 Found 771 hits with Last Name = 'liang' and Initial = 'z'
Found 771 hits with Last Name = 'liang' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50492518
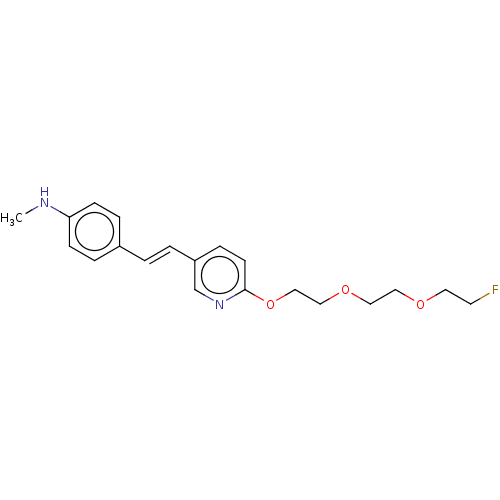
(Florbetapir | US10906900, AV45)Show InChI InChI=1S/C20H25FN2O3/c1-22-19-7-4-17(5-8-19)2-3-18-6-9-20(23-16-18)26-15-14-25-13-12-24-11-10-21/h2-9,16,22H,10-15H2,1H3/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500796

(CHEMBL3760007)Show InChI InChI=1S/C17H20FNO2/c1-19(2)15-5-9-17(10-6-15)21-13-14-3-7-16(8-4-14)20-12-11-18/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500797

(CHEMBL3758709)Show InChI InChI=1S/C17H20FNO2/c1-19(2)15-5-3-14(4-6-15)13-21-17-9-7-16(8-10-17)20-12-11-18/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50122787
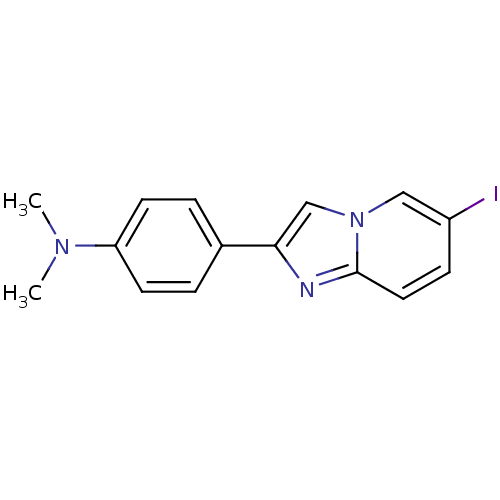
(2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...)Show InChI InChI=1S/C15H14IN3/c1-18(2)13-6-3-11(4-7-13)14-10-19-9-12(16)5-8-15(19)17-14/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793
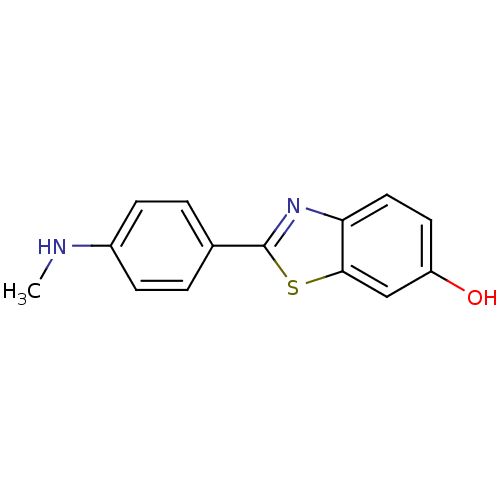
(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500791

(CHEMBL3759263)Show InChI InChI=1S/C16H17FO3/c1-18-14-4-2-13(3-5-14)12-20-16-8-6-15(7-9-16)19-11-10-17/h2-9H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500792

(CHEMBL3758500)Show InChI InChI=1S/C16H17FO3/c1-18-14-6-8-16(9-7-14)20-12-13-2-4-15(5-3-13)19-11-10-17/h2-9H,10-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500793

(CHEMBL3758630)Show InChI InChI=1S/C19H24FNO3/c1-21(2)17-5-3-16(4-6-17)15-24-19-9-7-18(8-10-19)23-14-13-22-12-11-20/h3-10H,11-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500798

(CHEMBL3759510)Show InChI InChI=1S/C21H28FNO4/c1-23(2)19-5-3-18(4-6-19)17-27-21-9-7-20(8-10-21)26-16-15-25-14-13-24-12-11-22/h3-10H,11-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500794

(CHEMBL3759176)Show InChI InChI=1S/C18H21FO4/c1-20-16-4-2-15(3-5-16)14-23-18-8-6-17(7-9-18)22-13-12-21-11-10-19/h2-9H,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50500795
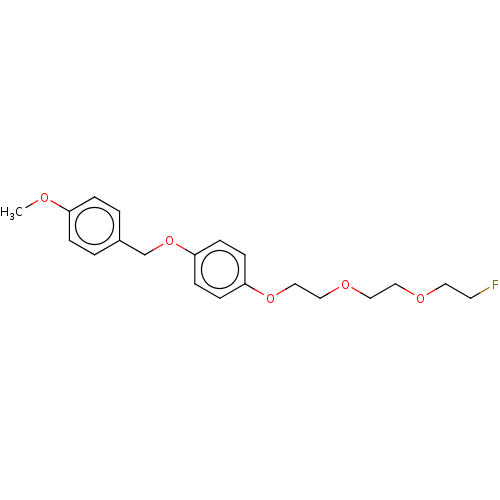
(CHEMBL3759854)Show InChI InChI=1S/C20H25FO5/c1-22-18-4-2-17(3-5-18)16-26-20-8-6-19(7-9-20)25-15-14-24-13-12-23-11-10-21/h2-9H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Normal University
Curated by ChEMBL
| Assay Description
Displacement of [125I]BOB-4 from Amyloid beta (1 to 42) (unknown origin) |
Eur J Med Chem 104: 86-96 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.028
BindingDB Entry DOI: 10.7270/Q22R3VP8 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397031

(CHEMBL2171190)Show SMILES [#8]-c1cc2ccccc2cc1-[#6](=O)-[#7]\[#7]=[#6]-1\[#6]=[#6]/[#6](/[#6]=[#6]-1)=[#7]\[#8]S(=O)(=O)c1ccccc1 |c:18,21| Show InChI InChI=1S/C23H17N3O5S/c27-22-15-17-7-5-4-6-16(17)14-21(22)23(28)25-24-18-10-12-19(13-11-18)26-31-32(29,30)20-8-2-1-3-9-20/h1-15,27H,(H,25,28)/b24-18-,26-19+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397030
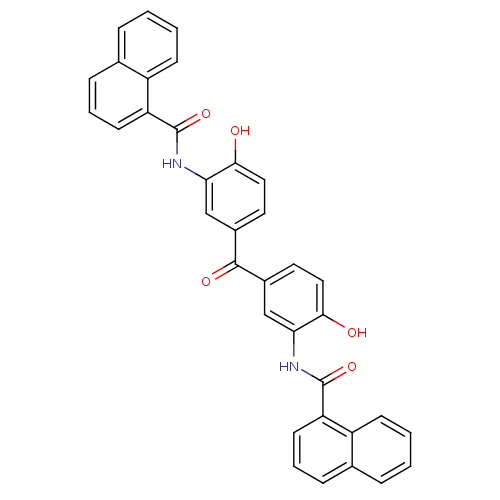
(CHEMBL2171189)Show SMILES Oc1ccc(cc1NC(=O)c1cccc2ccccc12)C(=O)c1ccc(O)c(NC(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C35H24N2O5/c38-31-17-15-23(19-29(31)36-34(41)27-13-5-9-21-7-1-3-11-25(21)27)33(40)24-16-18-32(39)30(20-24)37-35(42)28-14-6-10-22-8-2-4-12-26(22)28/h1-20,38-39H,(H,36,41)(H,37,42) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397030

(CHEMBL2171189)Show SMILES Oc1ccc(cc1NC(=O)c1cccc2ccccc12)C(=O)c1ccc(O)c(NC(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C35H24N2O5/c38-31-17-15-23(19-29(31)36-34(41)27-13-5-9-21-7-1-3-11-25(21)27)33(40)24-16-18-32(39)30(20-24)37-35(42)28-14-6-10-22-8-2-4-12-26(22)28/h1-20,38-39H,(H,36,41)(H,37,42) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 1 [11-371]
(Homo sapiens (Human)) | BDBM50397031

(CHEMBL2171190)Show SMILES [#8]-c1cc2ccccc2cc1-[#6](=O)-[#7]\[#7]=[#6]-1\[#6]=[#6]/[#6](/[#6]=[#6]-1)=[#7]\[#8]S(=O)(=O)c1ccccc1 |c:18,21| Show InChI InChI=1S/C23H17N3O5S/c27-22-15-17-7-5-4-6-16(17)14-21(22)23(28)25-24-18-10-12-19(13-11-18)26-31-32(29,30)20-8-2-1-3-9-20/h1-15,27H,(H,25,28)/b24-18-,26-19+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... |
J Med Chem 55: 7978-87 (2012)
Article DOI: 10.1021/jm300521m
BindingDB Entry DOI: 10.7270/Q2PN96R4 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50432878

(CHEMBL2376640)Show SMILES C[C@@H](Oc1c[nH]c(=O)c(c1)C(=O)NC1CCOCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C19H19Cl2FN2O4/c1-10(16-14(20)2-3-15(22)17(16)21)28-12-8-13(18(25)23-9-12)19(26)24-11-4-6-27-7-5-11/h2-3,8-11H,4-7H2,1H3,(H,23,25)(H,24,26)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) after 60 mins by ELISA |
Bioorg Med Chem Lett 23: 2408-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.037
BindingDB Entry DOI: 10.7270/Q2TQ62XH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50225416
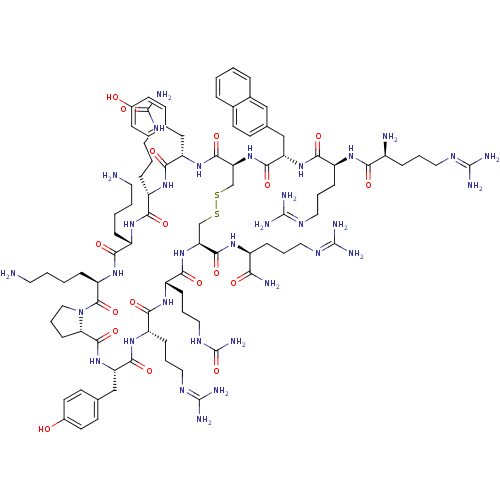
(CHEMBL393882 | TN-14003)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] Show InChI InChI=1S/C90H141N33O18S2/c91-35-5-3-17-59-75(130)117-64(18-4-6-36-92)84(139)123-43-13-24-70(123)83(138)120-66(46-51-28-33-56(125)34-29-51)79(134)116-61(21-10-40-108-88(101)102)74(129)114-63(23-12-42-110-90(104)141)77(132)121-68(81(136)111-58(71(94)126)19-8-38-106-86(97)98)48-142-143-49-69(82(137)119-65(45-50-26-31-55(124)32-27-50)78(133)115-62(73(128)113-59)22-11-41-109-89(103)140)122-80(135)67(47-52-25-30-53-14-1-2-15-54(53)44-52)118-76(131)60(20-9-39-107-87(99)100)112-72(127)57(93)16-7-37-105-85(95)96/h1-2,14-15,25-34,44,57-70,124-125H,3-13,16-24,35-43,45-49,91-93H2,(H2,94,126)(H,111,136)(H,112,127)(H,113,128)(H,114,129)(H,115,133)(H,116,134)(H,117,130)(H,118,131)(H,119,137)(H,120,138)(H,121,132)(H,122,135)(H4,95,96,105)(H4,97,98,106)(H4,99,100,107)(H4,101,102,108)(H3,103,109,140)(H3,104,110,141)/t57-,58-,59-,60-,61-,62-,63-,64+,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Inhibition of CXCR4 in MDA-MB-231 cells |
J Med Chem 50: 5655-64 (2007)
Article DOI: 10.1021/jm070679i
BindingDB Entry DOI: 10.7270/Q2X066RM |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50432894

(CHEMBL2376648 | US9126947, 1)Show SMILES C[C@@H](Oc1cc(nnc1N)C(=O)Nc1ccc(cc1)C(=O)N1CCN(C)CC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C25H25Cl2FN6O3/c1-14(21-17(26)7-8-18(28)22(21)27)37-20-13-19(31-32-23(20)29)24(35)30-16-5-3-15(4-6-16)25(36)34-11-9-33(2)10-12-34/h3-8,13-14H,9-12H2,1-2H3,(H2,29,32)(H,30,35)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-MET (unknown origin) |
Bioorg Med Chem Lett 23: 2408-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.037
BindingDB Entry DOI: 10.7270/Q2TQ62XH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50347490
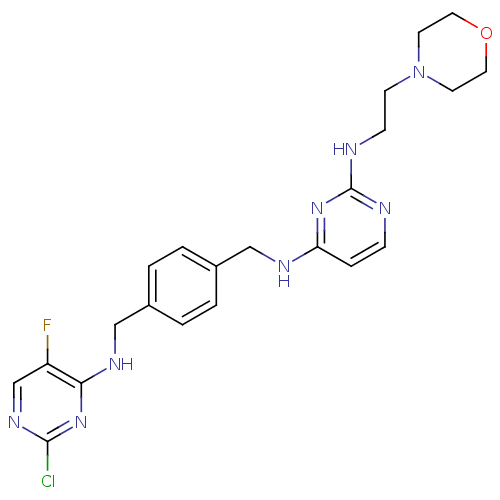
(CHEMBL1802333)Show SMILES Fc1cnc(Cl)nc1NCc1ccc(CNc2ccnc(NCCN3CCOCC3)n2)cc1 Show InChI InChI=1S/C22H26ClFN8O/c23-21-29-15-18(24)20(31-21)28-14-17-3-1-16(2-4-17)13-27-19-5-6-25-22(30-19)26-7-8-32-9-11-33-12-10-32/h1-6,15H,7-14H2,(H,28,29,31)(H2,25,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... |
J Med Chem 53: 8556-68 (2010)
Article DOI: 10.1021/jm100786g
BindingDB Entry DOI: 10.7270/Q2PK0GH6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113626

(CHEMBL3604634)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C27H36ClN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)14-29-27(32-25)31-22-12-17(3)20(13-24(22)38-19-6-7-19)18-8-10-34(4)11-9-18/h12-16,18-19H,6-11H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159537
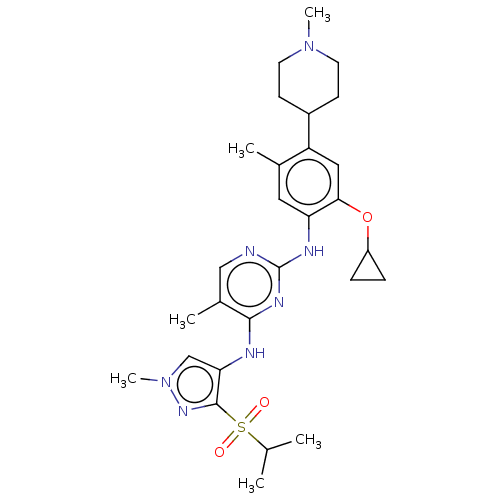
(CHEMBL3785722)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1C Show InChI InChI=1S/C28H39N7O3S/c1-17(2)39(36,37)27-24(16-35(6)33-27)30-26-19(4)15-29-28(32-26)31-23-13-18(3)22(14-25(23)38-21-7-8-21)20-9-11-34(5)12-10-20/h13-17,20-21H,7-12H2,1-6H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 60 mins by ELISA |
Bioorg Med Chem 21: 6804-20 (2013)
Article DOI: 10.1016/j.bmc.2013.07.032
BindingDB Entry DOI: 10.7270/Q27P90TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of c-Met incubated for 60 mins at spectrophotometry |
Bioorg Med Chem 20: 5169-80 (2012)
Article DOI: 10.1016/j.bmc.2012.07.007
BindingDB Entry DOI: 10.7270/Q2WH2R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50225415
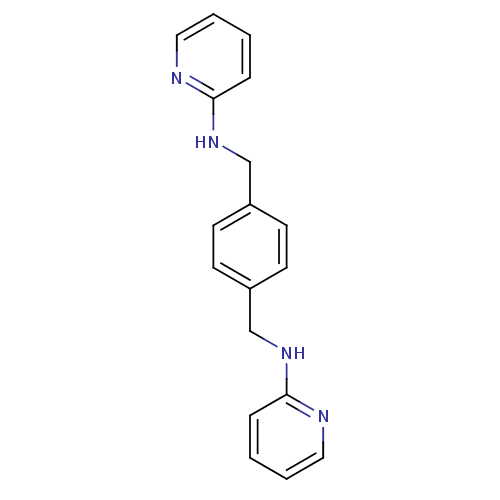
(CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...)Show InChI InChI=1S/C18H18N4/c1-3-11-19-17(5-1)21-13-15-7-9-16(10-8-15)14-22-18-6-2-4-12-20-18/h1-12H,13-14H2,(H,19,21)(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... |
J Med Chem 53: 8556-68 (2010)
Article DOI: 10.1021/jm100786g
BindingDB Entry DOI: 10.7270/Q2PK0GH6 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113627
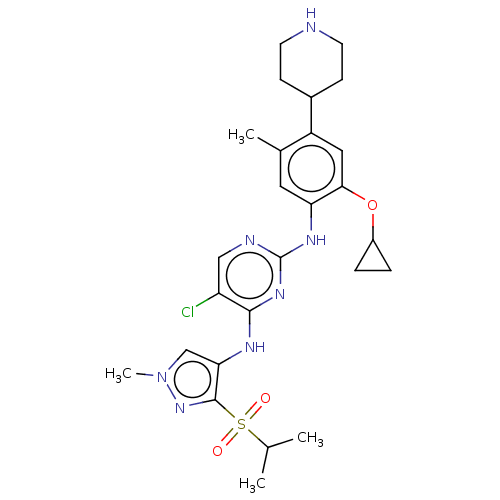
(CHEMBL3604633)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCNCC2)ncc1Cl Show InChI InChI=1S/C26H34ClN7O3S/c1-15(2)38(35,36)25-22(14-34(4)33-25)30-24-20(27)13-29-26(32-24)31-21-11-16(3)19(17-7-9-28-10-8-17)12-23(21)37-18-5-6-18/h11-15,17-18,28H,5-10H2,1-4H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113514

(CHEMBL3604652)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(CCN(C)C)cc2OC2CC2)ncc1Cl Show InChI InChI=1S/C25H34ClN7O3S/c1-15(2)37(34,35)24-21(14-33(6)31-24)28-23-19(26)13-27-25(30-23)29-20-11-16(3)17(9-10-32(4)5)12-22(20)36-18-7-8-18/h11-15,18H,7-10H2,1-6H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113508

(CHEMBL3604649)Show SMILES CC(C)N1CCC(CC1)c1cc(OC2CC2)c(Nc2ncc(Cl)c(Nc3cn(C)nc3S(=O)(=O)C(C)C)n2)cc1C Show InChI InChI=1S/C29H40ClN7O3S/c1-17(2)37-11-9-20(10-12-37)22-14-26(40-21-7-8-21)24(13-19(22)5)33-29-31-15-23(30)27(34-29)32-25-16-36(6)35-28(25)41(38,39)18(3)4/h13-18,20-21H,7-12H2,1-6H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159539

(CHEMBL3787598)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1F Show InChI InChI=1S/C27H36FN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)14-29-27(32-25)31-22-12-17(3)20(13-24(22)38-19-6-7-19)18-8-10-34(4)11-9-18/h12-16,18-19H,6-11H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159541
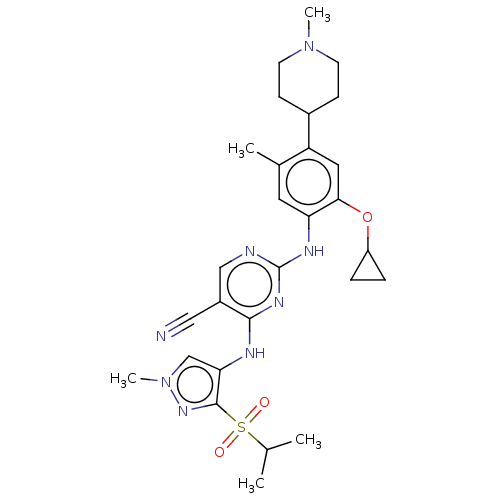
(CHEMBL3785890)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1C#N Show InChI InChI=1S/C28H36N8O3S/c1-17(2)40(37,38)27-24(16-36(5)34-27)31-26-20(14-29)15-30-28(33-26)32-23-12-18(3)22(13-25(23)39-21-6-7-21)19-8-10-35(4)11-9-19/h12-13,15-17,19,21H,6-11H2,1-5H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113518
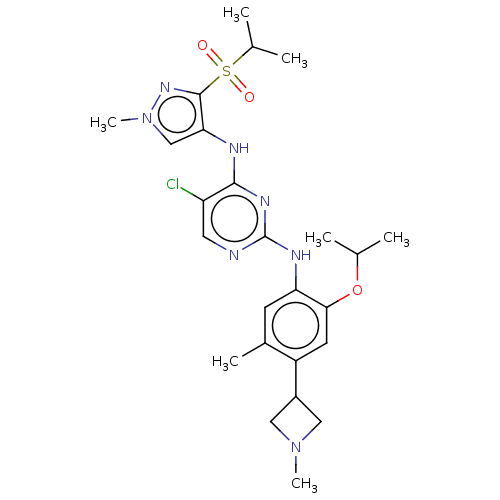
(CHEMBL3604646)Show SMILES CC(C)Oc1cc(C2CN(C)C2)c(C)cc1Nc1ncc(Cl)c(Nc2cn(C)nc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C25H34ClN7O3S/c1-14(2)36-22-9-18(17-11-32(6)12-17)16(5)8-20(22)29-25-27-10-19(26)23(30-25)28-21-13-33(7)31-24(21)37(34,35)15(3)4/h8-10,13-15,17H,11-12H2,1-7H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113626

(CHEMBL3604634)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C27H36ClN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)14-29-27(32-25)31-22-12-17(3)20(13-24(22)38-19-6-7-19)18-8-10-34(4)11-9-18/h12-16,18-19H,6-11H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113522

(CHEMBL3604642)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(F)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C26H33ClFN7O3S/c1-15(2)39(36,37)25-22(14-35(4)33-25)30-24-19(27)13-29-26(32-24)31-21-12-20(28)18(11-23(21)38-17-5-6-17)16-7-9-34(3)10-8-16/h11-17H,5-10H2,1-4H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113523

(CHEMBL3604641)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2ccc(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C26H34ClN7O3S/c1-16(2)38(35,36)25-22(15-34(4)32-25)29-24-20(27)14-28-26(31-24)30-21-8-5-18(13-23(21)37-19-6-7-19)17-9-11-33(3)12-10-17/h5,8,13-17,19H,6-7,9-12H2,1-4H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159531

(CHEMBL3786148)Show SMILES CN1CCC(CC1)c1cc(OC2CC2)c(Nc2ncc(Cl)c(Nc3cn(C)nc3C#N)n2)cc1C Show InChI InChI=1S/C25H29ClN8O/c1-15-10-20(23(35-17-4-5-17)11-18(15)16-6-8-33(2)9-7-16)30-25-28-13-19(26)24(31-25)29-22-14-34(3)32-21(22)12-27/h10-11,13-14,16-17H,4-9H2,1-3H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113510
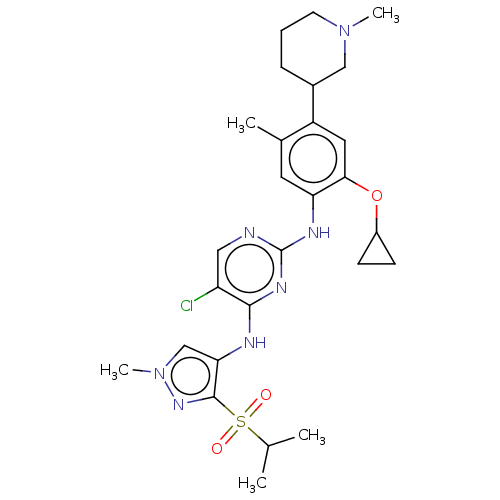
(CHEMBL3604656)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCCN(C)C2)ncc1Cl Show InChI InChI=1S/C27H36ClN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)13-29-27(32-25)31-22-11-17(3)20(12-24(22)38-19-8-9-19)18-7-6-10-34(4)14-18/h11-13,15-16,18-19H,6-10,14H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
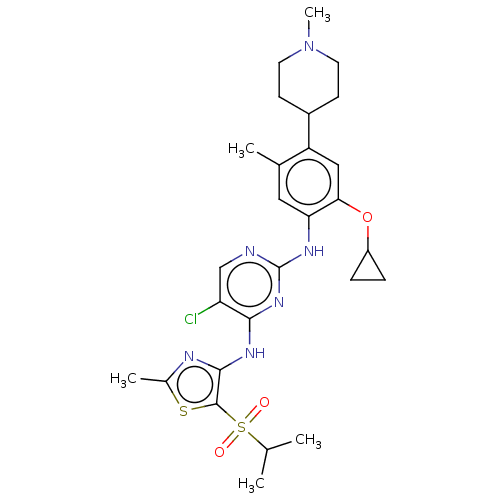
(Homo sapiens (Human)) | BDBM50159536
(CHEMBL3786916)Show SMILES CC(C)S(=O)(=O)c1sc(C)nc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C27H35ClN6O3S2/c1-15(2)39(35,36)26-25(30-17(4)38-26)32-24-21(28)14-29-27(33-24)31-22-12-16(3)20(13-23(22)37-19-6-7-19)18-8-10-34(5)11-9-18/h12-15,18-19H,6-11H2,1-5H3,(H2,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159522

(CHEMBL3785711)Show SMILES CC(C)CS(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC(C)C)C2CCNCC2)ncc1Cl Show InChI InChI=1S/C27H38ClN7O3S/c1-16(2)15-39(36,37)26-23(14-35(6)34-26)31-25-21(28)13-30-27(33-25)32-22-11-18(5)20(12-24(22)38-17(3)4)19-7-9-29-10-8-19/h11-14,16-17,19,29H,7-10,15H2,1-6H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159514
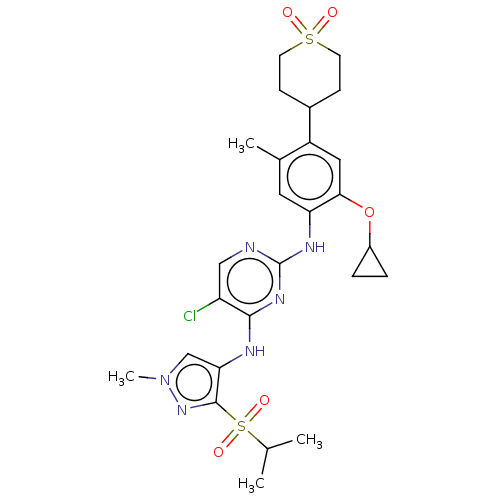
(CHEMBL3785607)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCS(=O)(=O)CC2)ncc1Cl Show InChI InChI=1S/C26H33ClN6O5S2/c1-15(2)40(36,37)25-22(14-33(4)32-25)29-24-20(27)13-28-26(31-24)30-21-11-16(3)19(12-23(21)38-18-5-6-18)17-7-9-39(34,35)10-8-17/h11-15,17-18H,5-10H2,1-4H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human wild type ALK using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113519
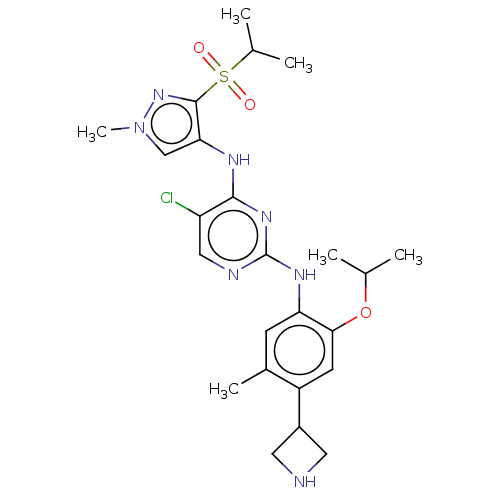
(CHEMBL3604645)Show SMILES CC(C)Oc1cc(C2CNC2)c(C)cc1Nc1ncc(Cl)c(Nc2cn(C)nc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C24H32ClN7O3S/c1-13(2)35-21-8-17(16-9-26-10-16)15(5)7-19(21)29-24-27-11-18(25)22(30-24)28-20-12-32(6)31-23(20)36(33,34)14(3)4/h7-8,11-14,16,26H,9-10H2,1-6H3,(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113626

(CHEMBL3604634)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C27H36ClN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)14-29-27(32-25)31-22-12-17(3)20(13-24(22)38-19-6-7-19)18-8-10-34(4)11-9-18/h12-16,18-19H,6-11H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ALK L1196M mutant using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113517
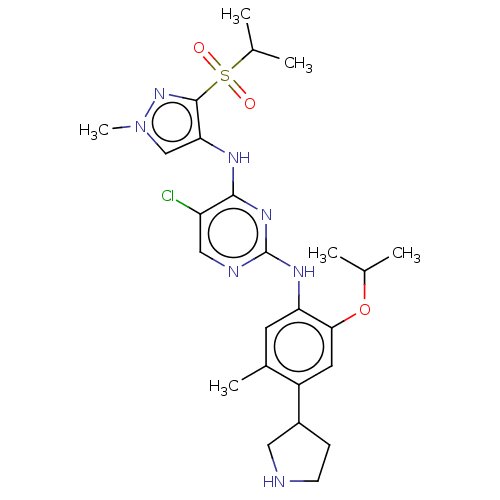
(CHEMBL3604647)Show SMILES CC(C)Oc1cc(C2CCNC2)c(C)cc1Nc1ncc(Cl)c(Nc2cn(C)nc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C25H34ClN7O3S/c1-14(2)36-22-10-18(17-7-8-27-11-17)16(5)9-20(22)30-25-28-12-19(26)23(31-25)29-21-13-33(6)32-24(21)37(34,35)15(3)4/h9-10,12-15,17,27H,7-8,11H2,1-6H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113522

(CHEMBL3604642)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(F)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C26H33ClFN7O3S/c1-15(2)39(36,37)25-22(14-35(4)33-25)30-24-19(27)13-29-26(32-24)31-21-12-20(28)18(11-23(21)38-17-5-6-17)16-7-9-34(3)10-8-16/h11-17H,5-10H2,1-4H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113507

(CHEMBL3604648)Show SMILES CC(C)Oc1cc(C2CCN(C)C2)c(C)cc1Nc1ncc(Cl)c(Nc2cn(C)nc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C26H36ClN7O3S/c1-15(2)37-23-11-19(18-8-9-33(6)13-18)17(5)10-21(23)30-26-28-12-20(27)24(31-26)29-22-14-34(7)32-25(22)38(35,36)16(3)4/h10-12,14-16,18H,8-9,13H2,1-7H3,(H2,28,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113516

(CHEMBL3604650)Show SMILES COCCc1cc(OC2CC2)c(Nc2ncc(Cl)c(Nc3cn(C)nc3S(=O)(=O)C(C)C)n2)cc1C Show InChI InChI=1S/C24H31ClN6O4S/c1-14(2)36(32,33)23-20(13-31(4)30-23)27-22-18(25)12-26-24(29-22)28-19-10-15(3)16(8-9-34-5)11-21(19)35-17-6-7-17/h10-14,17H,6-9H2,1-5H3,(H2,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113629

(CHEMBL3604632)Show SMILES CC(C)Oc1cc(C2CCN(C)CC2)c(C)cc1Nc1ncc(Cl)c(Nc2cn(C)nc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C27H38ClN7O3S/c1-16(2)38-24-13-20(19-8-10-34(6)11-9-19)18(5)12-22(24)31-27-29-14-21(28)25(32-27)30-23-15-35(7)33-26(23)39(36,37)17(3)4/h12-17,19H,8-11H2,1-7H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113521

(CHEMBL3604643)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(Cl)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C26H33Cl2N7O3S/c1-15(2)39(36,37)25-22(14-35(4)33-25)30-24-20(28)13-29-26(32-24)31-21-12-19(27)18(11-23(21)38-17-5-6-17)16-7-9-34(3)10-8-16/h11-17H,5-10H2,1-4H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50159541

(CHEMBL3785890)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1C#N Show InChI InChI=1S/C28H36N8O3S/c1-17(2)40(37,38)27-24(16-36(5)34-27)31-26-20(14-29)15-30-28(33-26)32-23-12-18(3)22(13-25(23)39-21-6-7-21)19-8-10-35(4)11-9-19/h12-13,15-17,19,21H,6-11H2,1-5H3,(H2,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ALK L1196M mutant using tyrosine kinase substrate-biotin after 30 mins by HTRF assay |
Bioorg Med Chem Lett 26: 1910-8 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.017
BindingDB Entry DOI: 10.7270/Q26M38QJ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113627

(CHEMBL3604633)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCNCC2)ncc1Cl Show InChI InChI=1S/C26H34ClN7O3S/c1-15(2)38(35,36)25-22(14-34(4)33-25)30-24-20(27)13-29-26(32-24)31-21-11-16(3)19(17-7-9-28-10-8-17)12-23(21)37-18-5-6-18/h11-15,17-18,28H,5-10H2,1-4H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113511
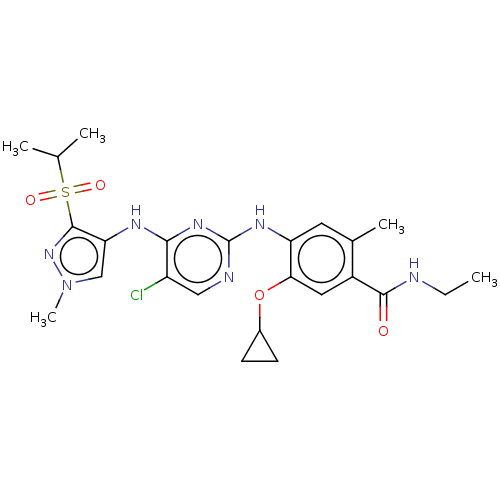
(CHEMBL3604655)Show SMILES CCNC(=O)c1cc(OC2CC2)c(Nc2ncc(Cl)c(Nc3cn(C)nc3S(=O)(=O)C(C)C)n2)cc1C Show InChI InChI=1S/C24H30ClN7O4S/c1-6-26-22(33)16-10-20(36-15-7-8-15)18(9-14(16)4)29-24-27-11-17(25)21(30-24)28-19-12-32(5)31-23(19)37(34,35)13(2)3/h9-13,15H,6-8H2,1-5H3,(H,26,33)(H2,27,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of wild type human recombinant ALK using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50113626

(CHEMBL3604634)Show SMILES CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)C2CCN(C)CC2)ncc1Cl Show InChI InChI=1S/C27H36ClN7O3S/c1-16(2)39(36,37)26-23(15-35(5)33-26)30-25-21(28)14-29-27(32-25)31-22-12-17(3)20(13-24(22)38-19-6-7-19)18-8-10-34(4)11-9-18/h12-16,18-19H,6-11H2,1-5H3,(H2,29,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Pearl Biotech Ltd
Curated by ChEMBL
| Assay Description
Inhibition of ALK L1196M mutant (unknown origin) using TK substrate-biotin incubated for 30 mins by HTRF assay |
Bioorg Med Chem Lett 25: 3738-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.021
BindingDB Entry DOI: 10.7270/Q208674B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data