

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427622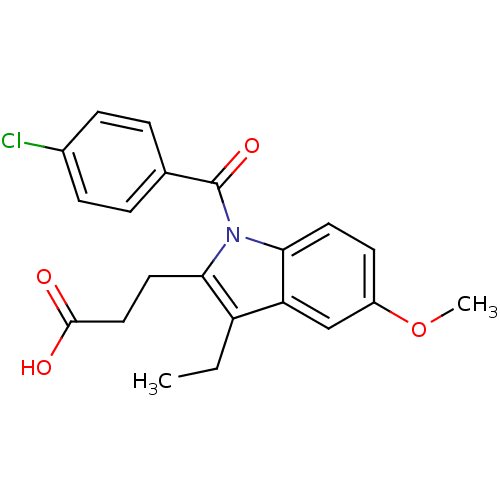 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427628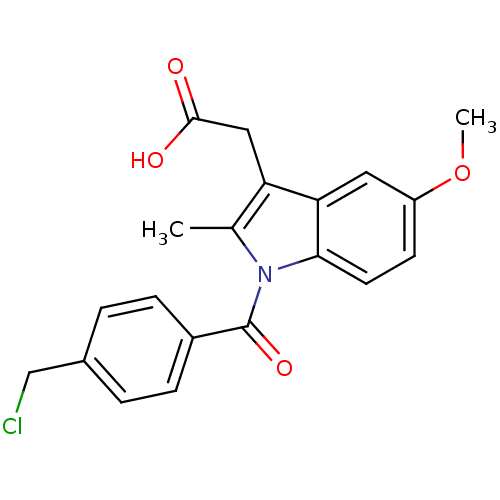 (CHEMBL2323472 | US9346803, Table 2, Compound 8: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427620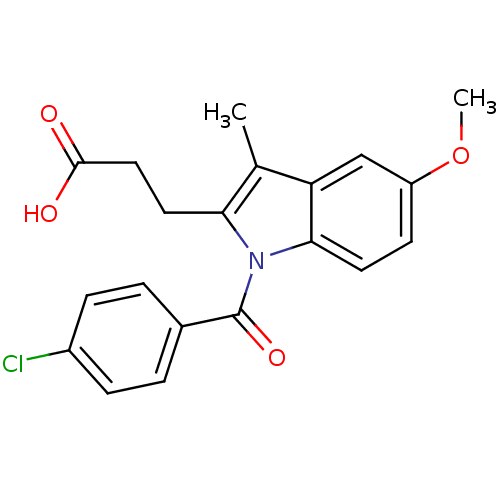 (CHEMBL2323507 | US9346803, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427629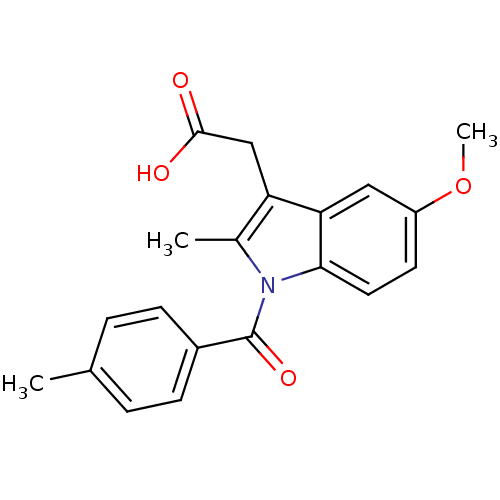 (CHEMBL179587 | US9346803, Table 2, Compound 7: 2-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427624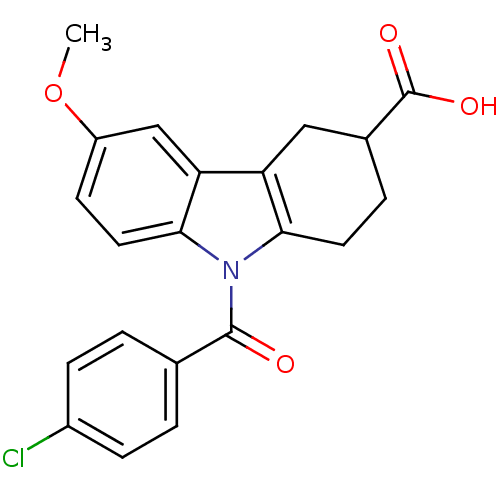 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427621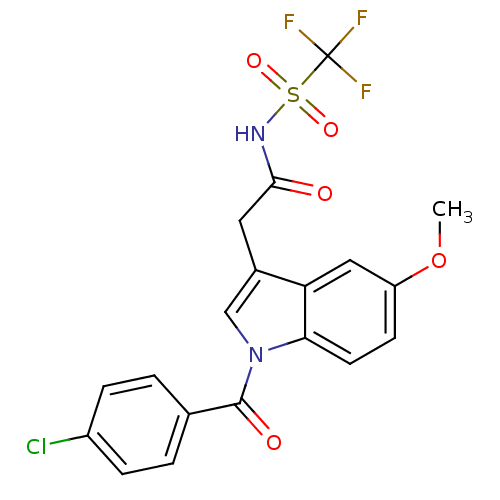 (CHEMBL2323490 | US9346803, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427625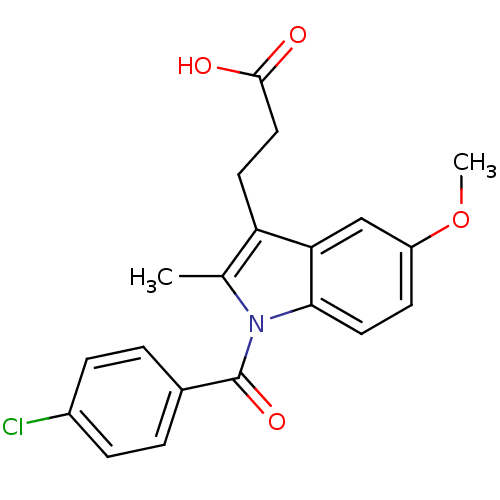 (CHEMBL178687 | US9346803, Table 2, Compound 6: 3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427627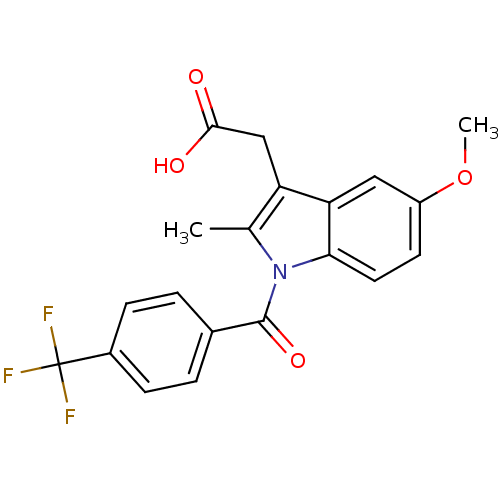 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427619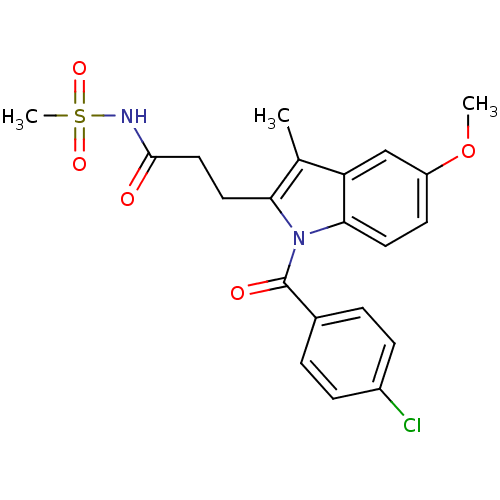 (CHEMBL2323511 | US9346803, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50427626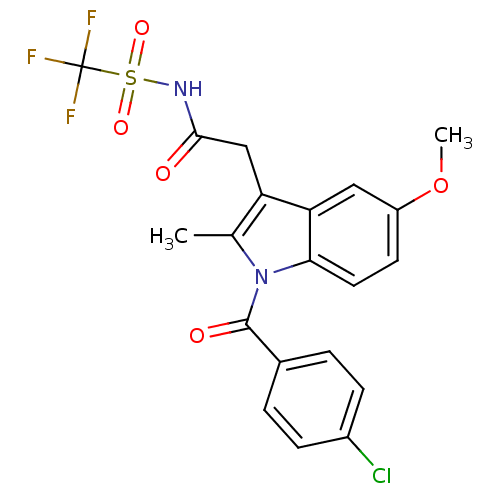 (CHEMBL2323481 | US9346803, Table 2, Compound 5: 2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50293598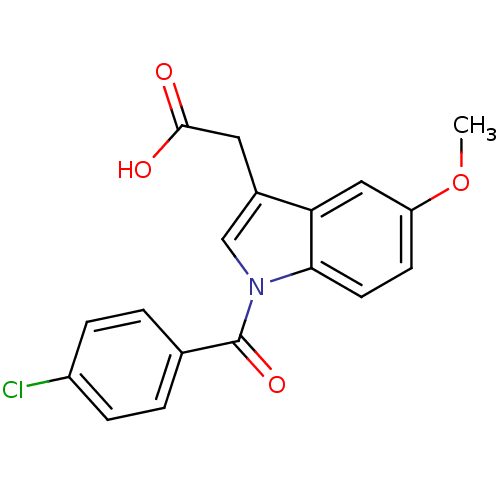 (2'-des-methyl indomethacin | CHEMBL503179 | US9346...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251993 (CHEMBL481952 | trans-4-{4-[4-(4-Fluoro-phenyl)-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251932 (CHEMBL516607 | trans-4-{4-[4-(4-Fluoro-phenyl)-2-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427624 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427620 (CHEMBL2323507 | US9346803, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251985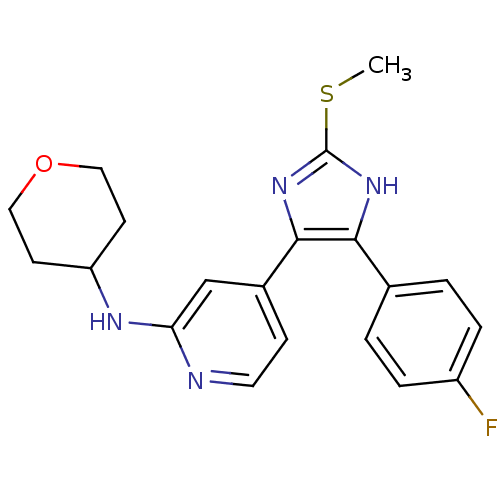 (4-(4-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251981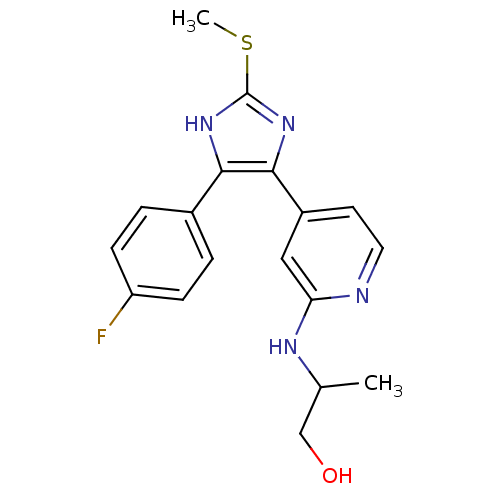 (2-{4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology Curated by ChEMBL | Assay Description Inhibition of COX1 | J Med Chem 55: 2287-300 (2012) Article DOI: 10.1021/jm201528b BindingDB Entry DOI: 10.7270/Q2WW7JQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251972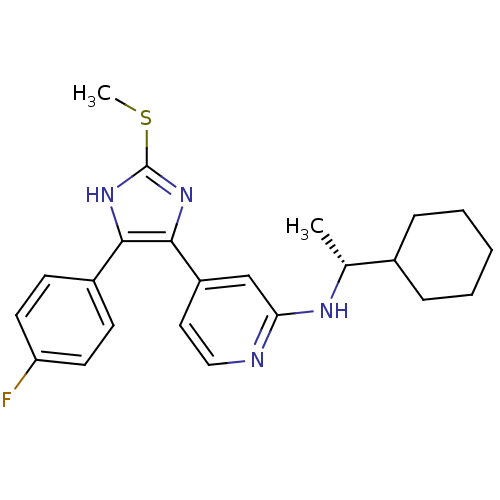 (((R)-1-Cyclohexyl-ethyl)-{4-[4-(4-fluoro-phenyl)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251982 (2-{4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251921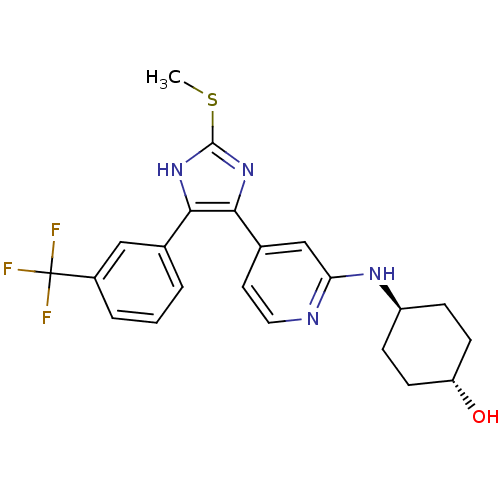 (CHEMBL481749 | trans-4-{4-[2-Methylsulfanyl-4-(3-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251978 ((1,2-Dimethyl-propyl)-{4-[4-(4-fluoro-phenyl)-2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761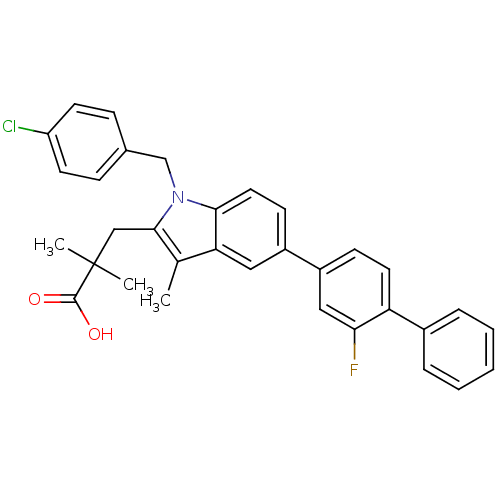 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta treated human A549 cell microsomal membrane assessed as residual enzyme activity after 1 min by measuring PGE2 level... | J Med Chem 52: 4968-72 (2009) Article DOI: 10.1021/jm900481c BindingDB Entry DOI: 10.7270/Q29C6XGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50427619 (CHEMBL2323511 | US9346803, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251975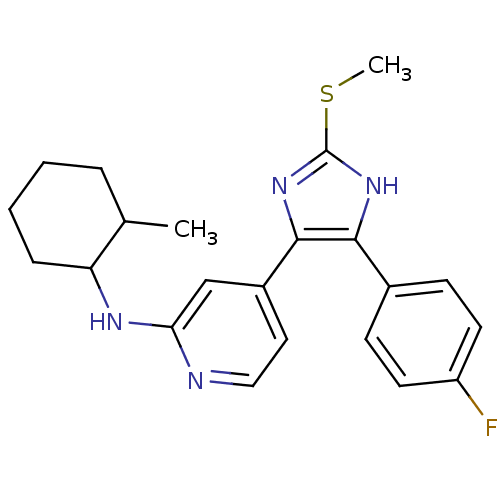 ((cis/trans) -{4-[4-(4-Fluoro-phenyl)-2-methylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50252665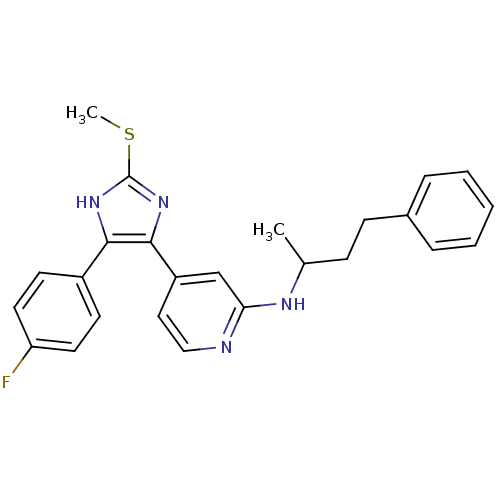 (CHEMBL493669 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427620 (CHEMBL2323507 | US9346803, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251984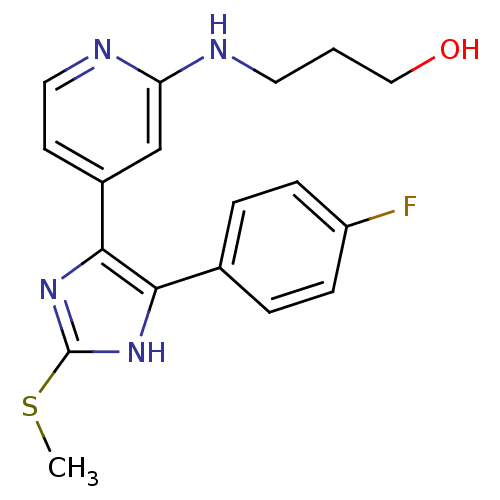 (3-{4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251924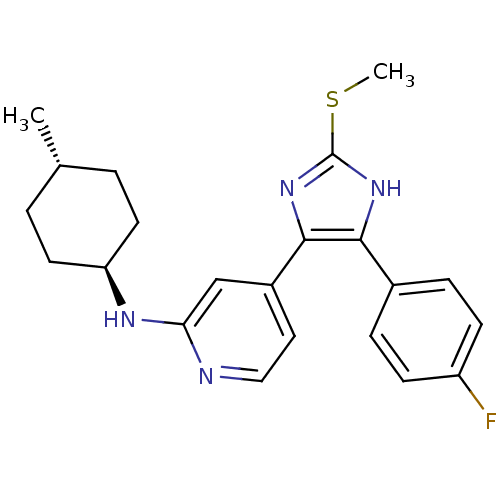 (CHEMBL481751 | trans-{4-[4-(4-Fluoro-phenyl)-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50252664 (CHEMBL523880 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427620 (CHEMBL2323507 | US9346803, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251974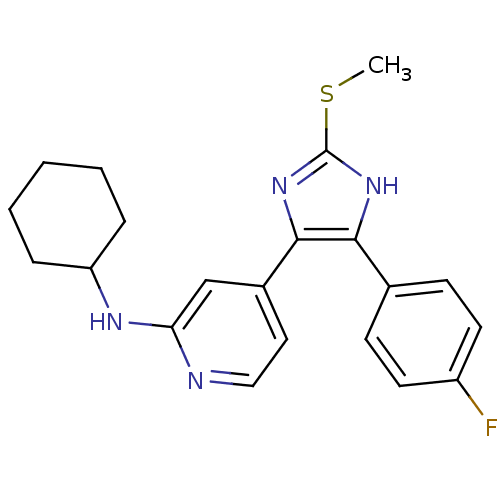 (CHEMBL482086 | Cyclohexyl-{4-[4-(4-fluoro-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251927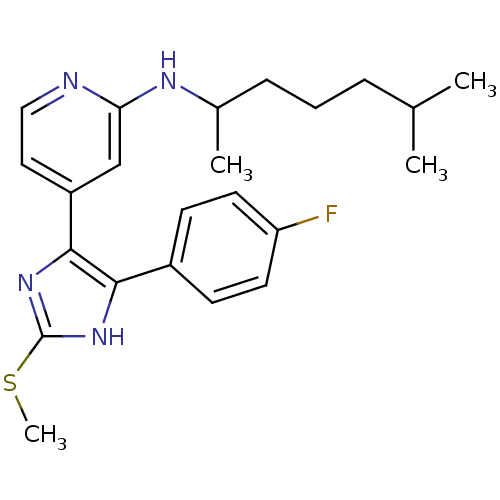 ((1,5-Dimethyl-hexyl)-{4-[4-(4-fluoro-phenyl)-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX1 (unknown origin)-mediated oxidation of N,N,N,Ntetramethyl-1,4-phenylenediamine using arachidonic acid as substrate by colorimetric... | J Med Chem 56: 2429-46 (2013) Article DOI: 10.1021/jm3017656 BindingDB Entry DOI: 10.7270/Q2X92CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251925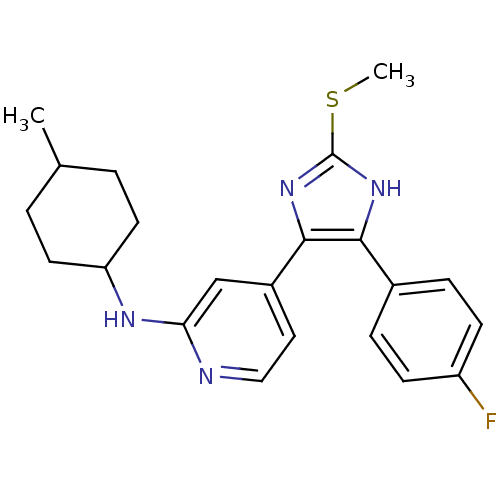 ((cis/trans)-{4-[4-(4-Fluoro-phenyl)-2-methylsulfan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251929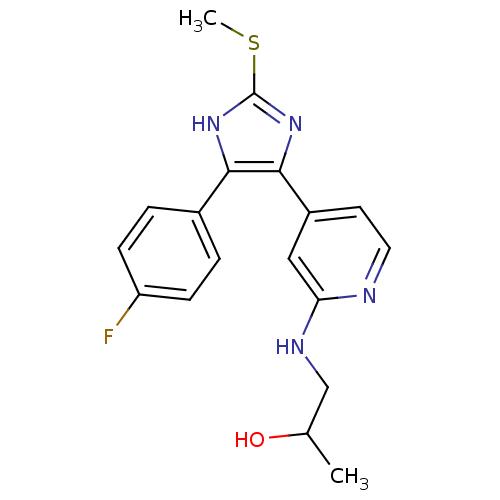 (1-{4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251912 (CHEMBL519617 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251926 (CHEMBL481919 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Binding affinity to FLAP | J Med Chem 52: 4968-72 (2009) Article DOI: 10.1021/jm900481c BindingDB Entry DOI: 10.7270/Q29C6XGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251980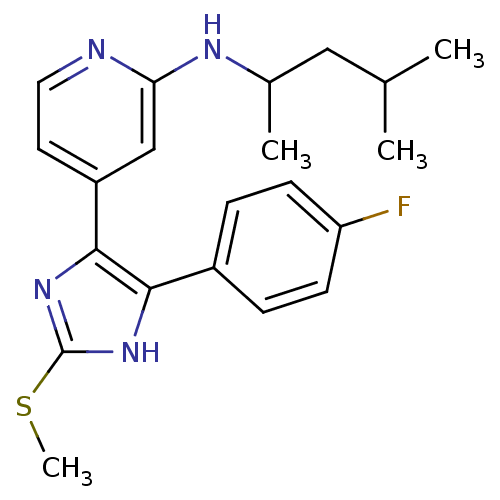 ((1,3-Dimethyl-butyl)-{4-[4-(4-fluoro-phenyl)-2-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50252617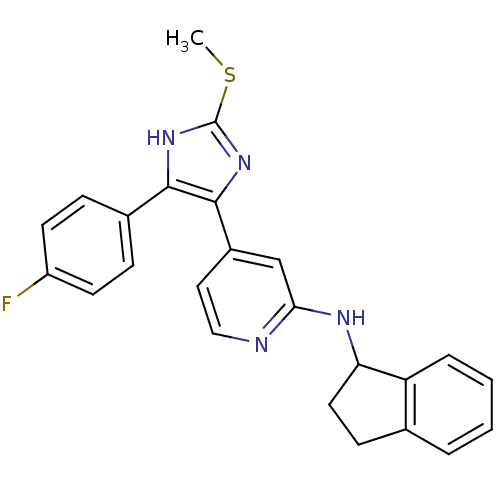 (CHEMBL494871 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427622 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251923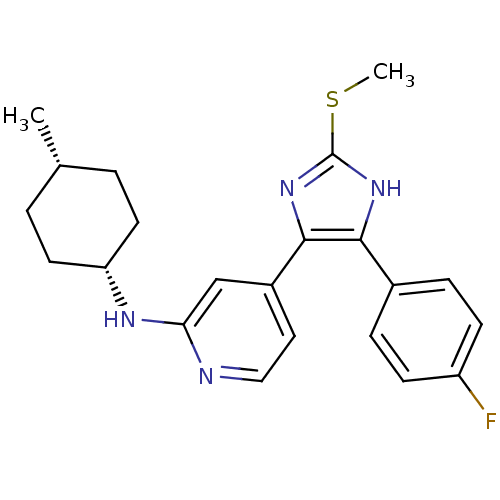 (CHEMBL481750 | cis-{4-[4-(4-Fluoro-phenyl)-2-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251935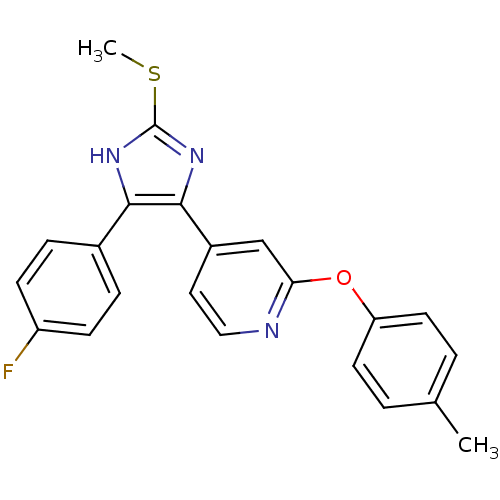 (4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251919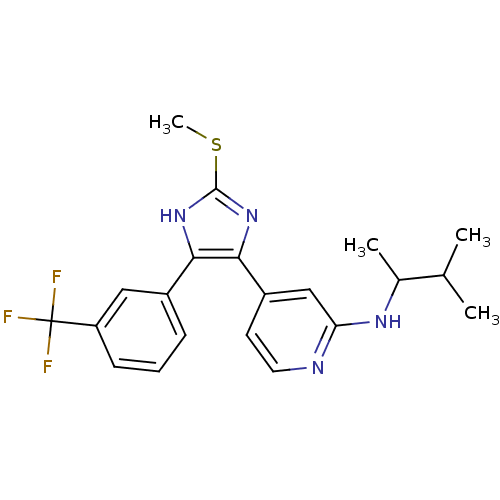 ((1,2-Dimethyl-propyl)-{4-[2-methylsulfanyl-4-(3-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251983 (2-{4-[4-(4-Fluoro-phenyl)-2-methylsulfanyl-1H-imid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251930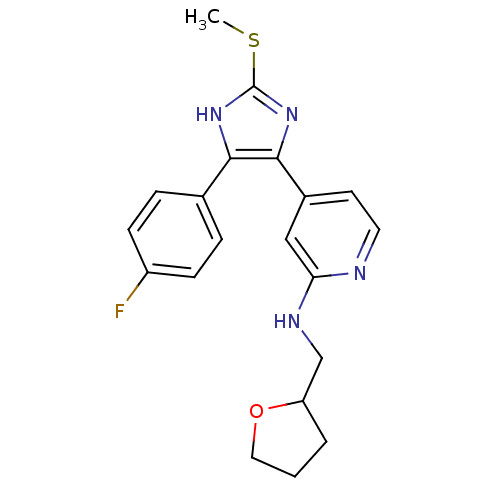 (CHEMBL480952 | {4-[4-(4-Fluoro-phenyl)-2-methylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50427627 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50251918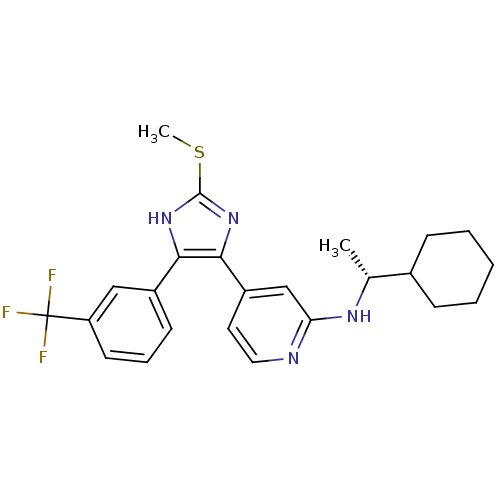 (((R)-1-Cyclohexyl-ethyl)-{4-[2-methylsulfanyl-4-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen Curated by ChEMBL | Assay Description Inhibition of p38alpha | J Med Chem 51: 4122-49 (2008) Article DOI: 10.1021/jm701529q BindingDB Entry DOI: 10.7270/Q2W37X67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 333 total ) | Next | Last >> |