

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8117 (Hydroxyethylene Sulfone 17b | N-[(2S,3R,5S)-1-(3-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 45 | -41.9 | 85 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8123 (Hydroxyethylene Sulfone 18a | N-((1S,2R,4S)-1-(3-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 370 | -36.7 | 530 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8124 (Hydroxyethylene Sulfone 18b | N-((1S,2R,4S)-1-(3-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | -35.8 | 790 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8118 (Hydroxyethylene Sulfone 17b- | N-[(2R,3S,5R)-1-(3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | -33.4 | 2.00E+3 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8121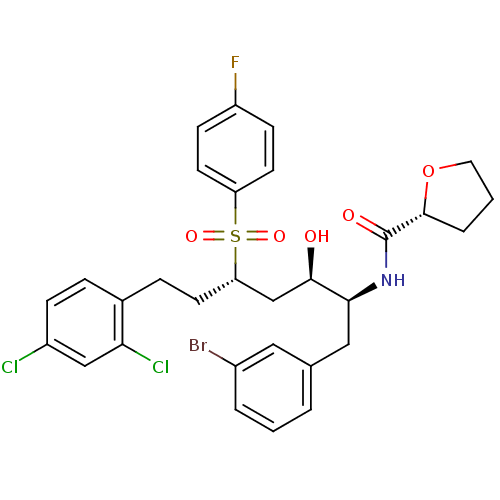 ((2R)-N-[(2S,3R,5S)-1-(3-bromophenyl)-7-(2,4-dichlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | -31.6 | 4.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8120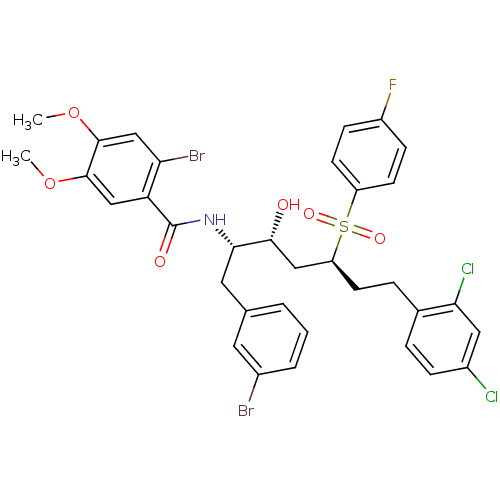 (2-bromo-N-[(2S,3R,5S)-1-(3-bromophenyl)-7-(2,4-dic...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+3 | -31.4 | 4.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8119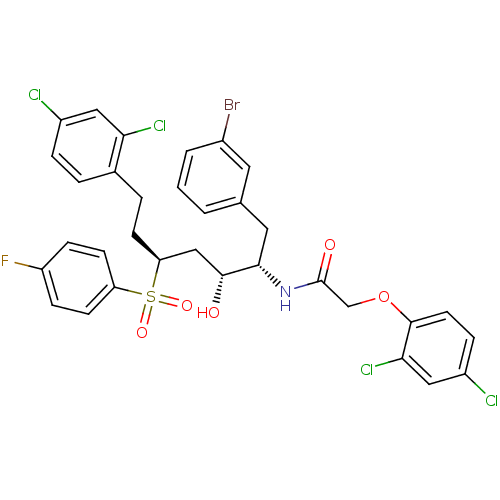 (Hydroxyethylene Sulfone 17c | N-[(2S,3R,5S)-1-(3-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | -29.4 | 1.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8122 ((2R)-N-[(2R,3S,5R)-1-(3-bromophenyl)-7-(2,4-dichlo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | >-25.1 | >4.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8116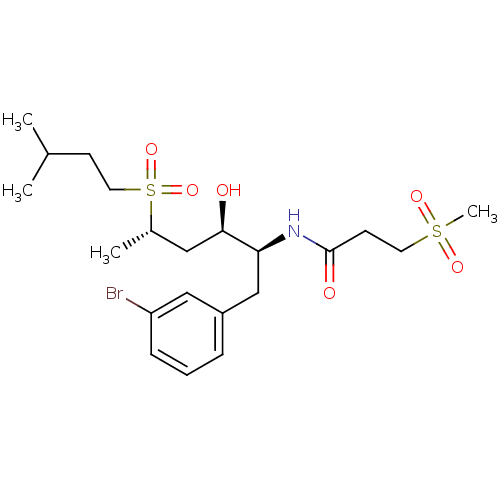 (Hydroxyethylene Sulfone 17a | N-[(2S,3R,5S)-1-(3-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >4.00E+4 | >-25.1 | >4.00E+4 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Philipps-Universitat Marburg | Assay Description The inhibition assays were performed by mixing enzyme and fluorogenic substrate in the presence of inhibitor compounds. The hydrolysis of the substra... | J Med Chem 48: 6607-19 (2005) Article DOI: 10.1021/jm050224y BindingDB Entry DOI: 10.7270/Q2416V8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM97451 (Aspartyl protease inhibitor, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM97450 (Aspartyl protease inhibitor, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM97447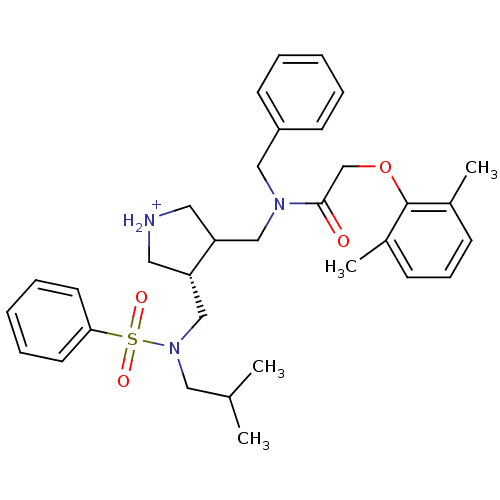 (Aspartyl protease inhibitor, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM97448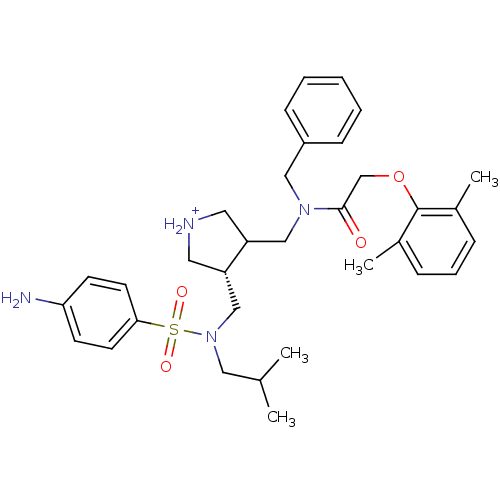 (Aspartyl protease inhibitor, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM97449 (Aspartyl protease inhibitor, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM97450 (Aspartyl protease inhibitor, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM97451 (Aspartyl protease inhibitor, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM97447 (Aspartyl protease inhibitor, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM97448 (Aspartyl protease inhibitor, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM97449 (Aspartyl protease inhibitor, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Philipps-Universitšt Marburg | Assay Description Binding constants for inhibition of HIV protease were determined as previously described. | ChemMedChem 1: 106-17 (2006) Article DOI: 10.1002/cmdc.200500008 BindingDB Entry DOI: 10.7270/Q228067M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||