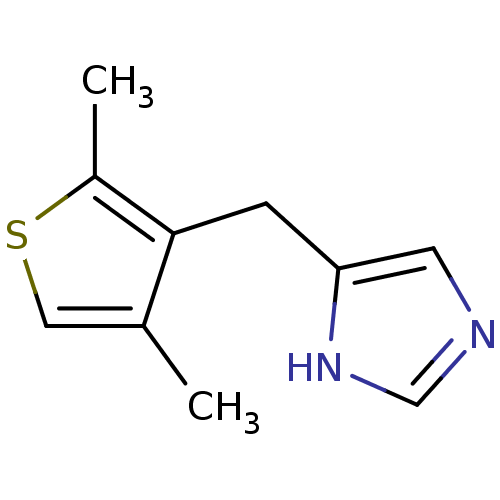 (CHEMBL1744272) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-2D adrenergic receptor of male Wistar rat | J Med Chem 44: 863-72 (2001) BindingDB Entry DOI: 10.7270/Q23R0TKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50146490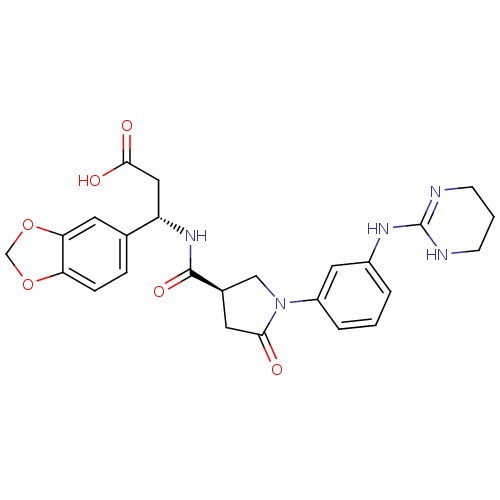 (3-(S)-Benzo[1,3]dioxol-5-yl-3-({(R)-5-oxo-1-[3-(1,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). | Bioorg Med Chem Lett 14: 2905-9 (2004) Article DOI: 10.1016/j.bmcl.2004.03.033 BindingDB Entry DOI: 10.7270/Q2416WJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50344098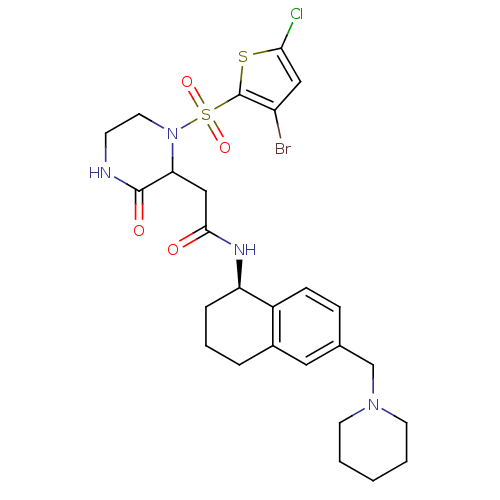 (2-(1-(3-bromo-5-chlorothiophen-2-ylsulfonyl)-3-oxo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human bradykinin B1 receptor | Bioorg Med Chem Lett 21: 3384-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.115 BindingDB Entry DOI: 10.7270/Q2FX79R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50344099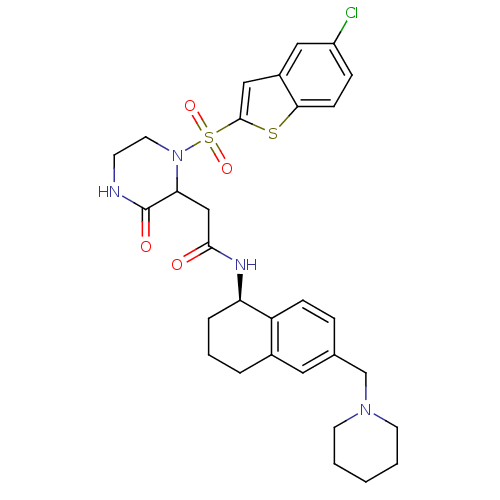 (2-(1-(5-chlorobenzo[b]thiophen-2-ylsulfonyl)-3-oxo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human bradykinin B1 receptor | Bioorg Med Chem Lett 21: 3384-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.115 BindingDB Entry DOI: 10.7270/Q2FX79R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272453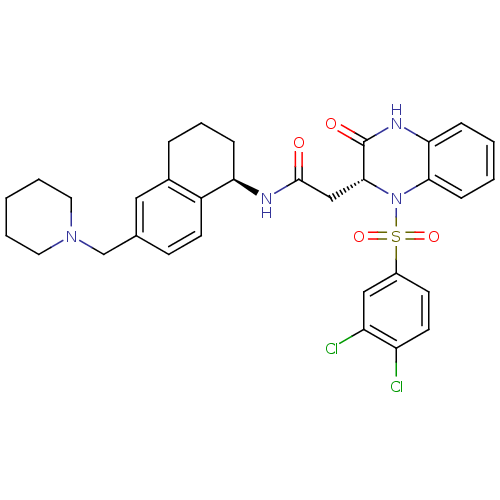 (2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human bradykinin B1 receptor | Bioorg Med Chem Lett 21: 3384-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.115 BindingDB Entry DOI: 10.7270/Q2FX79R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50344111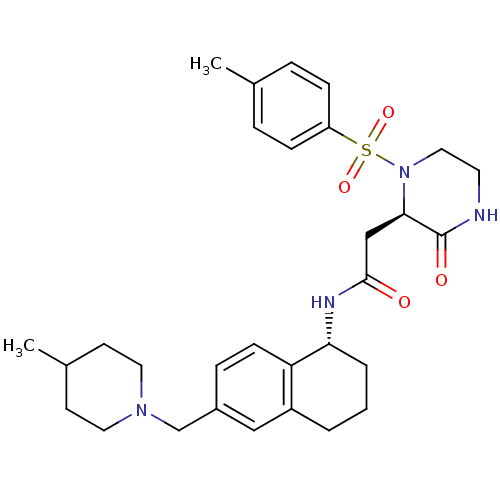 (CHEMBL1777969 | N-((R)-6-((4-methylpiperidin-1-yl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human bradykinin B1 receptor | Bioorg Med Chem Lett 21: 3384-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.115 BindingDB Entry DOI: 10.7270/Q2FX79R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 28708 total ) | Next | Last >> |