 Found 2121 hits with Last Name = 'lim' and Initial = 'h'
Found 2121 hits with Last Name = 'lim' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50317007
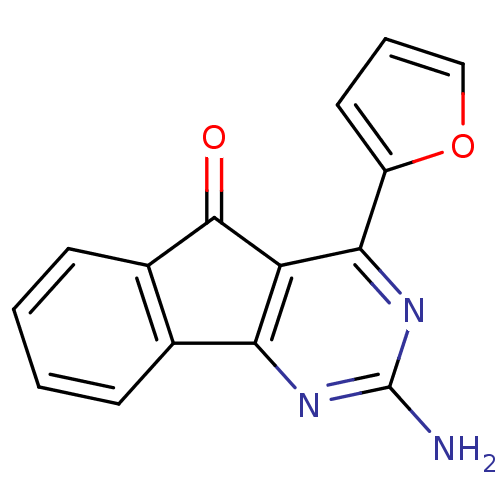
(2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...)Show InChI InChI=1S/C15H9N3O2/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant adenosine receptor A2a by cAMP assay |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394718
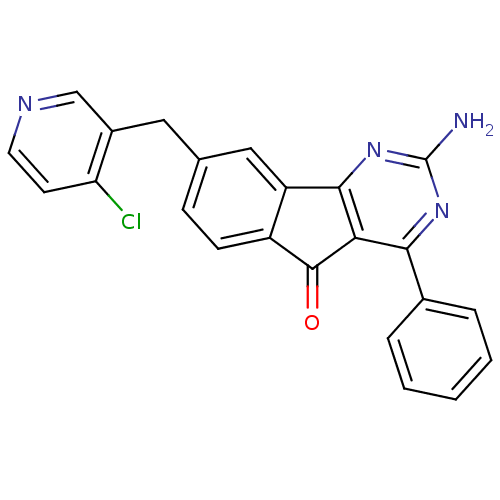
(CHEMBL2165801)Show SMILES Nc1nc2-c3cc(Cc4cnccc4Cl)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-8-9-26-12-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50504750
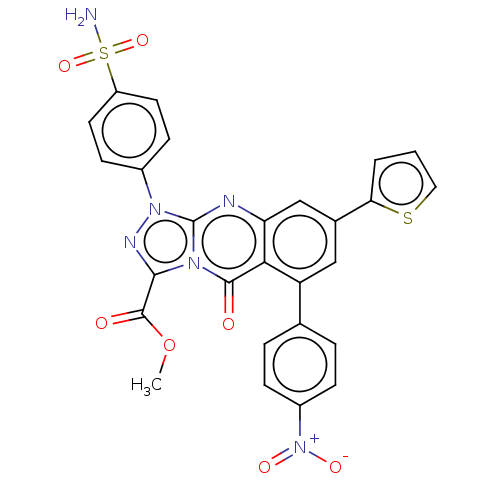
(CHEMBL4536202)Show SMILES COC(=O)c1nn(-c2ccc(cc2)S(N)(=O)=O)c2nc3cc(cc(-c4ccc(cc4)[N+]([O-])=O)c3c(=O)n12)-c1cccs1 Show InChI InChI=1S/C27H18N6O7S2/c1-40-26(35)24-30-32(17-8-10-19(11-9-17)42(28,38)39)27-29-21-14-16(22-3-2-12-41-22)13-20(23(21)25(34)31(24)27)15-4-6-18(7-5-15)33(36)37/h2-14H,1H3,(H2,28,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111843
BindingDB Entry DOI: 10.7270/Q2WW7N05 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394722
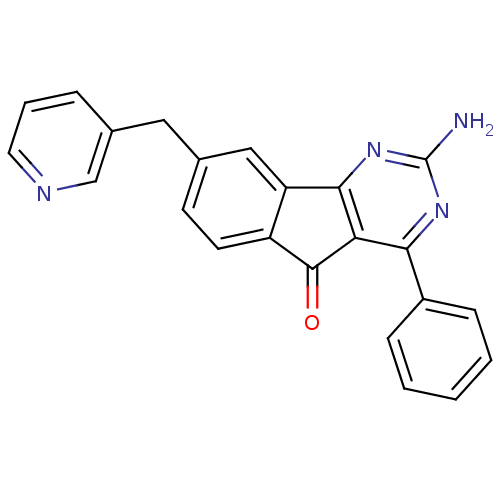
(CHEMBL2165807)Show SMILES Nc1nc2-c3cc(Cc4cccnc4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H16N4O/c24-23-26-20(16-6-2-1-3-7-16)19-21(27-23)18-12-14(8-9-17(18)22(19)28)11-15-5-4-10-25-13-15/h1-10,12-13H,11H2,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM21221

((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 Show InChI InChI=1S/C18H18ClIN6O4/c1-21-16(29)13-11(27)12(28)17(30-13)26-7-23-10-14(24-18(19)25-15(10)26)22-6-8-3-2-4-9(20)5-8/h2-5,7,11-13,17,27-28H,6H2,1H3,(H,21,29)(H,22,24,25)/t11-,12+,13-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Binding affinity for rat Adenosine A3 receptor |
Bioorg Med Chem Lett 13: 817-20 (2003)
BindingDB Entry DOI: 10.7270/Q2M32WB7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50237064
(CHEMBL401895 | N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES CN(C)Cc1cccc(OCC(=O)Nc2cc(nc(n2)-c2ccc(C)o2)-n2nc(C)cc2C)c1 Show InChI InChI=1S/C25H28N6O3/c1-16-11-17(2)31(29-16)23-13-22(27-25(28-23)21-10-9-18(3)34-21)26-24(32)15-33-20-8-6-7-19(12-20)14-30(4)5/h6-13H,14-15H2,1-5H3,(H,26,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM26256
(N-[2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-[6-(4-methox...)Show SMILES COC1CCN(CC1)c1cccc(n1)-c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C Show InChI InChI=1S/C22H27N7O2/c1-14-12-15(2)29(27-14)22-25-19(13-20(26-22)23-16(3)30)18-6-5-7-21(24-18)28-10-8-17(31-4)9-11-28/h5-7,12-13,17H,8-11H2,1-4H3,(H,23,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50317007

(2-amino-4-(furan-2-yl)-5H-indeno[1,2-d]pyrimidin-5...)Show InChI InChI=1S/C15H9N3O2/c16-15-17-12-8-4-1-2-5-9(8)14(19)11(12)13(18-15)10-6-3-7-20-10/h1-7H,(H2,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant adenosine A1 receptor by cAMP assay |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50364063
(CHEMBL1950649)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(nc12)C#CC[C@H]1CC[C@@H](CC1)C(=O)OC |r,wU:7.12,5.4,28.34,wD:8.8,10.11,25.27,(-2.37,-48.53,;-1.04,-49.31,;.3,-48.55,;1.62,-49.33,;1.61,-50.87,;2.93,-48.59,;2.94,-47.1,;4.43,-46.64,;5.27,-47.83,;6.72,-47.84,;4.37,-49.06,;4.85,-50.53,;4.89,-45.21,;3.96,-43.88,;4.91,-42.6,;6.23,-43.05,;7.53,-42.29,;7.52,-40.78,;8.97,-43.12,;8.97,-44.75,;7.59,-45.54,;6.24,-44.75,;10.31,-45.52,;11.64,-46.29,;12.97,-47.06,;14.3,-46.29,;15.63,-47.07,;16.96,-46.31,;16.97,-44.77,;15.64,-43.99,;14.3,-44.76,;18.31,-44,;18.32,-42.46,;19.64,-44.78,;20.98,-44.02,)| Show InChI InChI=1S/C23H30N6O6/c1-3-25-21(32)18-16(30)17(31)22(35-18)29-11-26-15-19(24)27-14(28-20(15)29)6-4-5-12-7-9-13(10-8-12)23(33)34-2/h11-13,16-18,22,30-31H,3,5,7-10H2,1-2H3,(H,25,32)(H2,24,27,28)/t12-,13-,16-,17+,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50394718

(CHEMBL2165801)Show SMILES Nc1nc2-c3cc(Cc4cnccc4Cl)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-8-9-26-12-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394717
(CHEMBL2165802)Show SMILES Nc1nc2-c3cc(ccc3C(=O)c2c(n1)-c1ccccc1)C(=O)c1cccnc1 Show InChI InChI=1S/C23H14N4O2/c24-23-26-19(13-5-2-1-3-6-13)18-20(27-23)17-11-14(8-9-16(17)22(18)29)21(28)15-7-4-10-25-12-15/h1-12H,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50232154
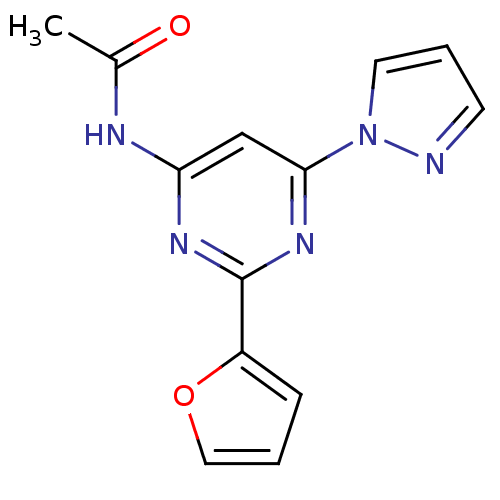
(CHEMBL401321 | N-(2-(furan-2-yl)-6-(1H-pyrazol-1-y...)Show InChI InChI=1S/C13H11N5O2/c1-9(19)15-11-8-12(18-6-3-5-14-18)17-13(16-11)10-4-2-7-20-10/h2-8H,1H3,(H,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202986
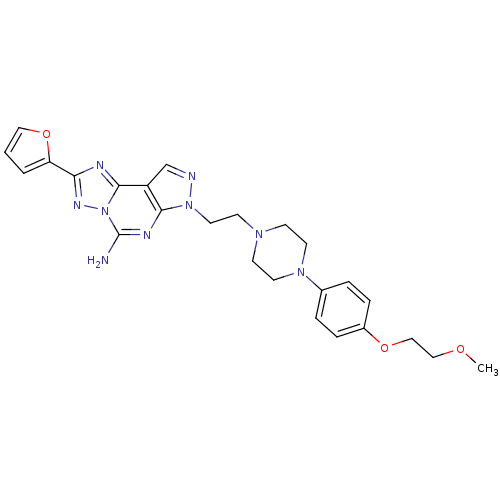
(2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(17-27-33)24-28-22(21-3-2-14-37-21)30-34(24)25(26)29-23/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202986

(2-furan-2-yl-7-(2-{4-[4-(2-methoxy-ethoxy)-phenyl]...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(17-27-33)24-28-22(21-3-2-14-37-21)30-34(24)25(26)29-23/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22916
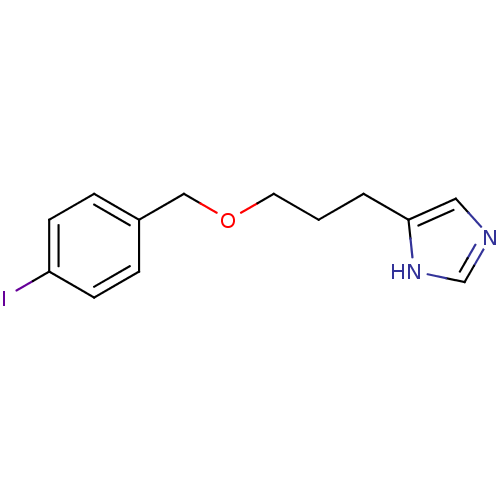
(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22916

(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50504776
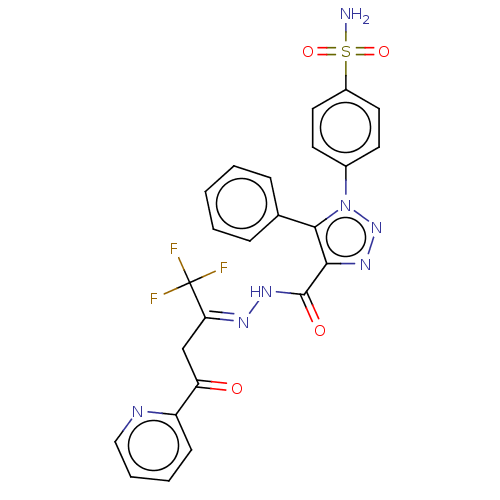
(CHEMBL4441461)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nnc(C(=O)N\N=C(\CC(=O)c2ccccn2)C(F)(F)F)c1-c1ccccc1 Show InChI InChI=1S/C24H18F3N7O4S/c25-24(26,27)20(14-19(35)18-8-4-5-13-29-18)30-32-23(36)21-22(15-6-2-1-3-7-15)34(33-31-21)16-9-11-17(12-10-16)39(28,37)38/h1-13H,14H2,(H,32,36)(H2,28,37,38)/b30-20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Egyptian Russian University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase-9 incubated for 15 mins by stopped flow CO2 hydrase assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111843
BindingDB Entry DOI: 10.7270/Q2WW7N05 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394719
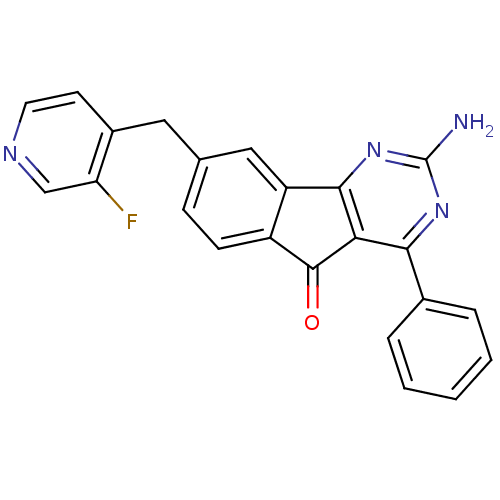
(CHEMBL2165800)Show SMILES Nc1nc2-c3cc(Cc4ccncc4F)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-18-12-26-9-8-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22910
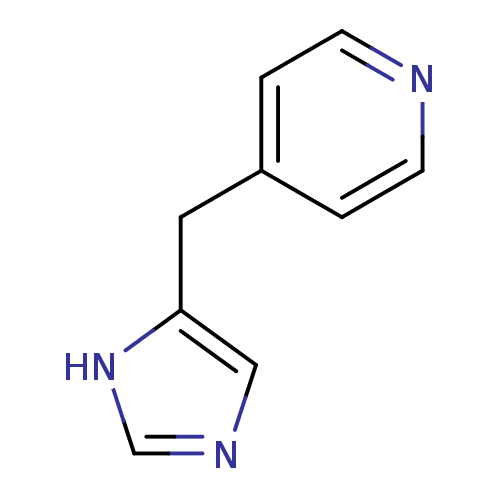
(4-(1H-imidazol-5-ylmethyl)pyridine | Immethridine)Show InChI InChI=1S/C9H9N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h1-4,6-7H,5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202773
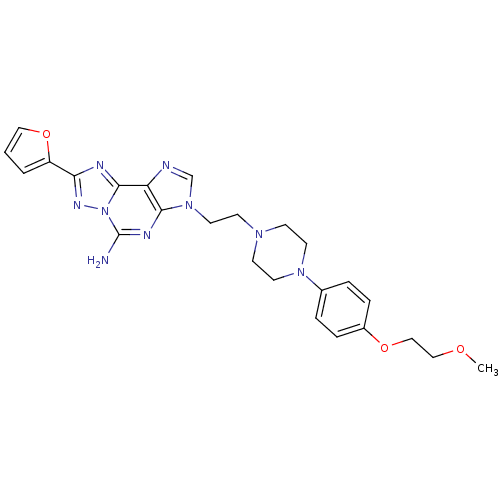
(8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2cnc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-17-27-21-23(33)29-25(26)34-24(21)28-22(30-34)20-3-2-14-37-20/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493039
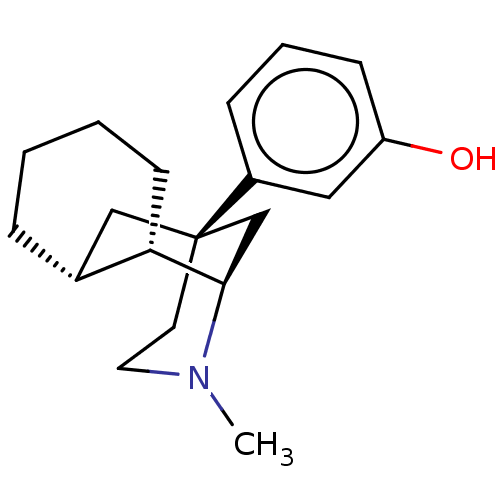
(CHEMBL2418733)Show SMILES [H][C@]12CCCC[C@]1([H])[C@@]1([H])C[C@](CCN1C)(C2)c1cccc(O)c1 |r| Show InChI InChI=1S/C19H27NO/c1-20-10-9-19(15-6-4-7-16(21)11-15)12-14-5-2-3-8-17(14)18(20)13-19/h4,6-7,11,14,17-18,21H,2-3,5,8-10,12-13H2,1H3/t14-,17+,18-,19+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Eur J Med Chem 67: 335-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.030
BindingDB Entry DOI: 10.7270/Q2251N40 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50294132
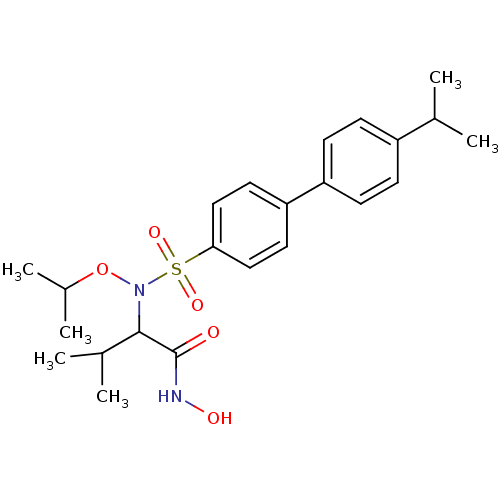
(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22909
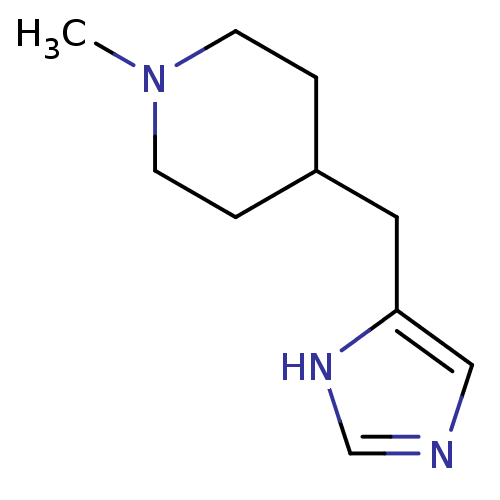
(4-(1H-imidazol-5-ylmethyl)-1-methylpiperidine | Me...)Show InChI InChI=1S/C10H17N3/c1-13-4-2-9(3-5-13)6-10-7-11-8-12-10/h7-9H,2-6H2,1H3,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50419448
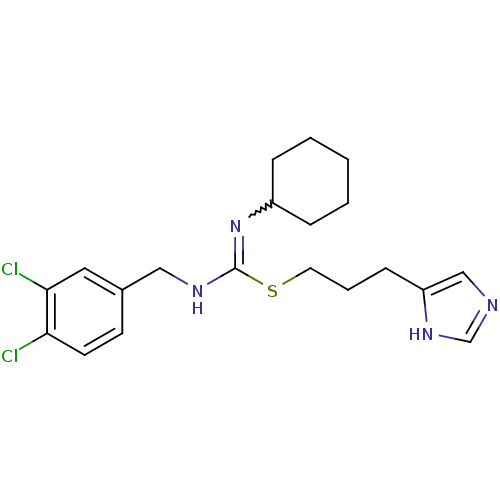
(CHEMBL1923026)Show SMILES Clc1ccc(CNC(SCCCc2cnc[nH]2)=NC2CCCCC2)cc1Cl |w:17.18| Show InChI InChI=1S/C20H26Cl2N4S/c21-18-9-8-15(11-19(18)22)12-24-20(26-16-5-2-1-3-6-16)27-10-4-7-17-13-23-14-25-17/h8-9,11,13-14,16H,1-7,10,12H2,(H,23,25)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50419444
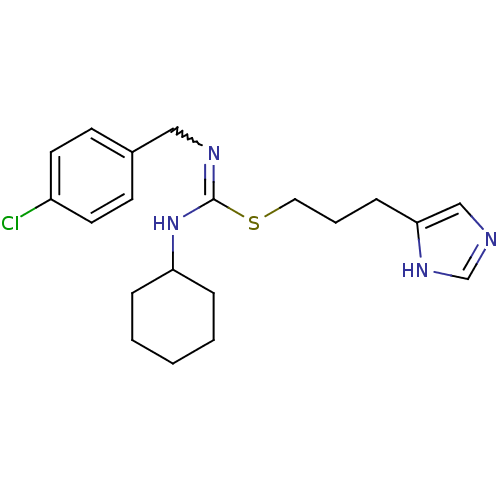
(CHEMBL43934 | VUF-5228)Show SMILES Clc1ccc(CN=C(NC2CCCCC2)SCCCc2cnc[nH]2)cc1 |w:6.5| Show InChI InChI=1S/C20H27ClN4S/c21-17-10-8-16(9-11-17)13-23-20(25-18-5-2-1-3-6-18)26-12-4-7-19-14-22-15-24-19/h8-11,14-15,18H,1-7,12-13H2,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
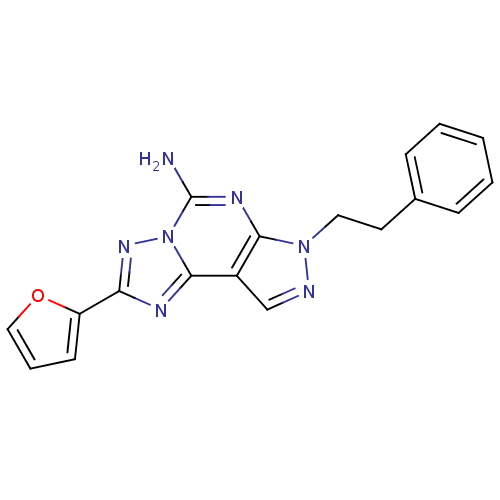
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50239036
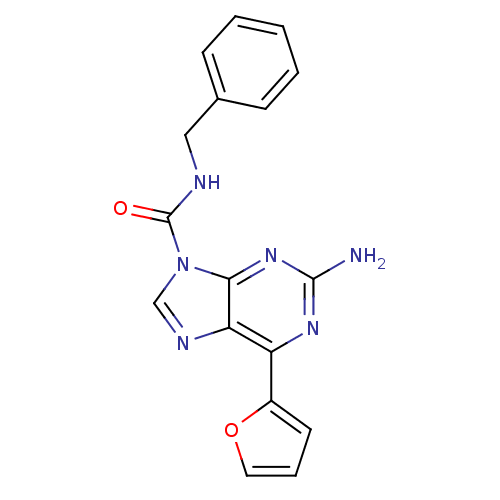
(2-amino-N-benzyl-6-(furan-2-yl)-9H-purine-9-carbox...)Show InChI InChI=1S/C17H14N6O2/c18-16-21-13(12-7-4-8-25-12)14-15(22-16)23(10-20-14)17(24)19-9-11-5-2-1-3-6-11/h1-8,10H,9H2,(H,19,24)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50419456
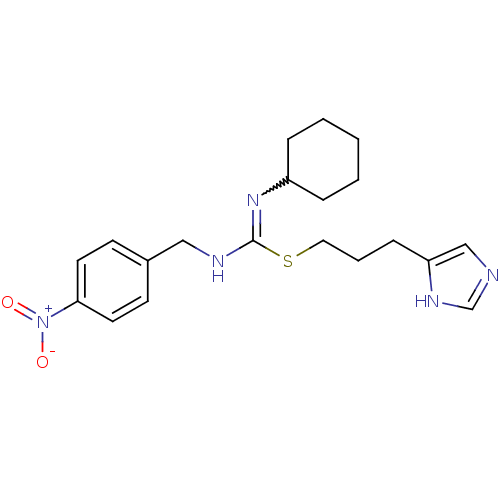
(CHEMBL1923034)Show SMILES [O-][N+](=O)c1ccc(CNC(SCCCc2cnc[nH]2)=NC2CCCCC2)cc1 |w:19.20| Show InChI InChI=1S/C20H27N5O2S/c26-25(27)19-10-8-16(9-11-19)13-22-20(24-17-5-2-1-3-6-17)28-12-4-7-18-14-21-15-23-18/h8-11,14-15,17H,1-7,12-13H2,(H,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Eur J Med Chem 67: 335-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.030
BindingDB Entry DOI: 10.7270/Q2251N40 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50254013
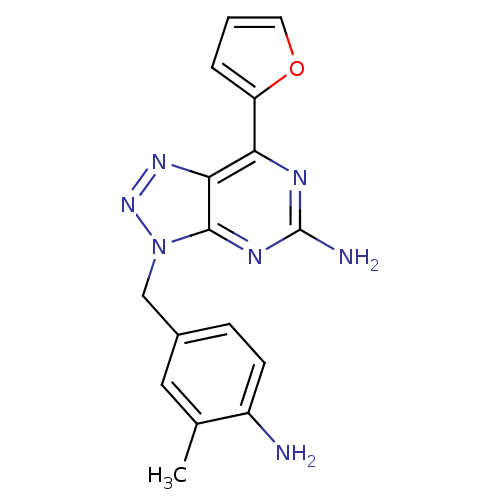
(3-(4-Amino-3-methylbenzyl)-7-(2-furyl)-3H-[1,2,3]t...)Show InChI InChI=1S/C16H15N7O/c1-9-7-10(4-5-11(9)17)8-23-15-14(21-22-23)13(19-16(18)20-15)12-3-2-6-24-12/h2-7H,8,17H2,1H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394720
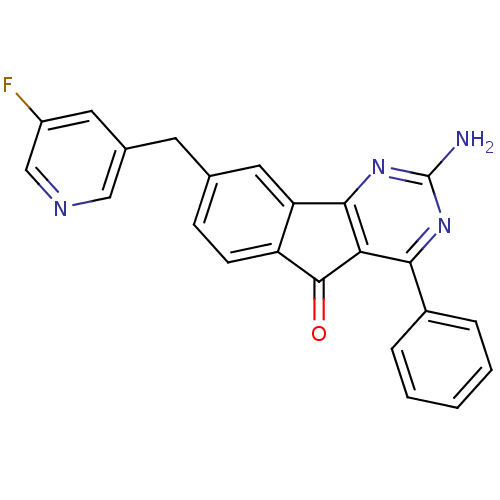
(CHEMBL2165799)Show SMILES Nc1nc2-c3cc(Cc4cncc(F)c4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-16-9-14(11-26-12-16)8-13-6-7-17-18(10-13)21-19(22(17)29)20(27-23(25)28-21)15-4-2-1-3-5-15/h1-7,9-12H,8H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
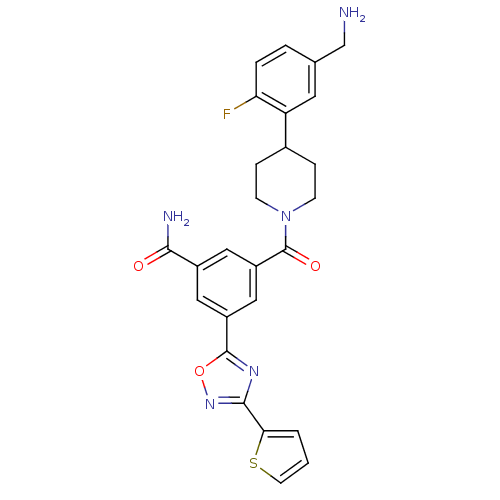
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108680
(CHEMBL3596517)Show SMILES COc1cccc2c3nc(CN4CCN(C[C@H]4C)c4sc(C)nc4C)nn3c(N)nc12 |r| Show InChI InChI=1S/C21H26N8OS/c1-12-10-28(20-13(2)23-14(3)31-20)9-8-27(12)11-17-24-19-15-6-5-7-16(30-4)18(15)25-21(22)29(19)26-17/h5-7,12H,8-11H2,1-4H3,(H2,22,25)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine A2A receptor |
Bioorg Med Chem Lett 25: 2958-62 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.036
BindingDB Entry DOI: 10.7270/Q23F4RFG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50238959
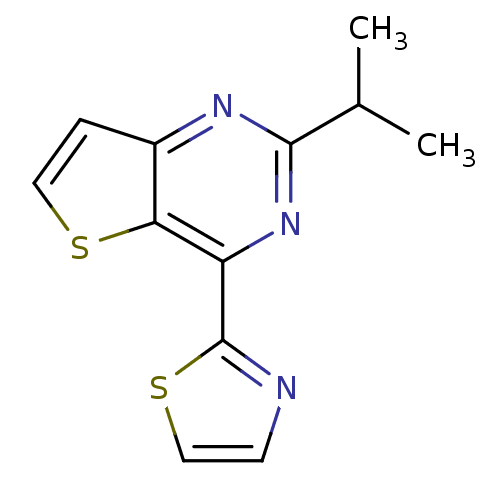
(2-isopropyl-4-(thiazol-2-yl)thieno[3,2-d]pyrimidin...)Show InChI InChI=1S/C12H11N3S2/c1-7(2)11-14-8-3-5-16-10(8)9(15-11)12-13-4-6-17-12/h3-7H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50009525
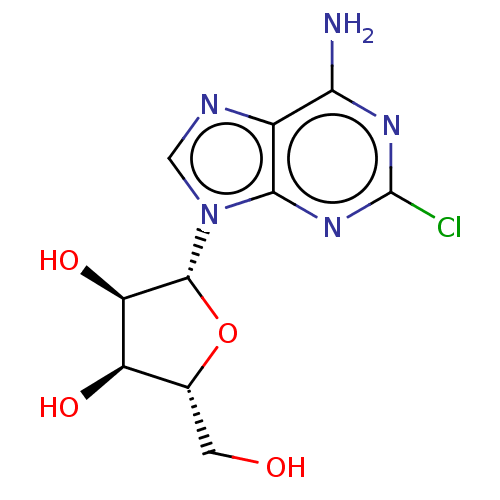
(2-CI Adenosine | 2-Chloroadenosine | 2-Chloroado |...)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12ClN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A1 receptor |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394721
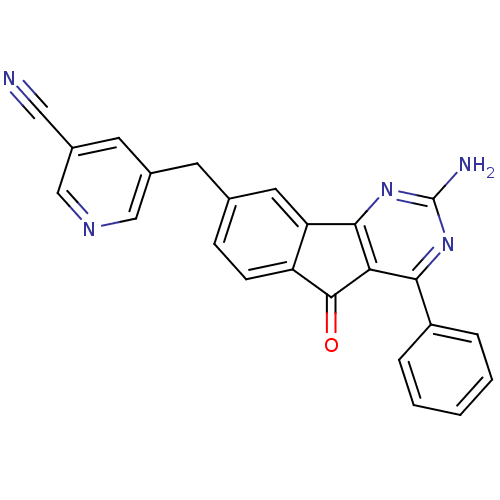
(CHEMBL2165808)Show SMILES Nc1nc2-c3cc(Cc4cncc(c4)C#N)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C24H15N5O/c25-11-16-9-15(12-27-13-16)8-14-6-7-18-19(10-14)22-20(23(18)30)21(28-24(26)29-22)17-4-2-1-3-5-17/h1-7,9-10,12-13H,8H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
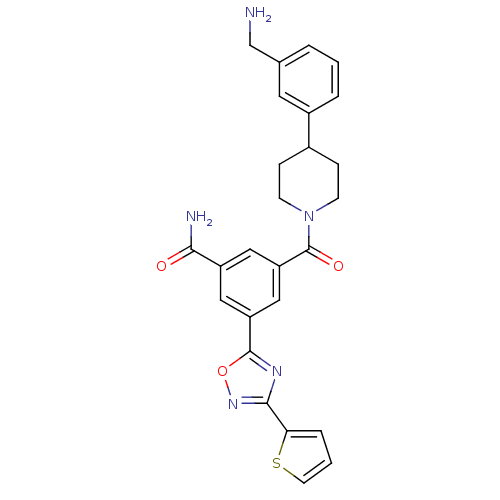
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50294132

(CHEMBL561963 | N-hydroxy-2-(N-isopropoxy-4'-isopro...)Show SMILES CC(C)ON(C(C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(C)C Show InChI InChI=1S/C23H32N2O5S/c1-15(2)18-7-9-19(10-8-18)20-11-13-21(14-12-20)31(28,29)25(30-17(5)6)22(16(3)4)23(26)24-27/h7-17,22,27H,1-6H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of MMP13-mediated collagen degradation by SDS-PAGE |
J Med Chem 52: 4757-73 (2009)
Article DOI: 10.1021/jm900261f
BindingDB Entry DOI: 10.7270/Q2JW8DXN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493034
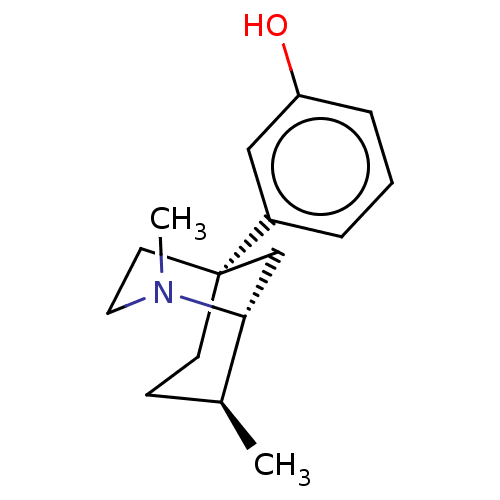
(CHEMBL2418571)Show SMILES [H][C@@]12C[C@@](CC[C@@H]1C)(CCN2C)c1cccc(O)c1 |r| Show InChI InChI=1S/C16H23NO/c1-12-6-7-16(8-9-17(2)15(12)11-16)13-4-3-5-14(18)10-13/h3-5,10,12,15,18H,6-9,11H2,1-2H3/t12-,15+,16+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 2 hrs by liquid scintillation counting |
Eur J Med Chem 67: 335-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.06.030
BindingDB Entry DOI: 10.7270/Q2251N40 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22911

(2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells by liquid scintillation counting |
Bioorg Med Chem 17: 3987-94 (2009)
Article DOI: 10.1016/j.bmc.2009.04.007
BindingDB Entry DOI: 10.7270/Q2SQ91MW |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50414391
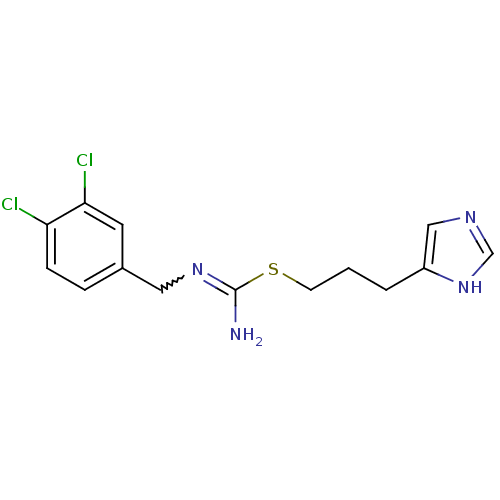
(CHEMBL1202332 | CHEMBL553423)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)c(Cl)c1 |w:11.12| Show InChI InChI=1S/C14H16Cl2N4S/c15-12-4-3-10(6-13(12)16)7-19-14(17)21-5-1-2-11-8-18-9-20-11/h3-4,6,8-9H,1-2,5,7H2,(H2,17,19)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in human SK-N-MC cells by liquid scintillation counting |
Bioorg Med Chem 17: 3987-94 (2009)
Article DOI: 10.1016/j.bmc.2009.04.007
BindingDB Entry DOI: 10.7270/Q2SQ91MW |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50414391

(CHEMBL1202332 | CHEMBL553423)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)c(Cl)c1 |w:11.12| Show InChI InChI=1S/C14H16Cl2N4S/c15-12-4-3-10(6-13(12)16)7-19-14(17)21-5-1-2-11-8-18-9-20-11/h3-4,6,8-9H,1-2,5,7H2,(H2,17,19)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Agonistic activity at histamine H4 receptor |
J Med Chem 54: 1693-703 (2011)
Article DOI: 10.1021/jm1013488
BindingDB Entry DOI: 10.7270/Q20G3MDD |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394716
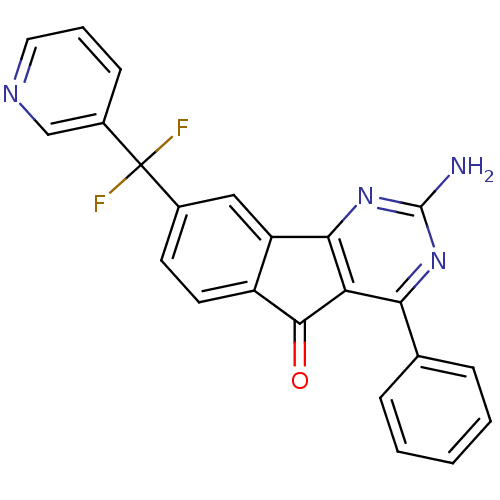
(CHEMBL2165803)Show SMILES Nc1nc2-c3cc(ccc3C(=O)c2c(n1)-c1ccccc1)C(F)(F)c1cccnc1 Show InChI InChI=1S/C23H14F2N4O/c24-23(25,15-7-4-10-27-12-15)14-8-9-16-17(11-14)20-18(21(16)30)19(28-22(26)29-20)13-5-2-1-3-6-13/h1-12H,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50419455
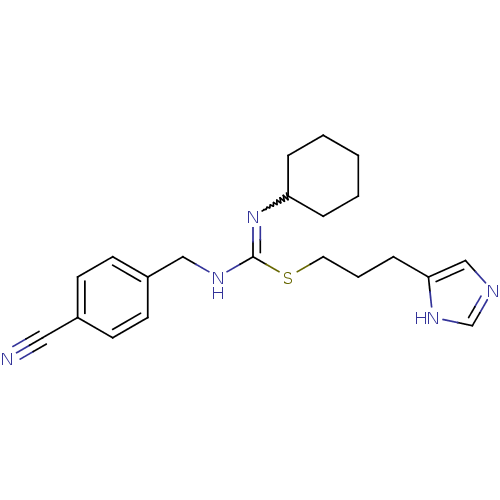
(CHEMBL1923033)Show SMILES N#Cc1ccc(CNC(SCCCc2cnc[nH]2)=NC2CCCCC2)cc1 |w:18.19| Show InChI InChI=1S/C21H27N5S/c22-13-17-8-10-18(11-9-17)14-24-21(26-19-5-2-1-3-6-19)27-12-4-7-20-15-23-16-25-20/h8-11,15-16,19H,1-7,12,14H2,(H,23,25)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50377559
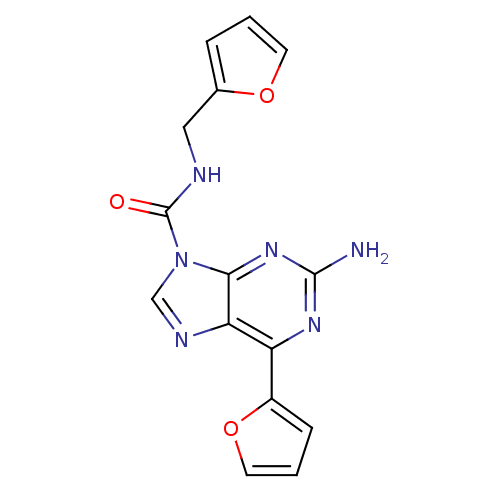
(CHEMBL259832)Show InChI InChI=1S/C15H12N6O3/c16-14-19-11(10-4-2-6-24-10)12-13(20-14)21(8-18-12)15(22)17-7-9-3-1-5-23-9/h1-6,8H,7H2,(H,17,22)(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data