

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106541 (4-Amino-2-(6-cyclopentylamino-purin-9-yl)-5-hydrox...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at Mutant (H272E) human adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574836 (CHEMBL4871105) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA or [3H]CGS 21680 from human adenosine A1 receptor in CHO cells | J Med Chem 48: 8103-7 (2005) Article DOI: 10.1021/jm050726b BindingDB Entry DOI: 10.7270/Q2CZ36QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50179182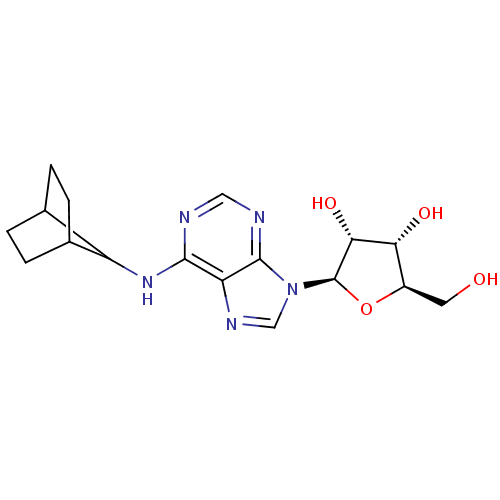 ((2R,3R,4S,5R)-2-(6-(bicyclo[2.2.1]heptan-7-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA or [3H]CGS 21680 from human adenosine A1 receptor in CHO cells | J Med Chem 48: 8103-7 (2005) Article DOI: 10.1021/jm050726b BindingDB Entry DOI: 10.7270/Q2CZ36QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106541 (4-Amino-2-(6-cyclopentylamino-purin-9-yl)-5-hydrox...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at wild-type Adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50366689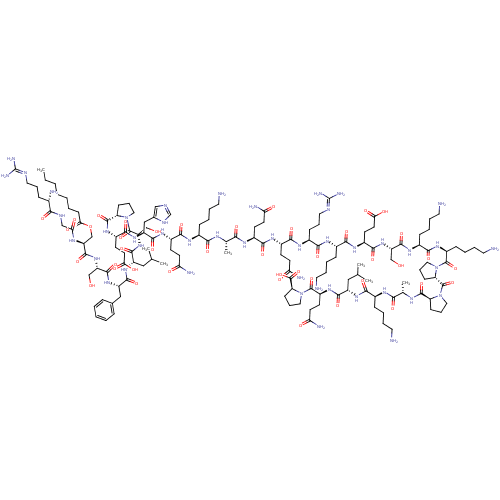 (GHRELIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Leipzig Curated by ChEMBL | Assay Description Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay | J Med Chem 55: 7437-49 (2012) Article DOI: 10.1021/jm300414b BindingDB Entry DOI: 10.7270/Q26M37Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50589922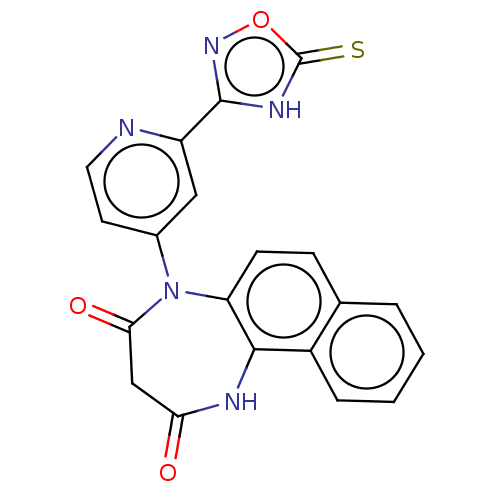 (CHEMBL5179436) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01197 BindingDB Entry DOI: 10.7270/Q2RX9H1T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA or [3H]CGS 21680 from human adenosine A1 receptor in CHO cells | J Med Chem 48: 8103-7 (2005) Article DOI: 10.1021/jm050726b BindingDB Entry DOI: 10.7270/Q2CZ36QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106535 ((2R,3R,4S,5S)-4-Amino-5-hydroxymethyl-2-[6-(3-iodo...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at wild-type Adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320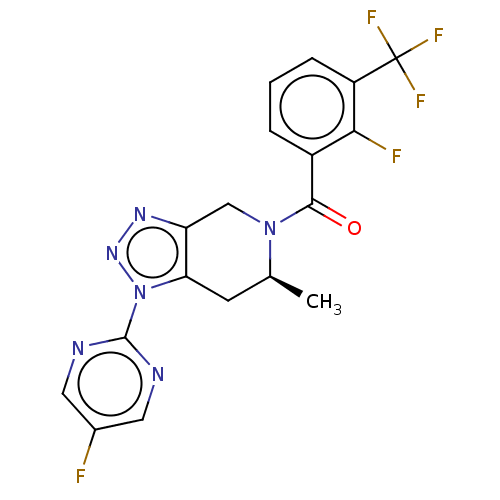 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106543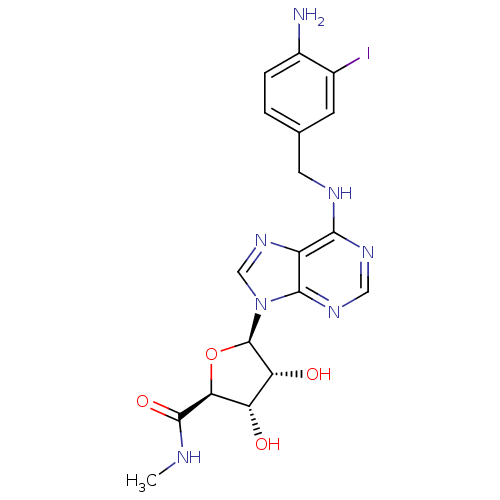 (5-[6-(4-Amino-3-iodo-benzylamino)-purin-9-yl]-3,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at wild-type Adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254320 (US10112937, Example 220 | US10150765, Example 220 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574837 (CHEMBL4855902) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured at second inhibition step by fluorome... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254247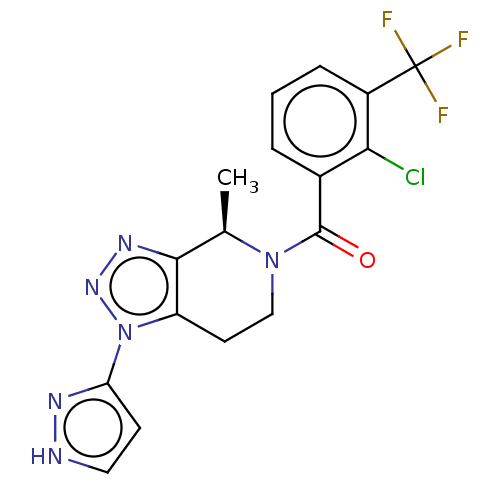 (US10112937, Example 133 | US10150765, Example 133 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254364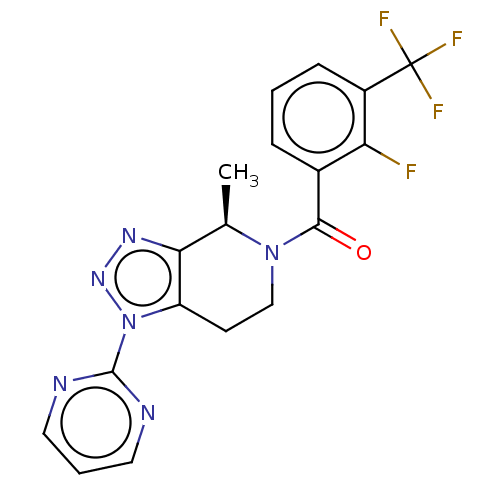 (US10112937, Example 271 | US10150765, Example 271 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254247 (US10112937, Example 133 | US10150765, Example 133 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106537 (4-[2-Chloro-6-(3-iodo-benzylamino)-purin-9-yl]-2,3...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at wild-type Adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1/2 (Rattus norvegicus) | BDBM50025450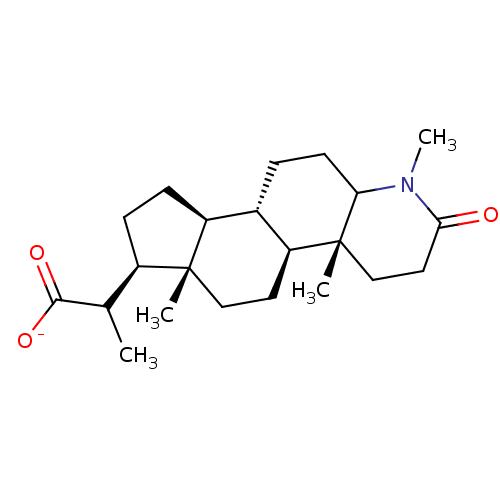 (CHEMBL1790294 | Sodium; 1,4a,6a-trimethyl-2-oxo-he...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined | J Med Chem 29: 2298-315 (1986) BindingDB Entry DOI: 10.7270/Q2XG9RQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254310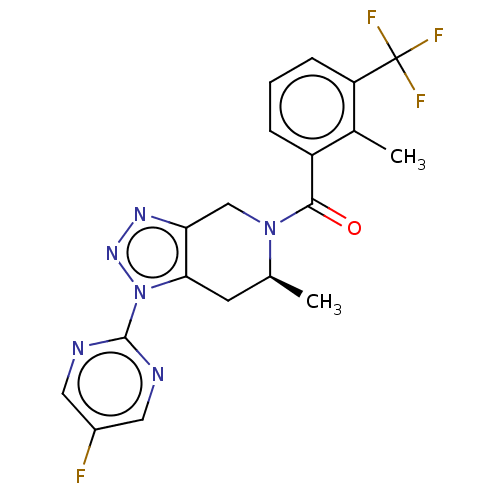 (US10112937, Example 208 | US10150765, Example 208 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254310 (US10112937, Example 208 | US10150765, Example 208 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106543 (5-[6-(4-Amino-3-iodo-benzylamino)-purin-9-yl]-3,4-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at Mutant (H272E) human adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254344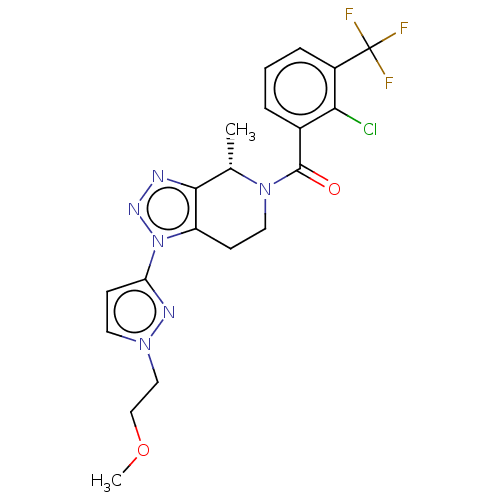 (US10112937, Example 249 | US10150765, Example 249 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254344 (US10112937, Example 249 | US10150765, Example 249 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254374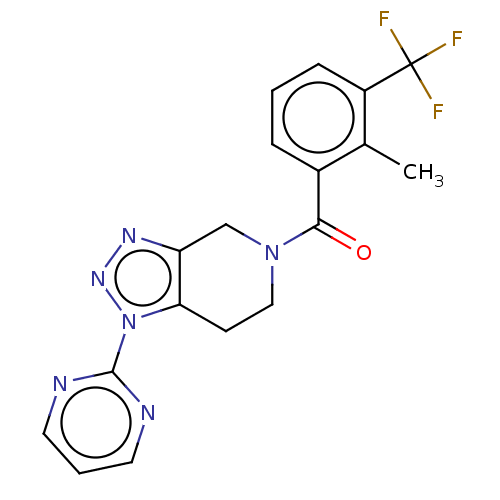 (US10112937, Example 281 | US10703749, Example 281 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254374 (US10112937, Example 281 | US10703749, Example 281 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50106542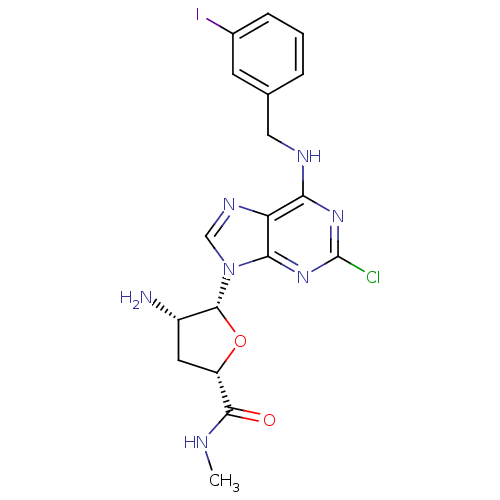 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at Mutant (H272E) human adenosine A3 receptor expressed in COS-7 cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254234 (US10112937, Example 119 | US10150765, Example 119 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254291 (US10112937, Example 188 | US10150765, Example 188 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254319 (US10112937, Example 218 | US10150765, Example 218 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254271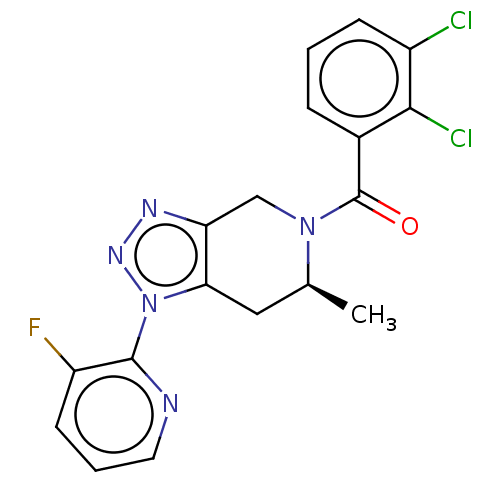 (US10112937, Example 166 | US10150765, Example 166 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254234 (US10112937, Example 119 | US10150765, Example 119 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254305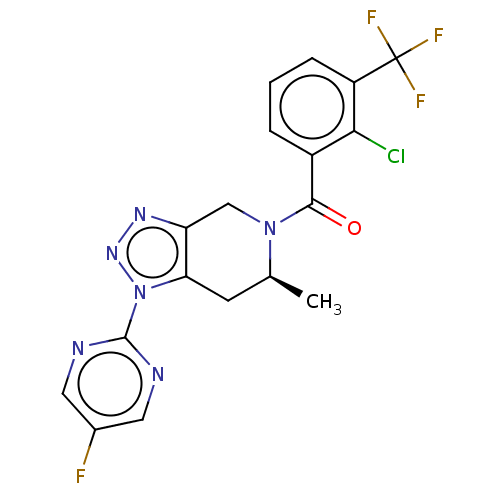 (US10112937, Example 202 | US10150765, Example 202 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254305 (US10112937, Example 202 | US10150765, Example 202 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254271 (US10112937, Example 166 | US10150765, Example 166 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254319 (US10112937, Example 218 | US10150765, Example 218 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254180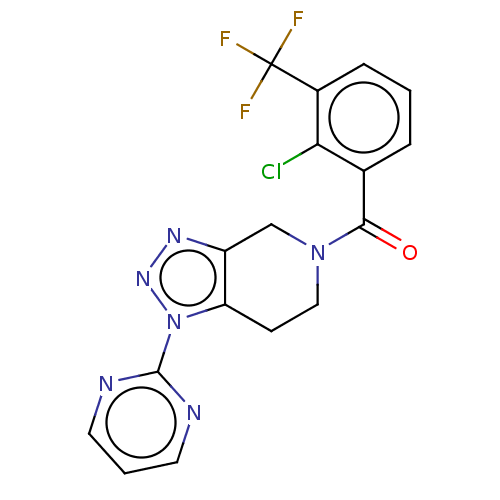 (US10112937, Example 65 | US10150765, Example 65 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254326 (US10112937, Example 228 | US10150765, Example 228 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50574818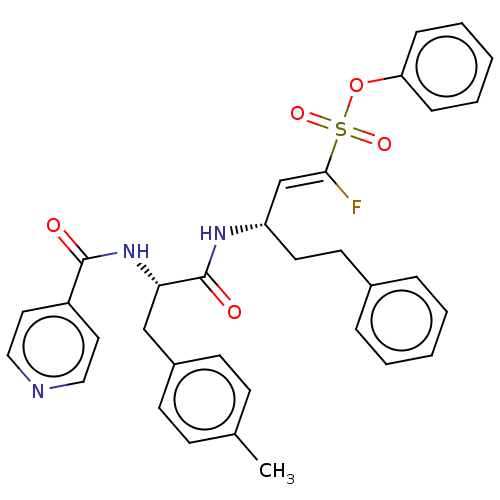 (CHEMBL4861941) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Trypanosoma brucei rhodesain assessed as fluorescence using Cbz-Phe-Arg-AMC as substrate measured using Dixon equation by fluorometric ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01002 BindingDB Entry DOI: 10.7270/Q2CC14G4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254332 (US10112937, Example 235 | US10150765, Example 235 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254332 (US10112937, Example 235 | US10150765, Example 235 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254254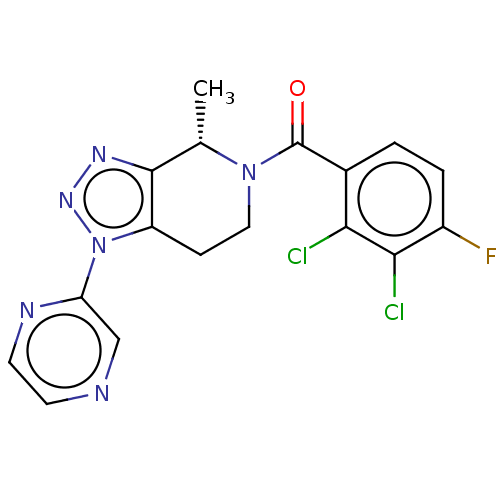 (US10112937, Example 144 | US10150765, Example 144 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254273 (US10112937, Example 168 | US10150765, Example 168 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254274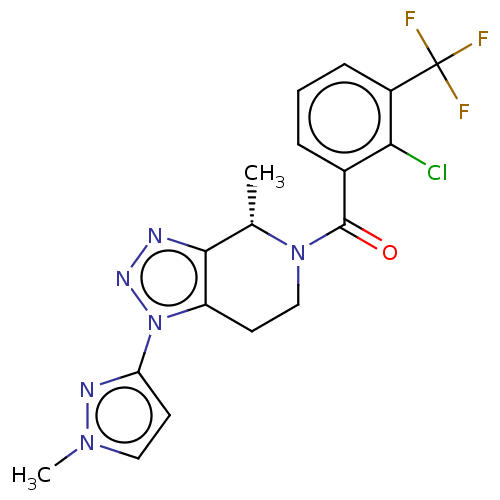 (US10112937, Example 170 | US10150765, Example 170 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254254 (US10112937, Example 144 | US10150765, Example 144 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254274 (US10112937, Example 170 | US10150765, Example 170 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254273 (US10112937, Example 168 | US10150765, Example 168 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50106542 (4-Amino-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity at rat adenosine A3 receptor in CHO cells | J Med Chem 44: 4125-36 (2001) BindingDB Entry DOI: 10.7270/Q2Z60PSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254282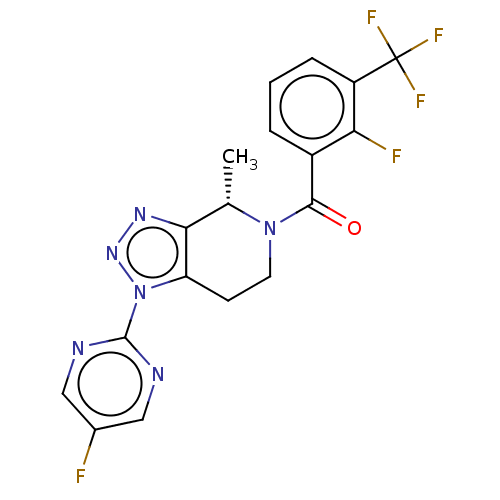 (US10112937, Example 178 | US10150765, Example 178 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description uman or rat P2X7-1321N1 cells were collected and frozen @−80° C. On the day of the experiment, cell membrane preparations were made according t... | US Patent US10112937 (2018) BindingDB Entry DOI: 10.7270/Q25H7J9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3261 total ) | Next | Last >> |