 Found 377 hits with Last Name = 'light' and Initial = 'dr'
Found 377 hits with Last Name = 'light' and Initial = 'dr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM17280

(1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N3CCCC(C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C28H28F2N6O5/c1-35-11-9-33-25(35)16-4-2-6-18(12-16)40-26-21(29)23(36-10-3-5-17(14-36)28(38)39)22(30)27(34-26)41-20-13-15(24(31)32)7-8-19(20)37/h2,4,6-8,12-13,17,37H,3,5,9-11,14H2,1H3,(H3,31,32)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Biochemistry 39: 12534-42 (2000)
Article DOI: 10.1021/bi001477q
BindingDB Entry DOI: 10.7270/Q2C827JM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284
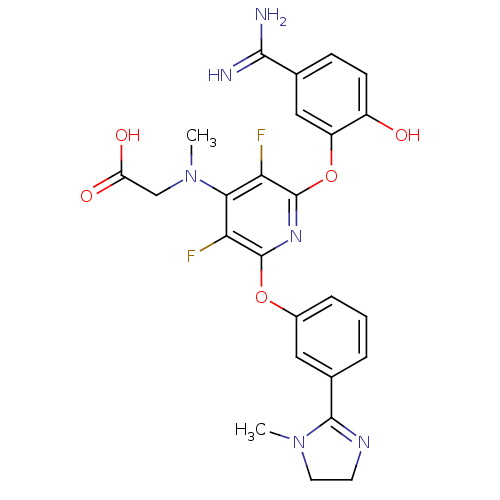
(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284

(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284

(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Biochemistry 39: 12534-42 (2000)
Article DOI: 10.1021/bi001477q
BindingDB Entry DOI: 10.7270/Q2C827JM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17280

(1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N3CCCC(C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C28H28F2N6O5/c1-35-11-9-33-25(35)16-4-2-6-18(12-16)40-26-21(29)23(36-10-3-5-17(14-36)28(38)39)22(30)27(34-26)41-20-13-15(24(31)32)7-8-19(20)37/h2,4,6-8,12-13,17,37H,3,5,9-11,14H2,1H3,(H3,31,32)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066635
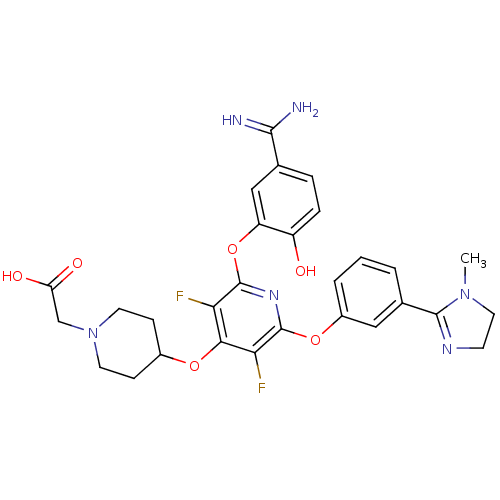
((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(OC3CCN(CC(O)=O)CC3)c2F)c1 |c:4| Show InChI InChI=1S/C29H30F2N6O6/c1-36-12-9-34-27(36)17-3-2-4-19(13-17)42-28-23(30)25(41-18-7-10-37(11-8-18)15-22(39)40)24(31)29(35-28)43-21-14-16(26(32)33)5-6-20(21)38/h2-6,13-14,18,38H,7-12,15H2,1H3,(H3,32,33)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066619

(({2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difluo...)Show SMILES CCOC(=O)CN(C)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:20| Show InChI InChI=1S/C27H28F2N6O5/c1-4-38-20(37)14-35(3)23-21(28)26(39-17-7-5-6-16(12-17)25-32-10-11-34(25)2)33-27(22(23)29)40-19-13-15(24(30)31)8-9-18(19)36/h5-9,12-13,36H,4,10-11,14H2,1-3H3,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17282

(7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2nc(C)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C27H30N6O/c1-17(28)32-11-9-23(10-12-32)34-24-7-8-25-26(15-24)33(18(2)31-25)16-19-3-4-20-5-6-21(27(29)30)14-22(20)13-19/h3-8,13-15,23,28H,9-12,16H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066634

((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...)Show SMILES CCOC(=O)CN1CCC(CC1)Oc1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:26| Show InChI InChI=1S/C31H34F2N6O6/c1-3-42-24(41)17-39-12-9-20(10-13-39)43-27-25(32)30(44-21-6-4-5-19(15-21)29-36-11-14-38(29)2)37-31(26(27)33)45-23-16-18(28(34)35)7-8-22(23)40/h4-8,15-16,20,40H,3,9-14,17H2,1-2H3,(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066641
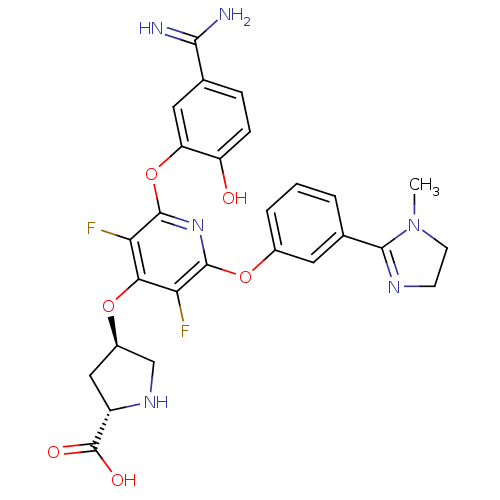
((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(O[C@H]3CN[C@@H](C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C27H26F2N6O6/c1-35-8-7-32-24(35)14-3-2-4-15(9-14)40-25-20(28)22(39-16-11-17(27(37)38)33-12-16)21(29)26(34-25)41-19-10-13(23(30)31)5-6-18(19)36/h2-6,9-10,16-17,33,36H,7-8,11-12H2,1H3,(H3,30,31)(H,37,38)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066623
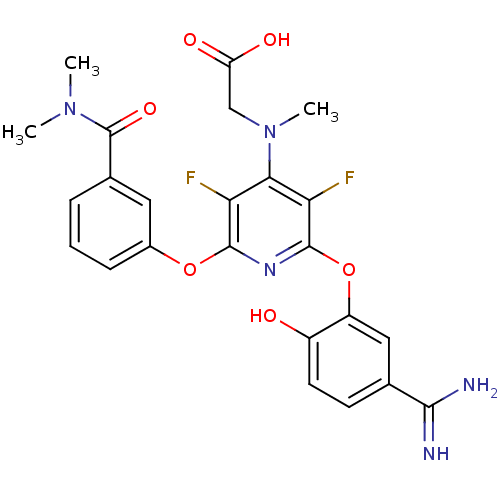
(CHEMBL72318 | {[2-(5-Carbamimidoyl-2-hydroxy-pheno...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N(C)CC(O)=O)c2F)c1 Show InChI InChI=1S/C24H23F2N5O6/c1-30(2)24(35)13-5-4-6-14(9-13)36-22-18(25)20(31(3)11-17(33)34)19(26)23(29-22)37-16-10-12(21(27)28)7-8-15(16)32/h4-10,32H,11H2,1-3H3,(H3,27,28)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066639
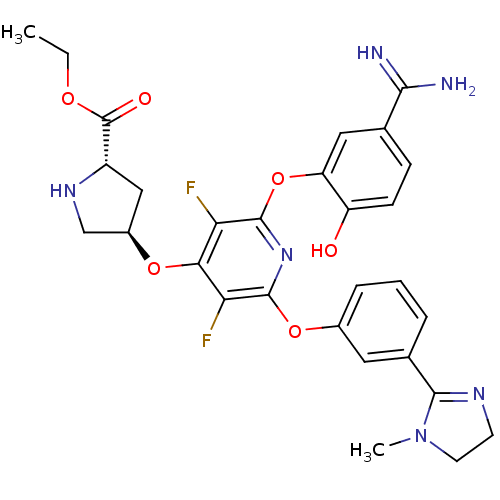
((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...)Show SMILES CCOC(=O)[C@@H]1C[C@H](CN1)Oc1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:24| Show InChI InChI=1S/C29H30F2N6O6/c1-3-40-29(39)19-13-18(14-35-19)41-24-22(30)27(42-17-6-4-5-16(11-17)26-34-9-10-37(26)2)36-28(23(24)31)43-21-12-15(25(32)33)7-8-20(21)38/h4-8,11-12,18-19,35,38H,3,9-10,13-14H2,1-2H3,(H3,32,33)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066625

(CHEMBL120438 | {4-[2-(5-Carbamimidoyl-2-hydroxy-ph...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(OC3CCN(CC(O)=O)CC3)c2F)c1 Show InChI InChI=1S/C28H29F2N5O7/c1-34(2)28(39)16-4-3-5-18(12-16)41-26-22(29)24(40-17-8-10-35(11-9-17)14-21(37)38)23(30)27(33-26)42-20-13-15(25(31)32)6-7-19(20)36/h3-7,12-13,17,36H,8-11,14H2,1-2H3,(H3,31,32)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277
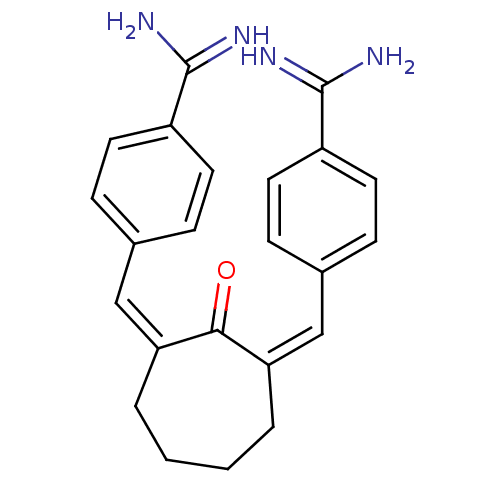
((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound (isomer) was tested in the absence of light for inhibitory activity against Human Coagulation factor X |
J Med Chem 41: 3551-6 (1998)
Article DOI: 10.1021/jm980281+
BindingDB Entry DOI: 10.7270/Q2QJ7GFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066628
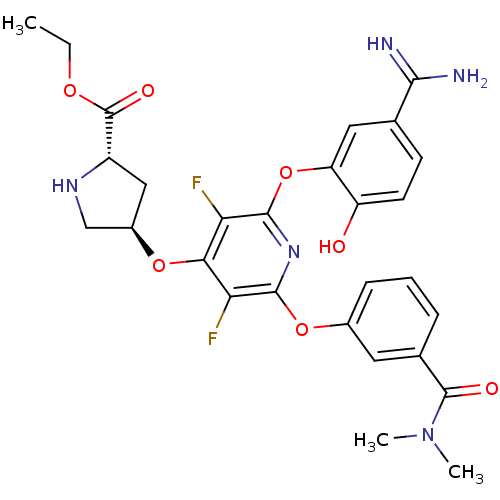
((2S,4R)-4-[2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6...)Show SMILES CCOC(=O)[C@@H]1C[C@H](CN1)Oc1c(F)c(Oc2cccc(c2)C(=O)N(C)C)nc(Oc2cc(ccc2O)C(N)=N)c1F Show InChI InChI=1S/C28H29F2N5O7/c1-4-39-28(38)18-12-17(13-33-18)40-23-21(29)25(41-16-7-5-6-15(10-16)27(37)35(2)3)34-26(22(23)30)42-20-11-14(24(31)32)8-9-19(20)36/h5-11,17-18,33,36H,4,12-13H2,1-3H3,(H3,31,32)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066638
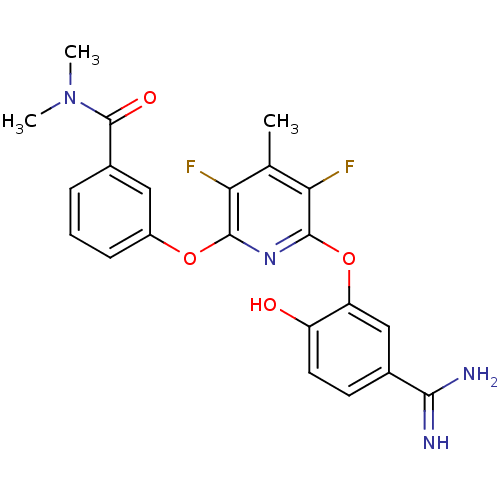
(3-[6-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-diflu...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(C)c2F)c1 Show InChI InChI=1S/C22H20F2N4O4/c1-11-17(23)20(31-14-6-4-5-13(9-14)22(30)28(2)3)27-21(18(11)24)32-16-10-12(19(25)26)7-8-15(16)29/h4-10,29H,1-3H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066637
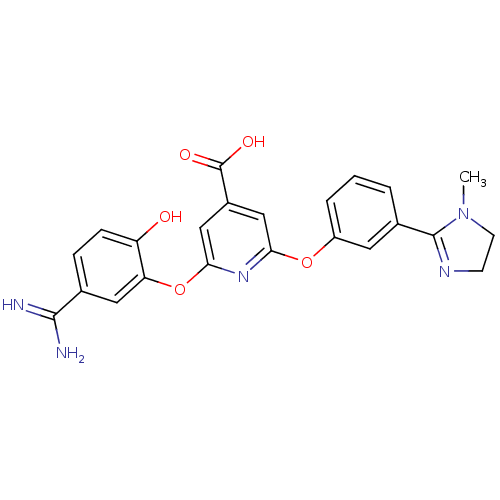
(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-[3-(1-meth...)Show SMILES CN1CCN=C1c1cccc(Oc2cc(cc(Oc3cc(ccc3O)C(N)=N)n2)C(O)=O)c1 |c:4| Show InChI InChI=1S/C23H21N5O5/c1-28-8-7-26-22(28)14-3-2-4-16(9-14)32-19-11-15(23(30)31)12-20(27-19)33-18-10-13(21(24)25)5-6-17(18)29/h2-6,9-12,29H,7-8H2,1H3,(H3,24,25)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084175
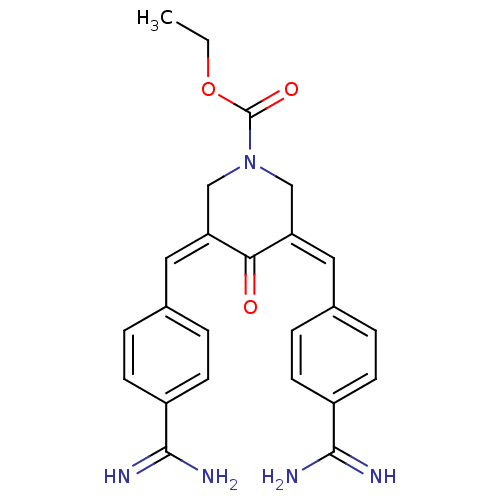
(3,5-Bis-[1-(4-carbamimidoyl-phenyl)-meth-(Z)-ylide...)Show SMILES CCOC(=O)N1C\C(=C\c2ccc(cc2)C(N)=N)C(=O)\C(C1)=C/c1ccc(cc1)C(N)=N Show InChI InChI=1S/C24H25N5O3/c1-2-32-24(31)29-13-19(11-15-3-7-17(8-4-15)22(25)26)21(30)20(14-29)12-16-5-9-18(10-6-16)23(27)28/h3-12H,2,13-14H2,1H3,(H3,25,26)(H3,27,28)/b19-11-,20-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084186

(2,9-di[1-[4-amino(imino)methylphenyl]-(Z)-methylid...)Show SMILES NC(=N)c1ccc(\C=C2\CCCCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C25H28N4O/c26-24(27)19-11-7-17(8-12-19)15-21-5-3-1-2-4-6-22(23(21)30)16-18-9-13-20(14-10-18)25(28)29/h7-16H,1-6H2,(H3,26,27)(H3,28,29)/b21-15-,22-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066622

(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-(3-dimethy...)Show SMILES CN(C)C(=O)c1cccc(Oc2cc(cc(Oc3cc(ccc3O)C(N)=N)n2)C(O)=O)c1 Show InChI InChI=1S/C22H20N4O6/c1-26(2)21(28)13-4-3-5-15(8-13)31-18-10-14(22(29)30)11-19(25-18)32-17-9-12(20(23)24)6-7-16(17)27/h3-11,27H,1-2H3,(H3,23,24)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084174
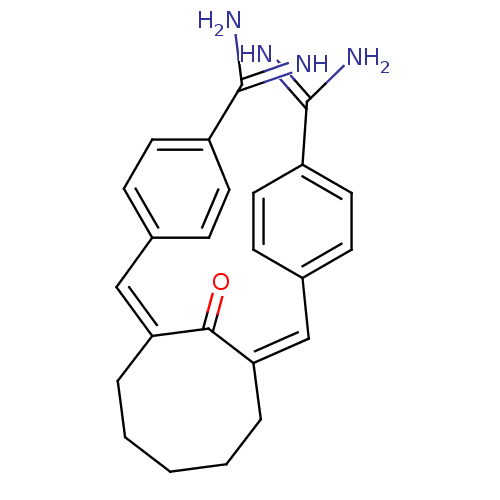
(2,8-di[1-[4-amino(imino)methylphenyl]-(Z)-methylid...)Show SMILES NC(=N)c1ccc(\C=C2\CCCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C24H26N4O/c25-23(26)18-10-6-16(7-11-18)14-20-4-2-1-3-5-21(22(20)29)15-17-8-12-19(13-9-17)24(27)28/h6-15H,1-5H2,(H3,25,26)(H3,27,28)/b20-14-,21-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084192

(3,5-Bis-[1-(4-carbamimidoyl-phenyl)-meth-(Z)-ylide...)Show SMILES COC(=O)C1C\C(=C\c2ccc(cc2)C(N)=N)C(=O)\C(C1)=C/c1ccc(cc1)C(N)=N Show InChI InChI=1S/C24H24N4O3/c1-31-24(30)20-12-18(10-14-2-6-16(7-3-14)22(25)26)21(29)19(13-20)11-15-4-8-17(9-5-15)23(27)28/h2-11,20H,12-13H2,1H3,(H3,25,26)(H3,27,28)/b18-10-,19-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084163
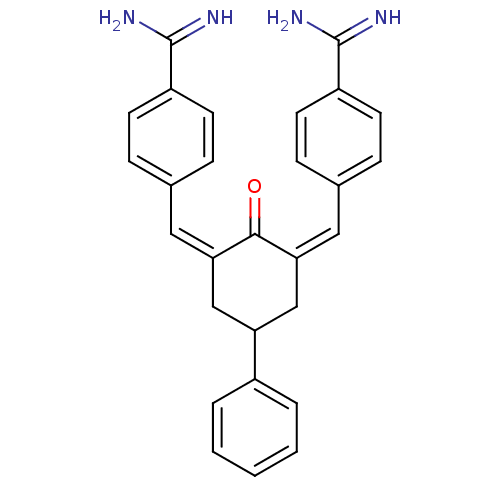
(2,6-di[1-[4-amino(imino)methylphenyl]-(Z)-methylid...)Show SMILES NC(=N)c1ccc(\C=C2\CC(C\C(=C\c3ccc(cc3)C(N)=N)C2=O)c2ccccc2)cc1 Show InChI InChI=1S/C28H26N4O/c29-27(30)21-10-6-18(7-11-21)14-24-16-23(20-4-2-1-3-5-20)17-25(26(24)33)15-19-8-12-22(13-9-19)28(31)32/h1-15,23H,16-17H2,(H3,29,30)(H3,31,32)/b24-14-,25-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066616
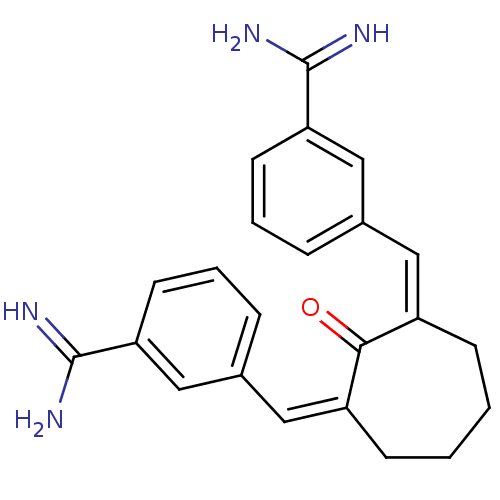
((Z,Z)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1cccc(\C=C2\CCCC\C(=C\c3cccc(c3)C(N)=N)C2=O)c1 Show InChI InChI=1S/C23H24N4O/c24-22(25)19-9-3-5-15(13-19)11-17-7-1-2-8-18(21(17)28)12-16-6-4-10-20(14-16)23(26)27/h3-6,9-14H,1-2,7-8H2,(H3,24,25)(H3,26,27)/b17-11-,18-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066616

((Z,Z)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1cccc(\C=C2\CCCC\C(=C\c3cccc(c3)C(N)=N)C2=O)c1 Show InChI InChI=1S/C23H24N4O/c24-22(25)19-9-3-5-15(13-19)11-17-7-1-2-8-18(21(17)28)12-16-6-4-10-20(14-16)23(26)27/h3-6,9-14H,1-2,7-8H2,(H3,24,25)(H3,26,27)/b17-11-,18-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound (isomer) was tested in the absence of light for inhibitory activity against Human Coagulation factor X |
J Med Chem 41: 3551-6 (1998)
Article DOI: 10.1021/jm980281+
BindingDB Entry DOI: 10.7270/Q2QJ7GFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084170
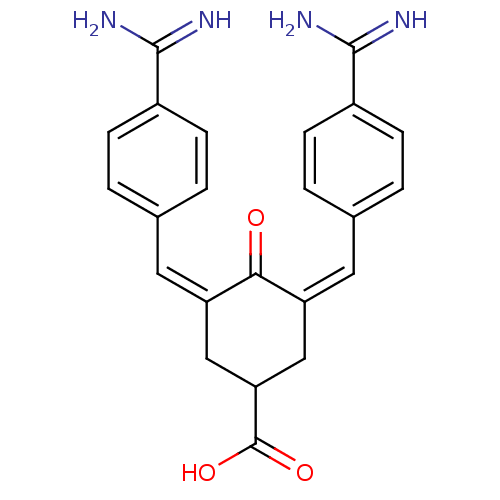
(3,5-Bis-[1-(4-carbamimidoyl-phenyl)-meth-(Z)-ylide...)Show SMILES NC(=N)c1ccc(\C=C2\CC(C\C(=C\c3ccc(cc3)C(N)=N)C2=O)C(O)=O)cc1 Show InChI InChI=1S/C23H22N4O3/c24-21(25)15-5-1-13(2-6-15)9-17-11-19(23(29)30)12-18(20(17)28)10-14-3-7-16(8-4-14)22(26)27/h1-10,19H,11-12H2,(H3,24,25)(H3,26,27)(H,29,30)/b17-9-,18-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066636
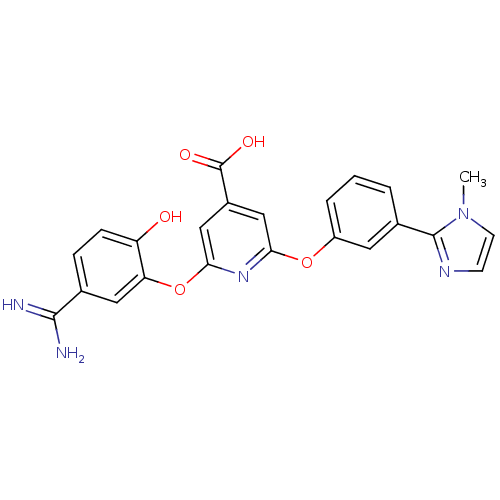
(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-[3-(1-meth...)Show SMILES Cn1ccnc1-c1cccc(Oc2cc(cc(Oc3cc(ccc3O)C(N)=N)n2)C(O)=O)c1 Show InChI InChI=1S/C23H19N5O5/c1-28-8-7-26-22(28)14-3-2-4-16(9-14)32-19-11-15(23(30)31)12-20(27-19)33-18-10-13(21(24)25)5-6-17(18)29/h2-12,29H,1H3,(H3,24,25)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081365
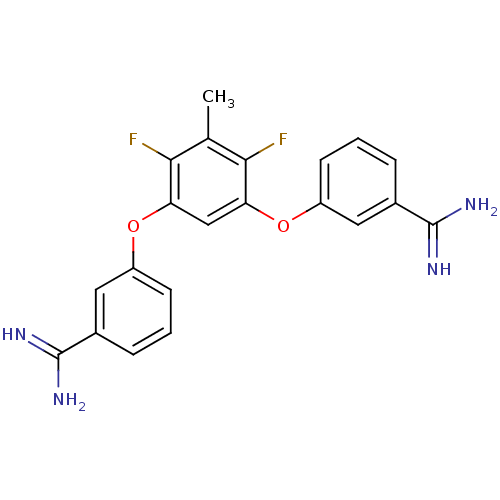
(3-(5-{3-[(Z)-amino(imino)methyl]phenoxy}-2,4-diflu...)Show SMILES Cc1c(F)c(Oc2cccc(c2)C(N)=N)cc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C21H18F2N4O2/c1-11-18(22)16(28-14-6-2-4-12(8-14)20(24)25)10-17(19(11)23)29-15-7-3-5-13(9-15)21(26)27/h2-10H,1H3,(H3,24,25)(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against Coagulation factor Xa (factor Xa) |
J Med Chem 42: 3910-8 (1999)
BindingDB Entry DOI: 10.7270/Q2X63M5W |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084164

(2-[1-[4-amino(imino)methylphenyl]-(Z)-methylidene]...)Show SMILES CNC(=N)c1ccc(C=C2CCCCC(=Cc3ccc(cc3)C(N)=N)C2=O)cc1 |w:8.7,15.15| Show InChI InChI=1S/C24H26N4O/c1-28-24(27)19-12-8-17(9-13-19)15-21-5-3-2-4-20(22(21)29)14-16-6-10-18(11-7-16)23(25)26/h6-15H,2-5H2,1H3,(H3,25,26)(H2,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17278
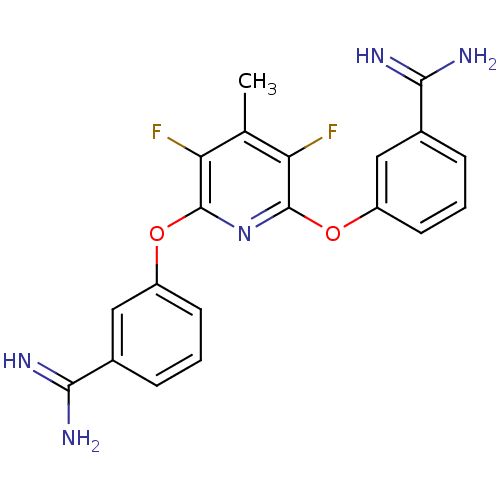
(3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...)Show SMILES Cc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C20H17F2N5O2/c1-10-15(21)19(28-13-6-2-4-11(8-13)17(23)24)27-20(16(10)22)29-14-7-3-5-12(9-14)18(25)26/h2-9H,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17278

(3-{[6-(3-carbamimidoylphenoxy)-3,5-difluoro-4-meth...)Show SMILES Cc1c(F)c(Oc2cccc(c2)C(N)=N)nc(Oc2cccc(c2)C(N)=N)c1F Show InChI InChI=1S/C20H17F2N5O2/c1-10-15(21)19(28-13-6-2-4-11(8-13)17(23)24)27-20(16(10)22)29-14-7-3-5-12(9-14)18(25)26/h2-9H,1H3,(H3,23,24)(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066627
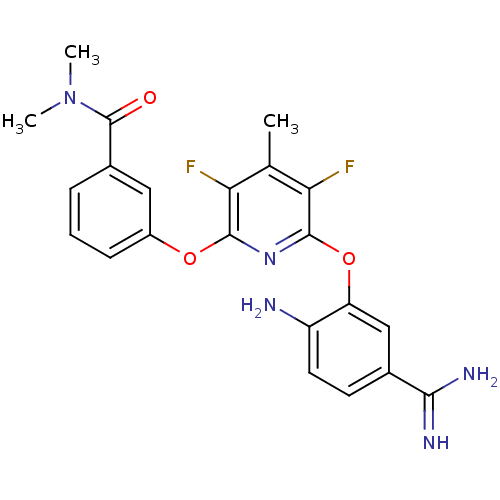
(3-[6-(2-Amino-5-carbamimidoyl-phenoxy)-3,5-difluor...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3N)C(N)=N)c(F)c(C)c2F)c1 Show InChI InChI=1S/C22H21F2N5O3/c1-11-17(23)20(31-14-6-4-5-13(9-14)22(30)29(2)3)28-21(18(11)24)32-16-10-12(19(26)27)7-8-15(16)25/h4-10H,25H2,1-3H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50084160

(2-[1-[4-amino(imino)methylphenyl]-(E)-methylidene]...)Show SMILES CN(C)C(=N)c1ccc(\C=C2\CCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C25H28N4O/c1-29(2)25(28)20-13-9-18(10-14-20)16-22-6-4-3-5-21(23(22)30)15-17-7-11-19(12-8-17)24(26)27/h7-16,28H,3-6H2,1-2H3,(H3,26,27)/b21-15+,22-16-,28-25? | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084176

(2,6-di[1-[4-amino(imino)methylphenyl]-(Z)-methylid...)Show SMILES NC(=N)c1ccc(\C=C2\CCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C22H22N4O/c23-21(24)16-8-4-14(5-9-16)12-18-2-1-3-19(20(18)27)13-15-6-10-17(11-7-15)22(25)26/h4-13H,1-3H2,(H3,23,24)(H3,25,26)/b18-12-,19-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17282

(7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2nc(C)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C27H30N6O/c1-17(28)32-11-9-23(10-12-32)34-24-7-8-25-26(15-24)33(18(2)31-25)16-19-3-4-20-5-6-21(27(29)30)14-22(20)13-19/h3-8,13-15,23,28H,9-12,16H2,1-2H3,(H3,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 18 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084177

(2-[1-[4-amino(imino)methylphenyl]-(Z)-methylidene]...)Show SMILES CN(C)C(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C25H28N4O/c1-29(2)25(28)20-13-9-18(10-14-20)16-22-6-4-3-5-21(23(22)30)15-17-7-11-19(12-8-17)24(26)27/h7-16,28H,3-6H2,1-2H3,(H3,26,27)/b21-15-,22-16-,28-25? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17283

((2S)-3-(7-carbamimidoylnaphthalen-2-yl)-2-(4-{[(3S...)Show SMILES CC(=N)N1CC[C@@H](C1)Oc1ccc(cc1)[C@H](Cc1ccc2ccc(cc2c1)C(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H28N4O3/c1-16(27)30-11-10-23(15-30)33-22-8-6-19(7-9-22)24(26(31)32)13-17-2-3-18-4-5-20(25(28)29)14-21(18)12-17/h2-9,12,14,23-24,27H,10-11,13,15H2,1H3,(H3,28,29)(H,31,32)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM50084175

(3,5-Bis-[1-(4-carbamimidoyl-phenyl)-meth-(Z)-ylide...)Show SMILES CCOC(=O)N1C\C(=C\c2ccc(cc2)C(N)=N)C(=O)\C(C1)=C/c1ccc(cc1)C(N)=N Show InChI InChI=1S/C24H25N5O3/c1-2-32-24(31)29-13-19(11-15-3-7-17(8-4-15)22(25)26)21(30)20(14-29)12-16-5-9-18(10-6-16)23(27)28/h3-12H,2,13-14H2,1H3,(H3,25,26)(H3,27,28)/b19-11-,20-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066640
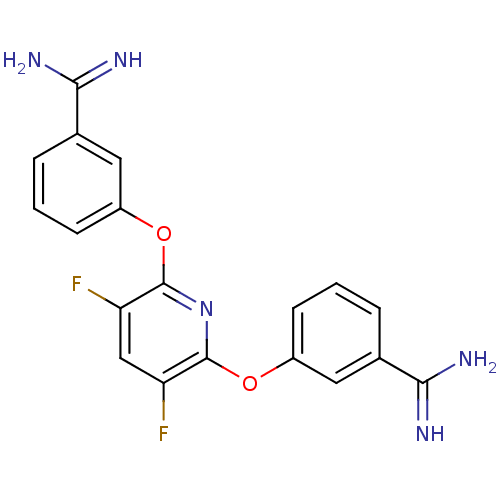
(3-{6-[3-amino(imino)methylphenoxy]-3,5-difluoro-2-...)Show SMILES NC(=N)c1cccc(Oc2nc(Oc3cccc(c3)C(N)=N)c(F)cc2F)c1 Show InChI InChI=1S/C19H15F2N5O2/c20-14-9-15(21)19(28-13-6-2-4-11(8-13)17(24)25)26-18(14)27-12-5-1-3-10(7-12)16(22)23/h1-9H,(H3,22,23)(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066617
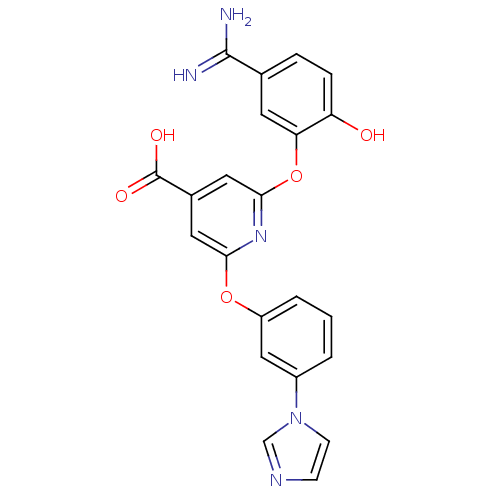
(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-(3-imidazo...)Show SMILES NC(=N)c1ccc(O)c(Oc2cc(cc(Oc3cccc(c3)-n3ccnc3)n2)C(O)=O)c1 Show InChI InChI=1S/C22H17N5O5/c23-21(24)13-4-5-17(28)18(8-13)32-20-10-14(22(29)30)9-19(26-20)31-16-3-1-2-15(11-16)27-7-6-25-12-27/h1-12,28H,(H3,23,24)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50066615
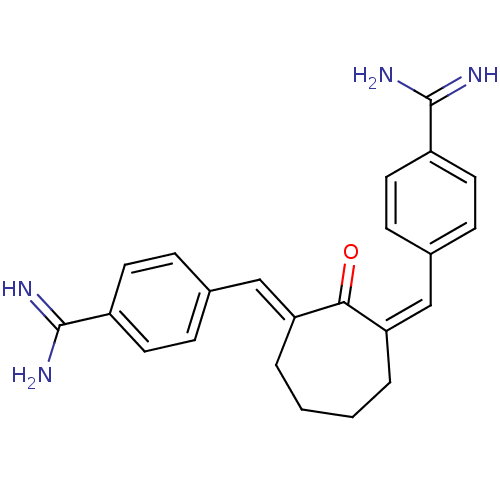
((E,Z)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound (isomer) was tested in the absence of light for inhibitory activity against trypsin. |
J Med Chem 41: 3551-6 (1998)
Article DOI: 10.1021/jm980281+
BindingDB Entry DOI: 10.7270/Q2QJ7GFX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50066615

((E,Z)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50084191
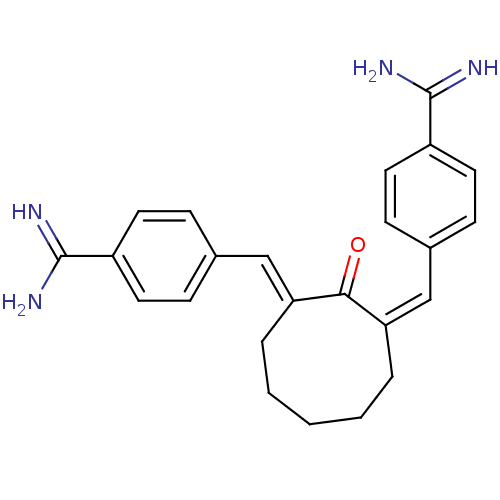
(2-[1-[4-amino(imino)methylphenyl]-(E)-methylidene]...)Show SMILES NC(=N)c1ccc(\C=C2\CCCCC\C(=C/c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C24H26N4O/c25-23(26)18-10-6-16(7-11-18)14-20-4-2-1-3-5-21(22(20)29)15-17-8-12-19(13-9-17)24(27)28/h6-15H,1-5H2,(H3,25,26)(H3,27,28)/b20-14-,21-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50084164

(2-[1-[4-amino(imino)methylphenyl]-(Z)-methylidene]...)Show SMILES CNC(=N)c1ccc(C=C2CCCCC(=Cc3ccc(cc3)C(N)=N)C2=O)cc1 |w:8.7,15.15| Show InChI InChI=1S/C24H26N4O/c1-28-24(27)19-12-8-17(9-13-19)15-21-5-3-2-4-20(22(21)29)14-16-6-10-18(11-7-16)23(25)26/h6-15H,2-5H2,1H3,(H3,25,26)(H2,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of bovine trypsin(Trp). |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine protease 1
(Bos taurus (bovine)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound (isomer) was tested in the absence of light for inhibitory activity against trypsin. |
J Med Chem 41: 3551-6 (1998)
Article DOI: 10.1021/jm980281+
BindingDB Entry DOI: 10.7270/Q2QJ7GFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data