

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176988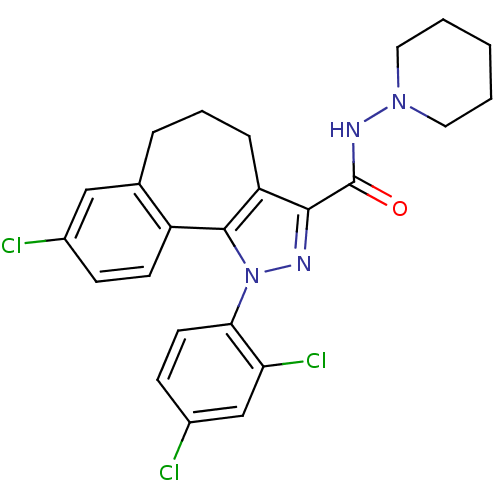 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain | J Med Chem 48: 7351-62 (2005) Article DOI: 10.1021/jm050317f BindingDB Entry DOI: 10.7270/Q2CF9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176980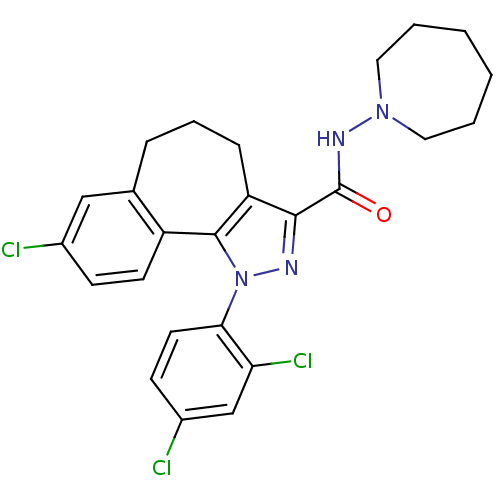 (8-chloro-1-(2',4'-dichlorophenyl)-N-homopiperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain | J Med Chem 48: 7351-62 (2005) Article DOI: 10.1021/jm050317f BindingDB Entry DOI: 10.7270/Q2CF9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030745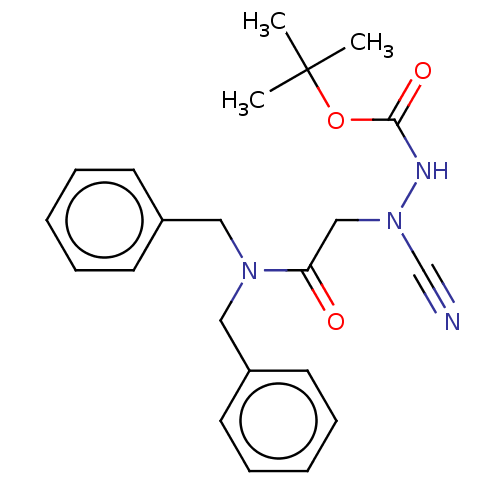 (CHEMBL3342185 | acs.jmedchem.1c00409_ST.412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293089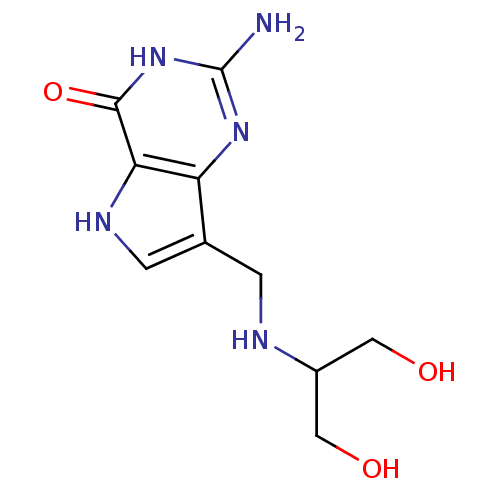 (2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50010932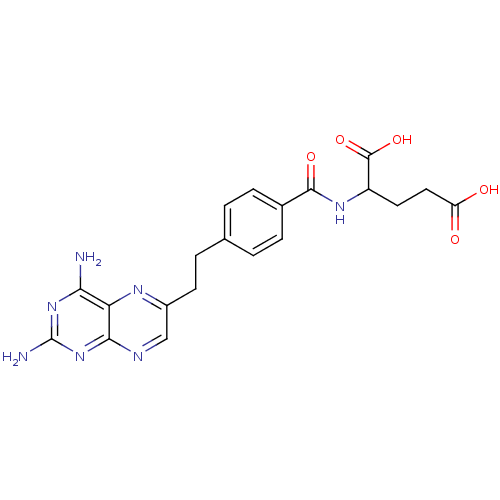 ((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016323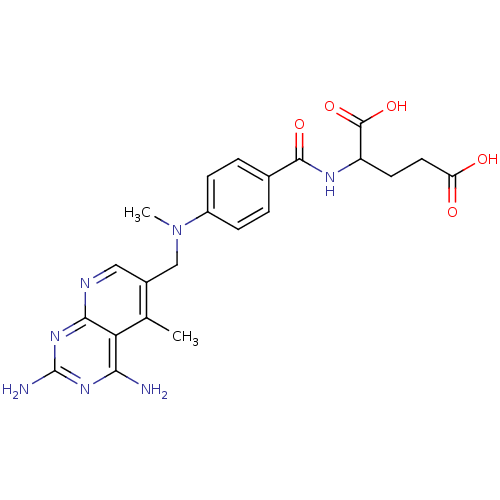 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme from mouse | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50025009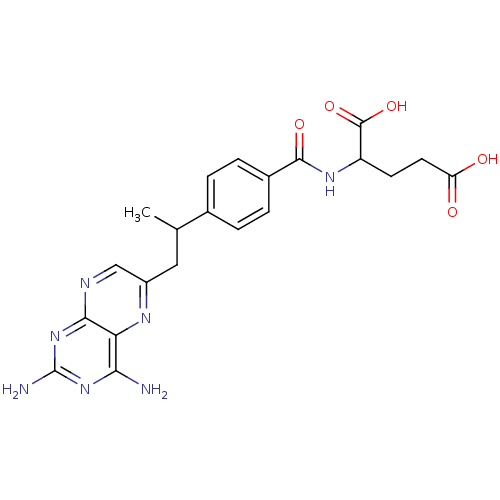 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-1-methyl-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028641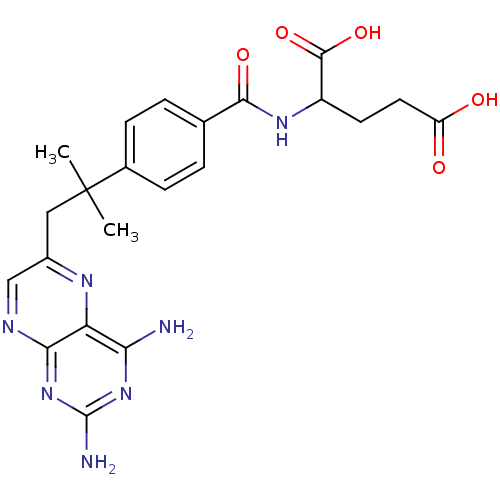 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-1,1-dimethyl-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016324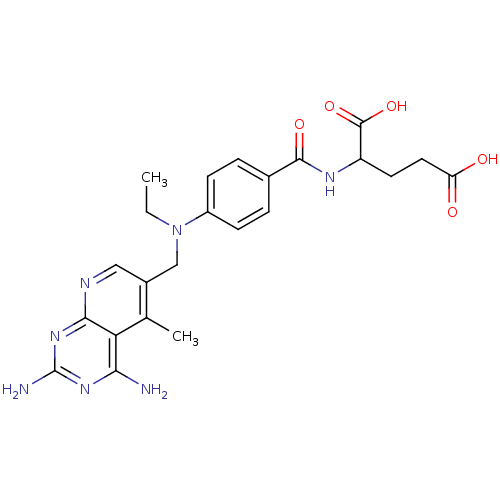 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016460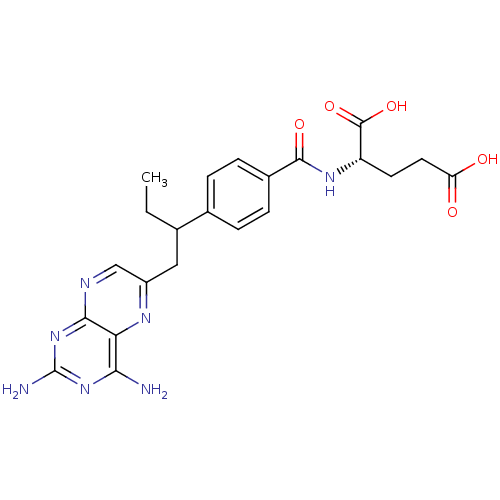 ((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50004544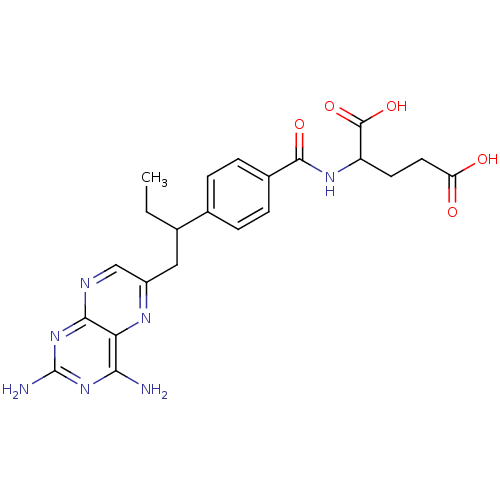 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016326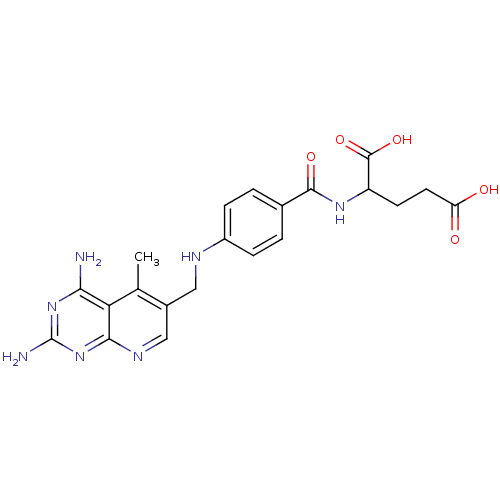 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293090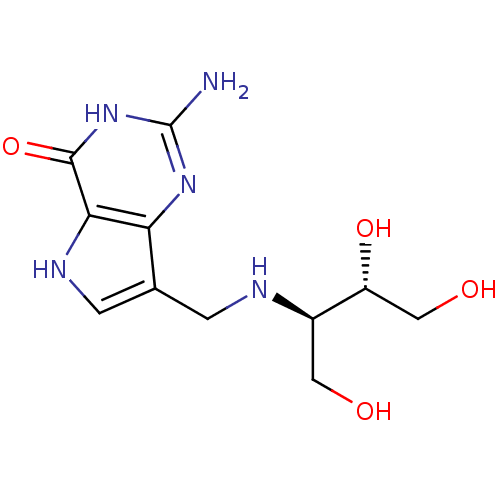 (2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400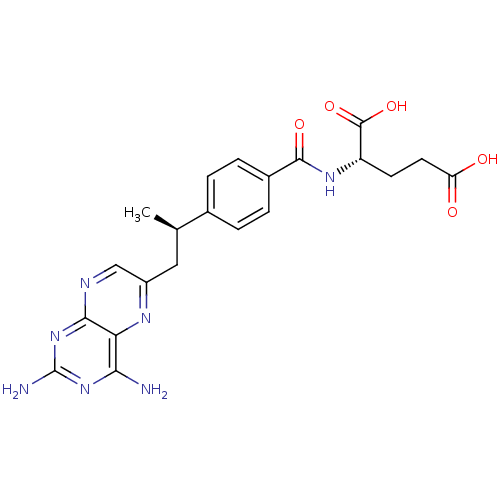 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028605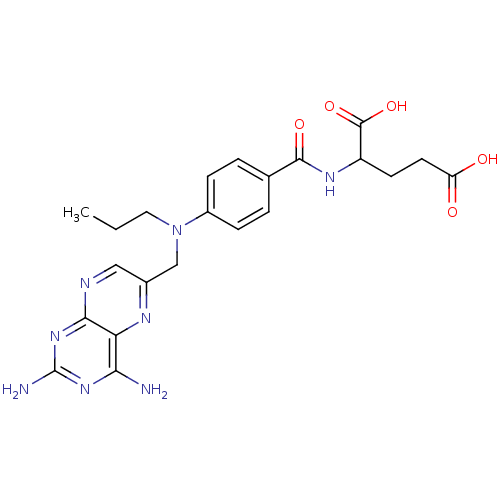 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-propyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of dihydrofolate reductase enzyme | J Med Chem 25: 1227-30 (1983) BindingDB Entry DOI: 10.7270/Q2WW7J72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50010932 ((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016325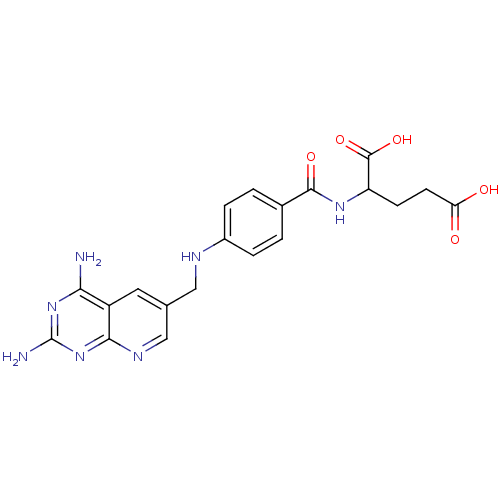 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50367055 (4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50226274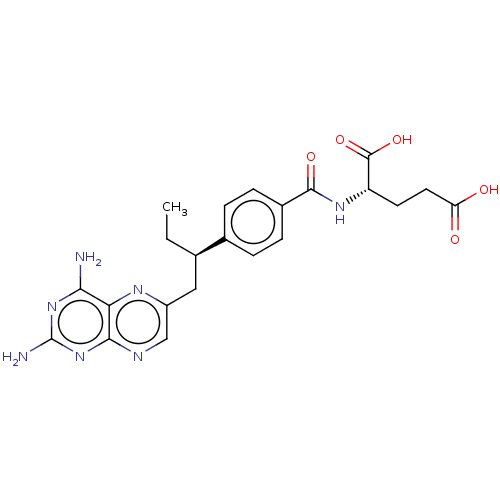 (CHEMBL3349020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176989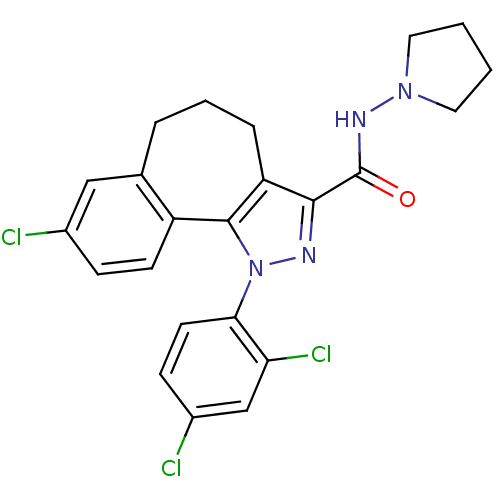 (8-chloro-1-(2',4'-dichlorophenyl)-N-pyrrolidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain | J Med Chem 48: 7351-62 (2005) Article DOI: 10.1021/jm050317f BindingDB Entry DOI: 10.7270/Q2CF9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030746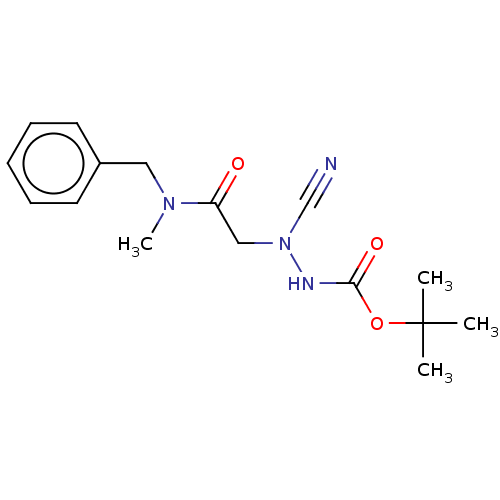 (CHEMBL3342184 | acs.jmedchem.1c00409_ST.413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593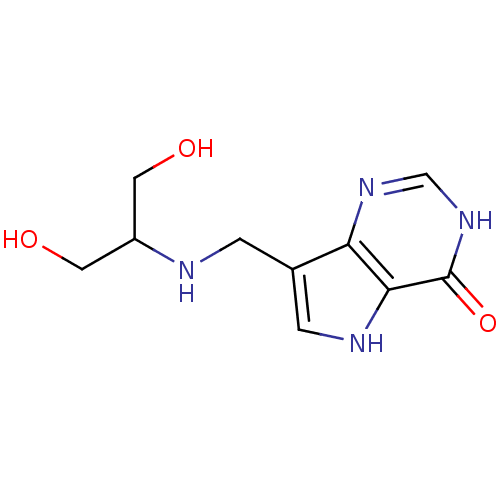 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176979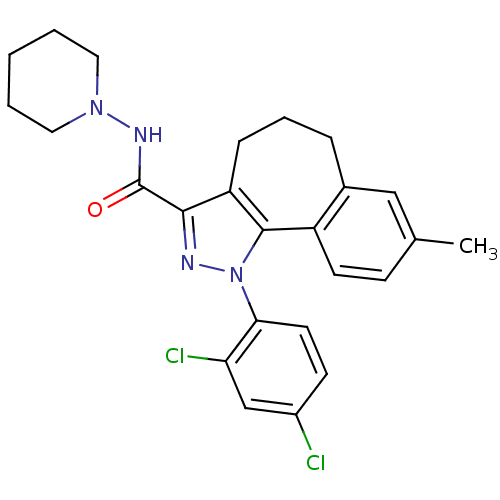 (8-methyl-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain | J Med Chem 48: 7351-62 (2005) Article DOI: 10.1021/jm050317f BindingDB Entry DOI: 10.7270/Q2CF9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014707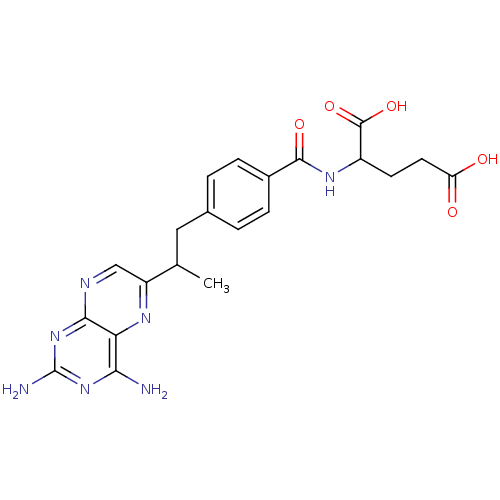 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-propyl]-benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016322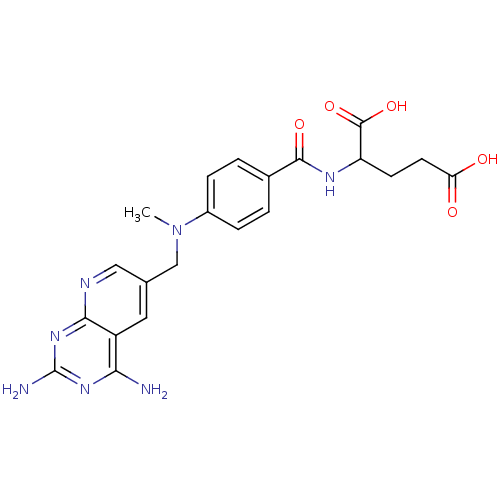 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells | J Med Chem 31: 2164-9 (1988) BindingDB Entry DOI: 10.7270/Q25H7F8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect on dihydrofolate reductase (DHFR) from L1210 cells at Inhibitory constant (n=3) | J Med Chem 29: 1080-7 (1986) BindingDB Entry DOI: 10.7270/Q2416XMQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014706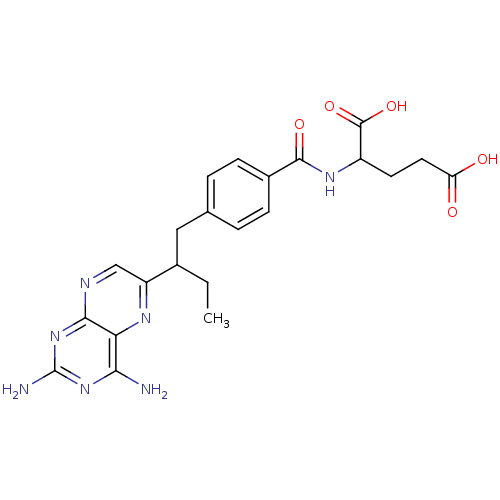 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-butyl]-benzoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting Dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against L1210 dihydrofolate reductase in rodent neoplastic cells | J Med Chem 25: 877-80 (1982) BindingDB Entry DOI: 10.7270/Q23F4Q6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008287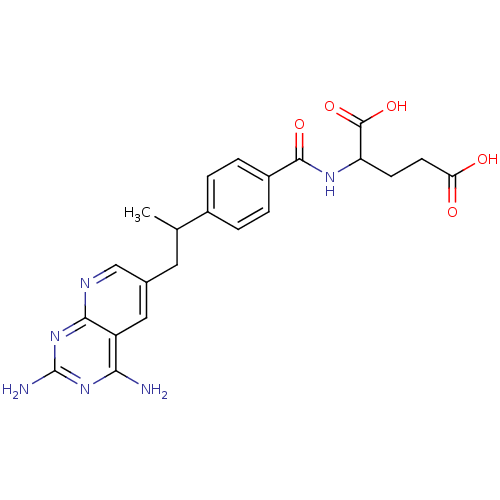 (2-{4-[2-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293087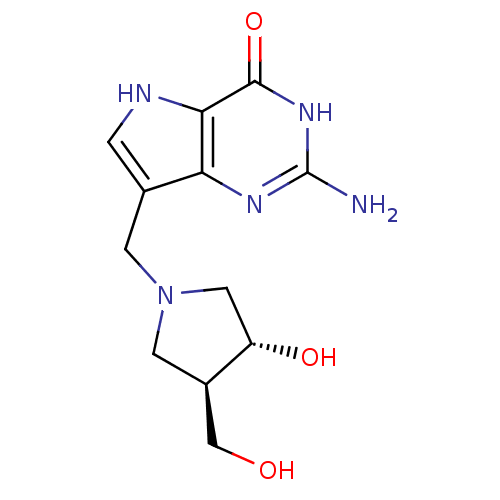 (2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176986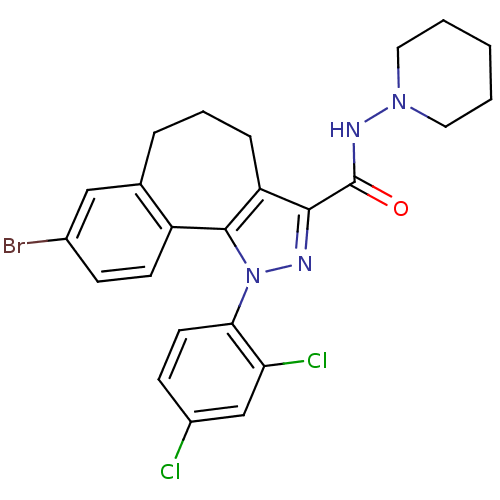 (8-bromo-1-(2',4'-dichlorophenyl)-N-piperidin-1-yl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in CD1 mouse brain | J Med Chem 48: 7351-62 (2005) Article DOI: 10.1021/jm050317f BindingDB Entry DOI: 10.7270/Q2CF9PNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109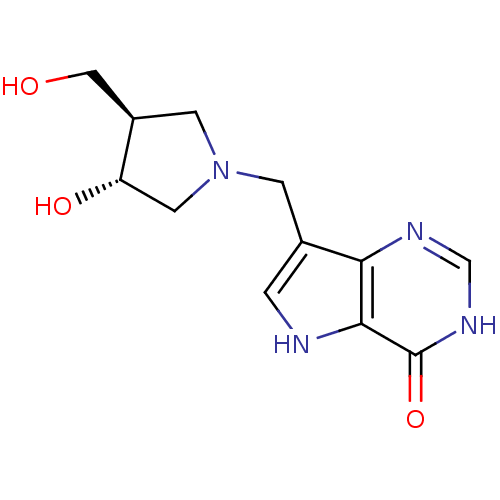 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293091 (7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM177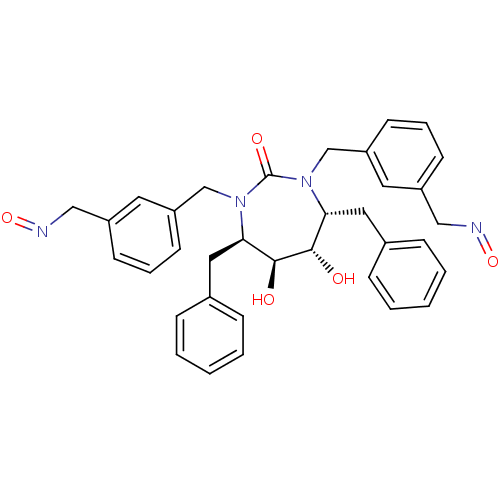 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM435577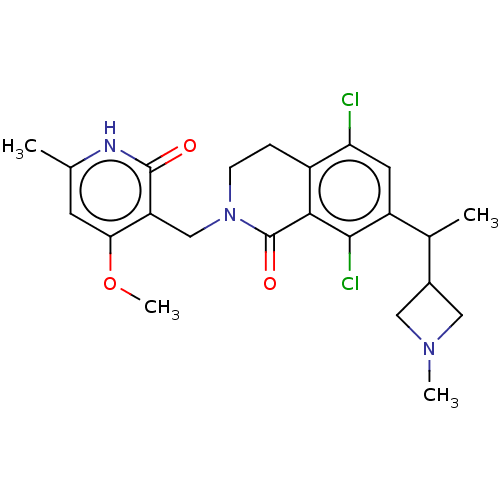 (US10570121, Example 87) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM162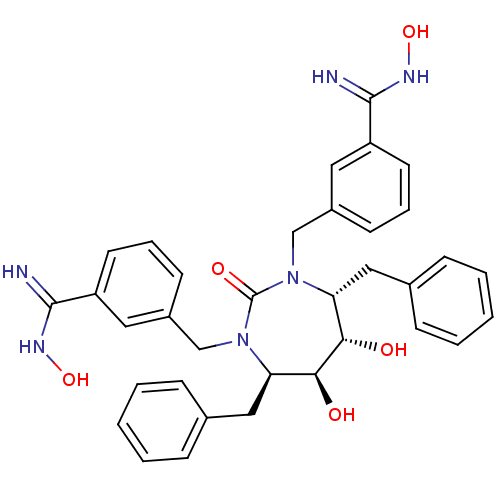 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM435691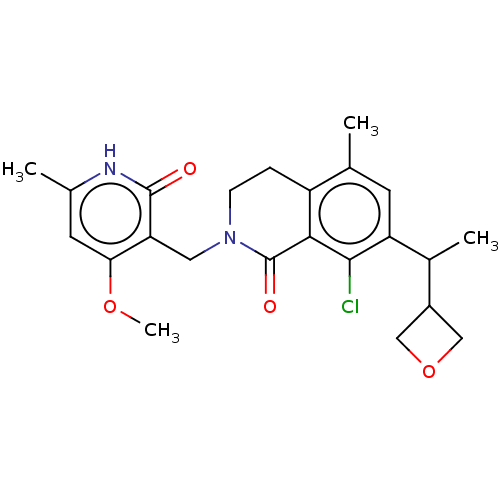 (US10570121, Example 197 | US10570121, Example 198) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299749 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM159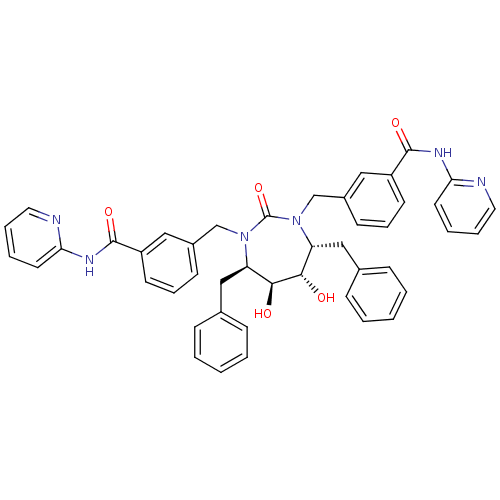 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648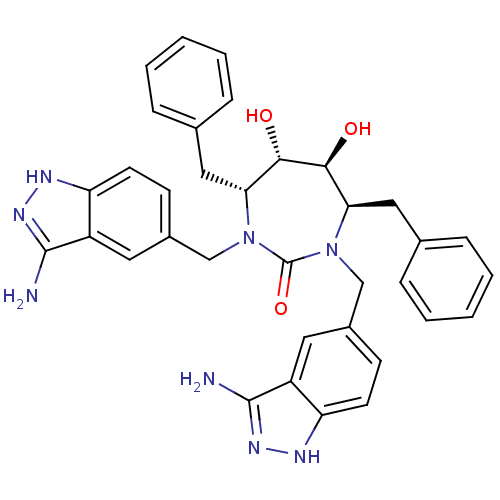 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117108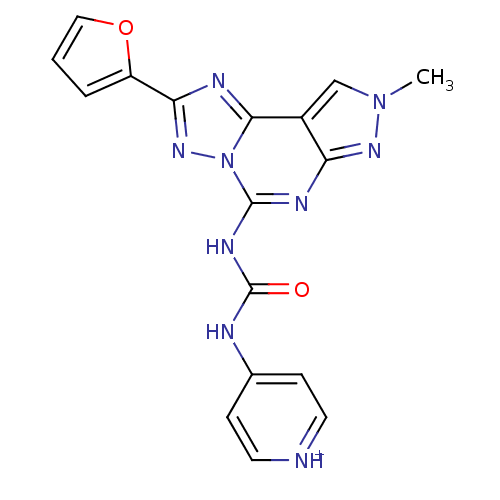 (4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]MRE-3008-F20 from human adenosine A3 receptor expressed in CHO cells after 120 mins by scintillation counter | J Med Chem 55: 5380-90 (2012) Article DOI: 10.1021/jm300323t BindingDB Entry DOI: 10.7270/Q2CN7509 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM177 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054159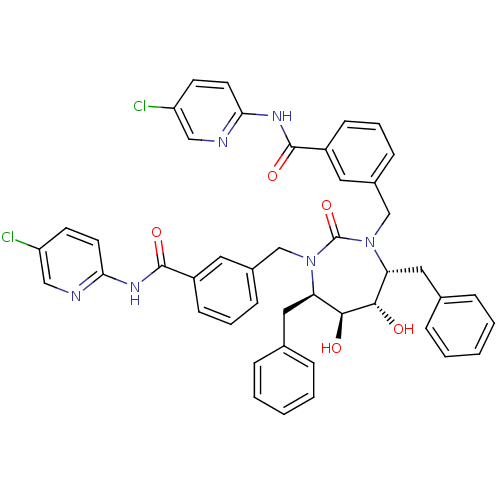 (5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 95896 total ) | Next | Last >> |