

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59242 (Benzotriazole ester, 8 | acs.jmedchem.1c00409_ST.6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59240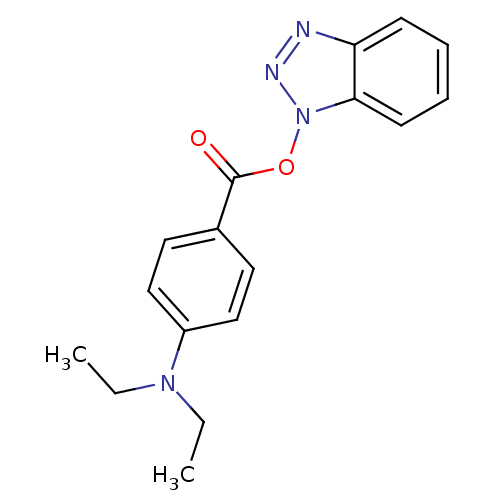 (Benzotriazole ester, 6 | acs.jmedchem.1c00409_ST.8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59239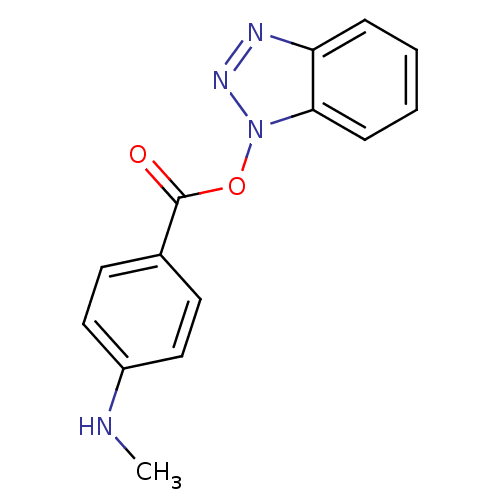 (Benzotriazole ester, 5 | acs.jmedchem.1c00409_ST.9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59243 (Benzotriazole ester, 9 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59244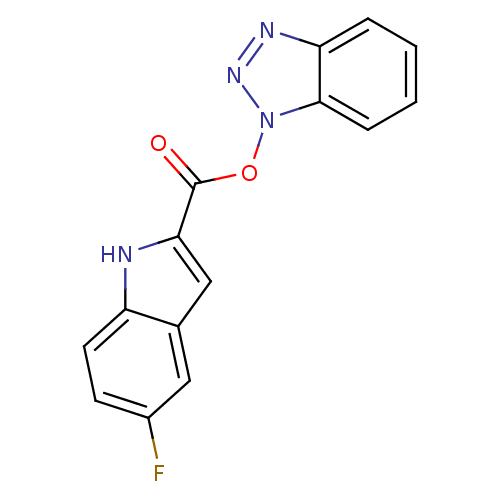 (Benzotriazole ester, 10 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59238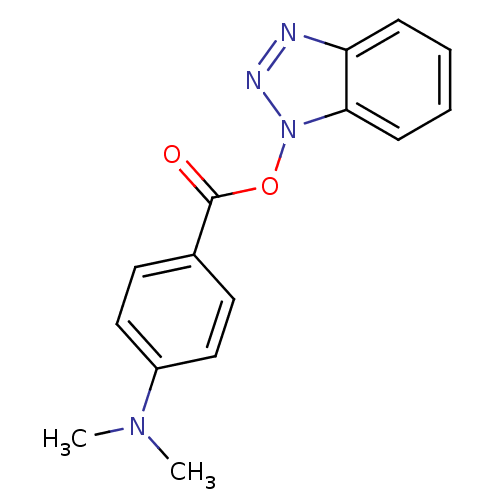 (Benzotriazole ester, 4 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59237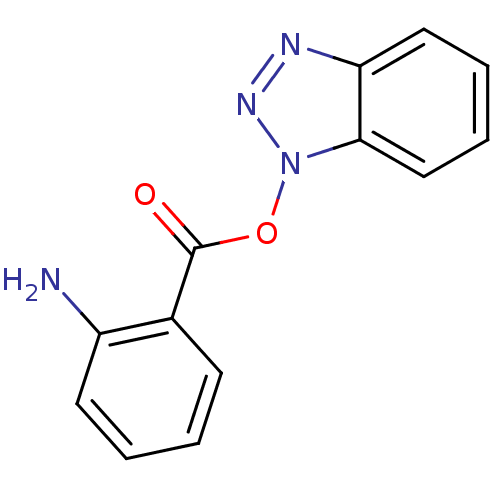 (Benzotriazole ester, 3 | acs.jmedchem.1c00409_ST.1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59241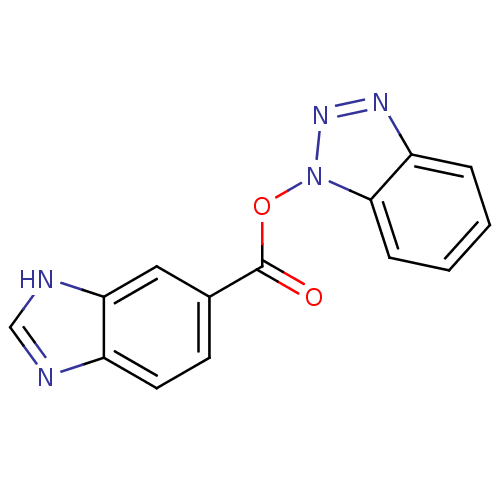 (Benzotriazole ester, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59246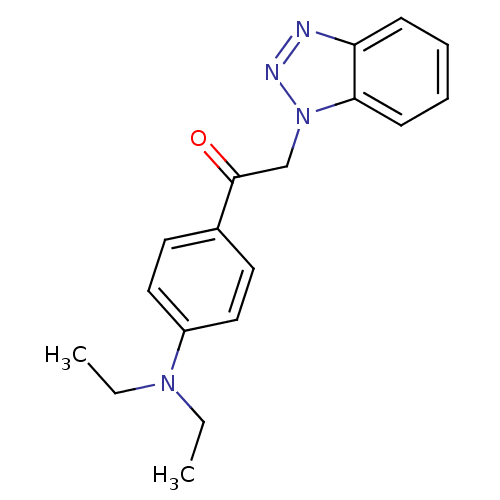 (Benzotriazole ester, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59249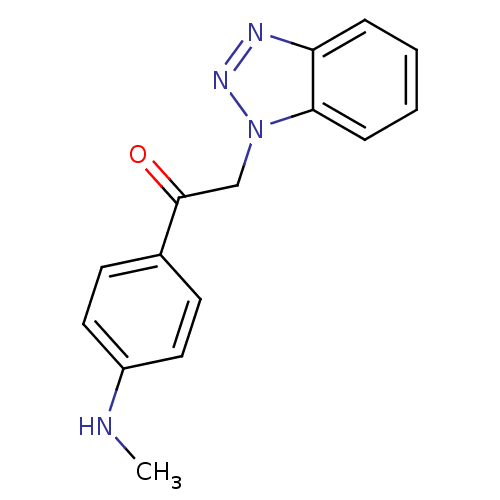 (Benzotriazole ester, 17 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59250 (Benzotriazole ester, 18 | acs.jmedchem.1c00409_ST....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59251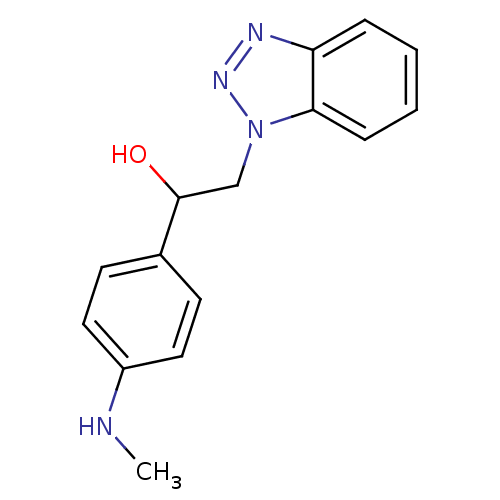 (Benzotriazole ester, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM59252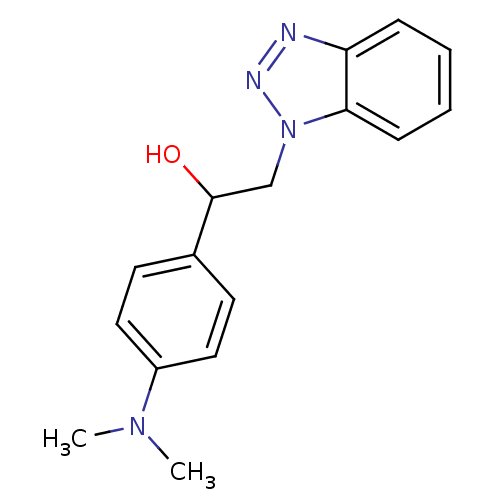 (Benzotriazole ester, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Academia Sinica | Assay Description Inhibition assay against SARS-CoV 3CL protease, a fluorometric assay was utilized to determine the inhibition constants of the samples. | Chem Biol 13: 261-8 (2006) Article DOI: 10.1016/j.chembiol.2005.12.008 BindingDB Entry DOI: 10.7270/Q21C1V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129810 (US8815926, 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129937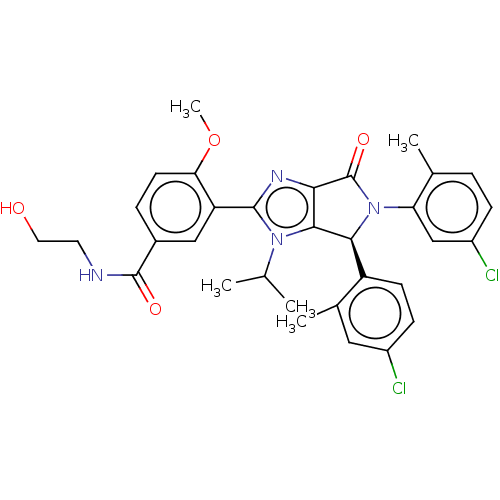 (US8815926, 218) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130042 (US8815926, 325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50264967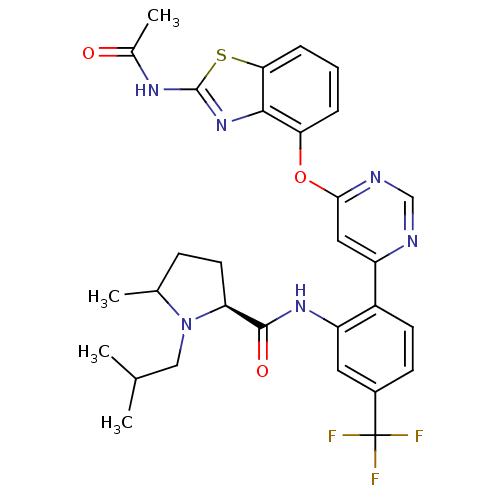 ((2S)-N-(2-(6-(2-acetamidobenzo[d]thiazol-4-yloxy)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced 45Ca2+ influx by FLIPR assay | Bioorg Med Chem Lett 18: 5118-22 (2008) Article DOI: 10.1016/j.bmcl.2008.07.112 BindingDB Entry DOI: 10.7270/Q27D2TXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129730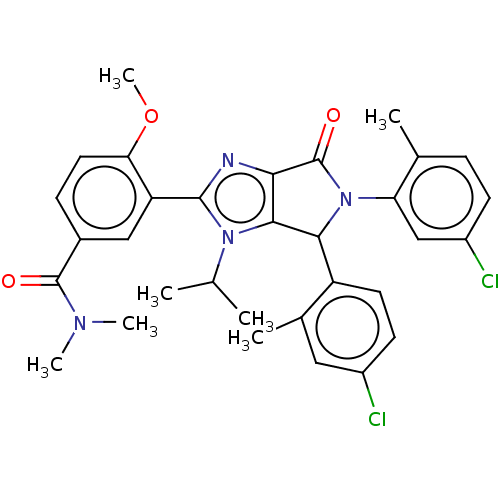 (US8815926, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129796 (US8815926, 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129738 (US8815926, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129945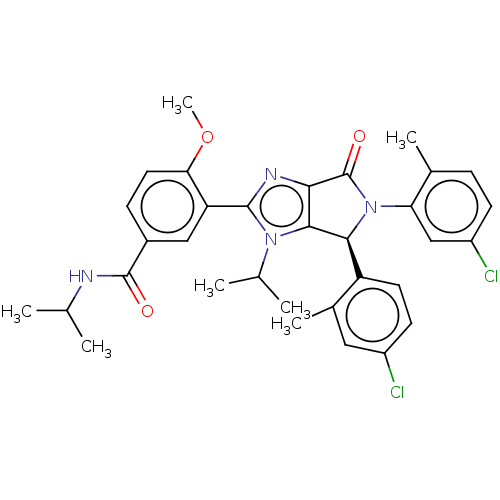 (US8815926, 226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129966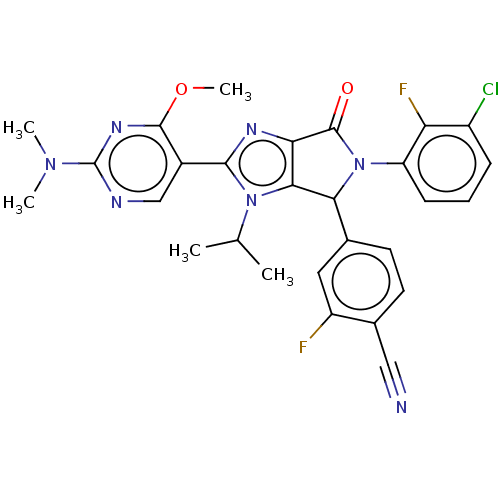 (US8815926, 247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129947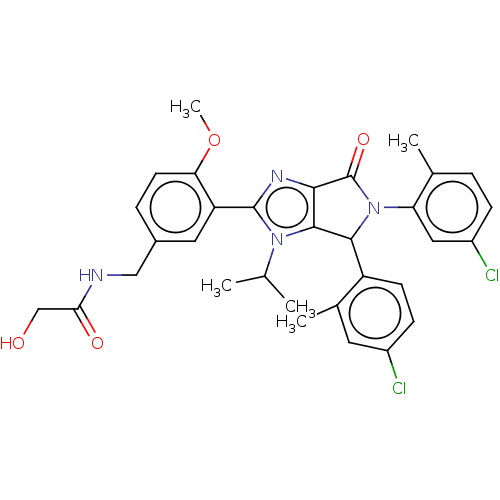 (US8815926, 228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129892 (US8815926, 173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130000 (US8815926, 281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130035 (US8815926, 317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129737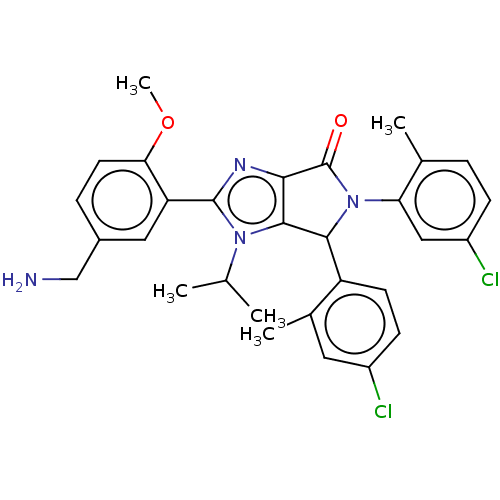 (US8815926, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129838 (US8815926, 117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129942 (US8815926, 223) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129928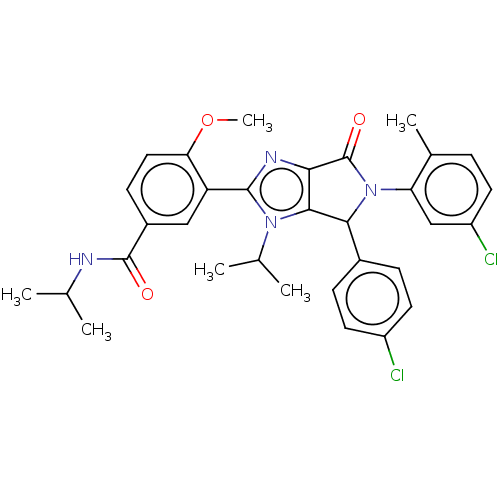 (US8815926, 209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129894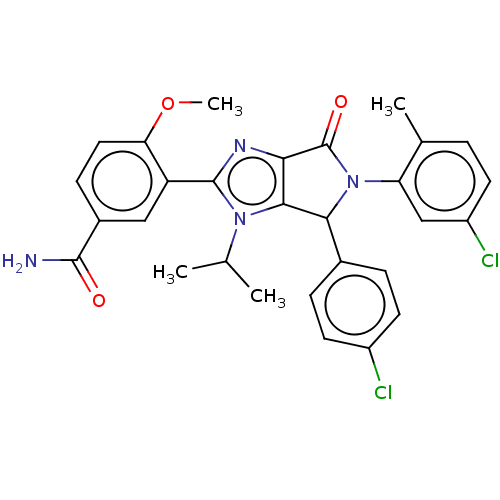 (US8815926, 175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 [388-802] (Homo sapiens (Human)) | BDBM209350 (US9266883, 149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description Liquid handling and incubation steps were done on an Innovadyne Nanodrop Express equipped with a robotic arm (Thermo CatX, Caliper Twister II) and an... | US Patent US9266883 (2016) BindingDB Entry DOI: 10.7270/Q2CC0ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129884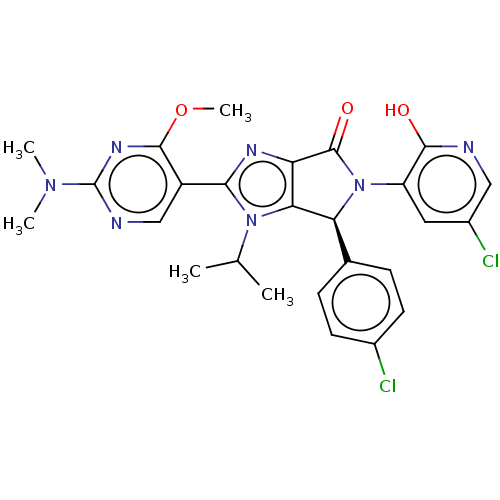 (US8815926, 164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 4 [388-802] (Homo sapiens (Human)) | BDBM209374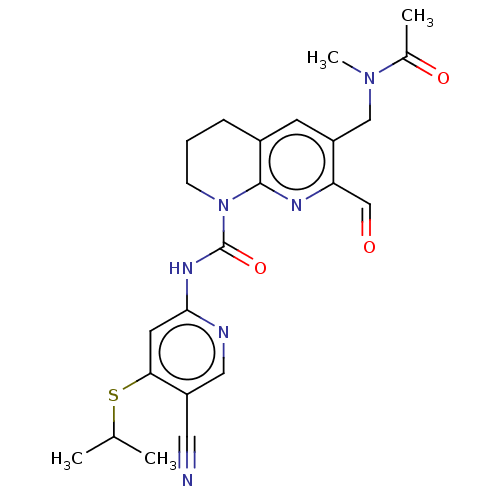 (US9266883, 240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description Liquid handling and incubation steps were done on an Innovadyne Nanodrop Express equipped with a robotic arm (Thermo CatX, Caliper Twister II) and an... | US Patent US9266883 (2016) BindingDB Entry DOI: 10.7270/Q2CC0ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130001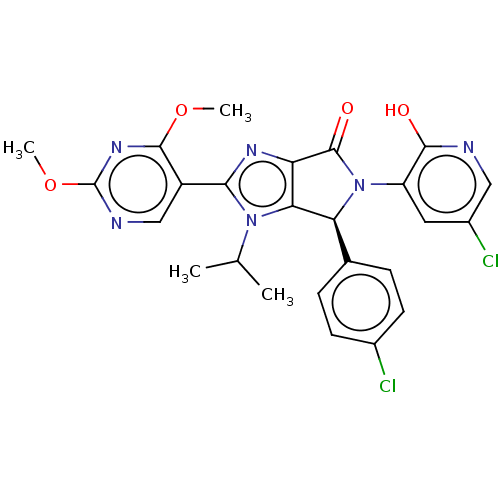 (US8815926, 282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130034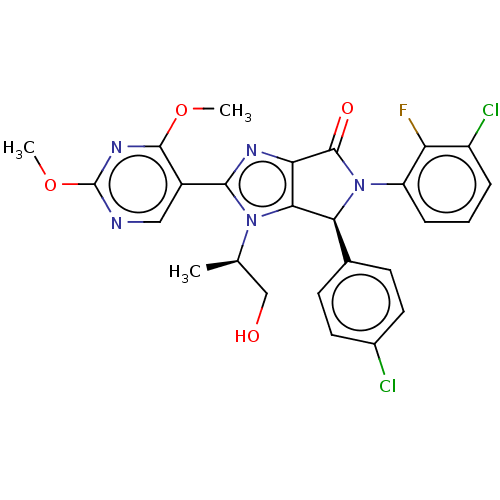 (US8815926, 316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129763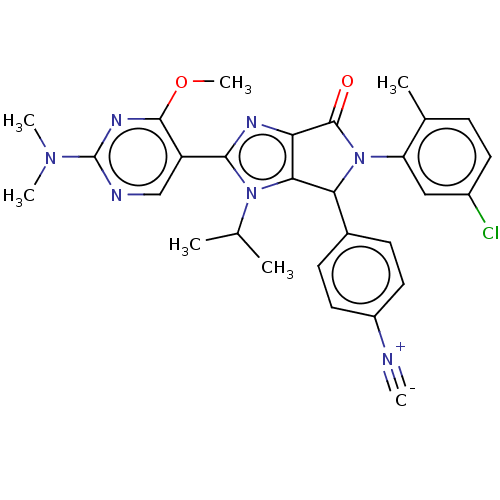 (US8815926, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129916 (US8815926, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129924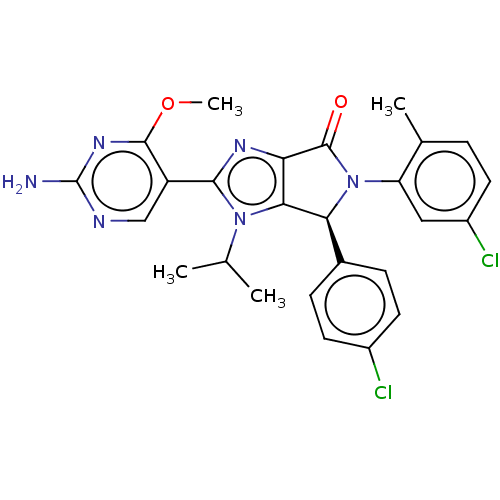 (US8815926, 205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129893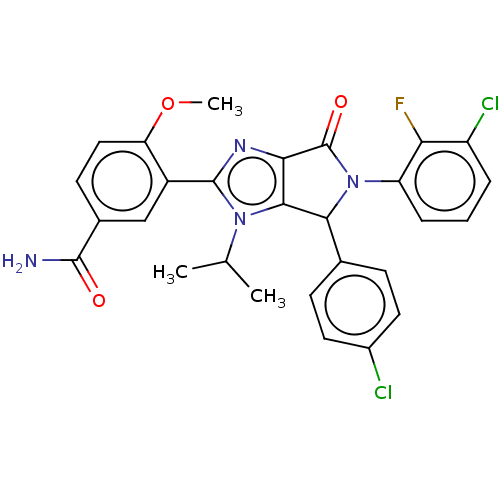 (US8815926, 174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129834 (US8815926, 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129888 (US8815926, 169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129890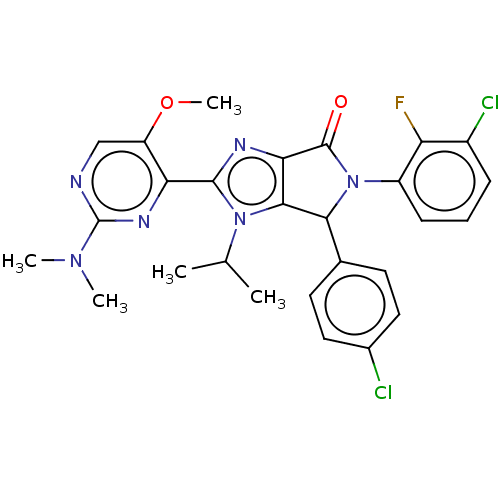 (US8815926, 171 | US8815926, 174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130006 (US8815926, 287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129742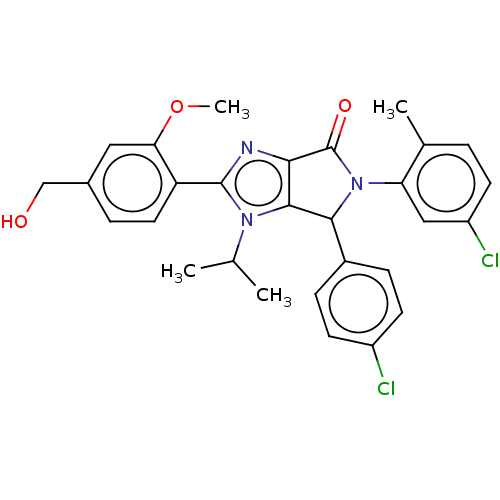 (US8815926, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129964 (US8815926, 245 | US8815926, 275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129964 (US8815926, 245 | US8815926, 275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM130025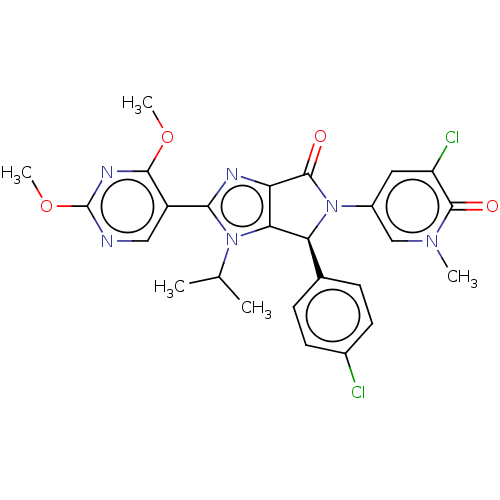 (US8815926, 307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129743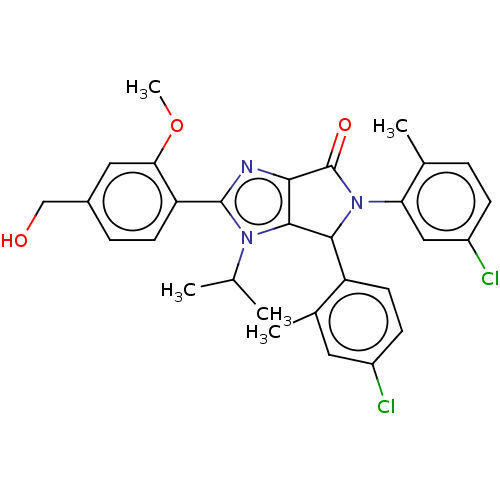 (US8815926, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM129744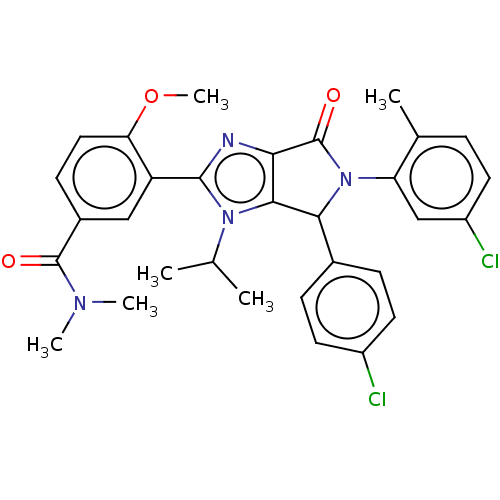 (US8815926, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-MDM2 and p53-MDM4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US8815926 (2014) BindingDB Entry DOI: 10.7270/Q29P309G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1896 total ) | Next | Last >> |