

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562581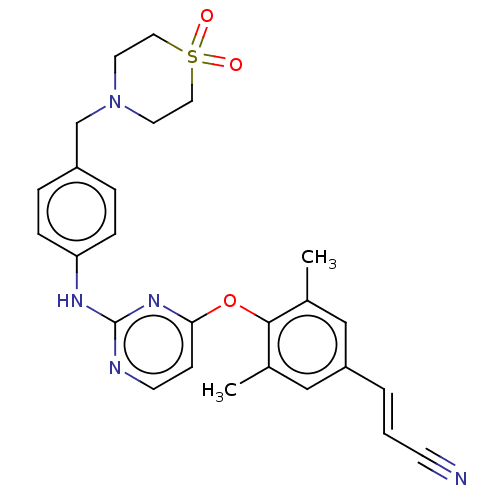 (CHEMBL4795744) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562581 (CHEMBL4795744) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562580 (CHEMBL4741019) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562580 (CHEMBL4741019) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562583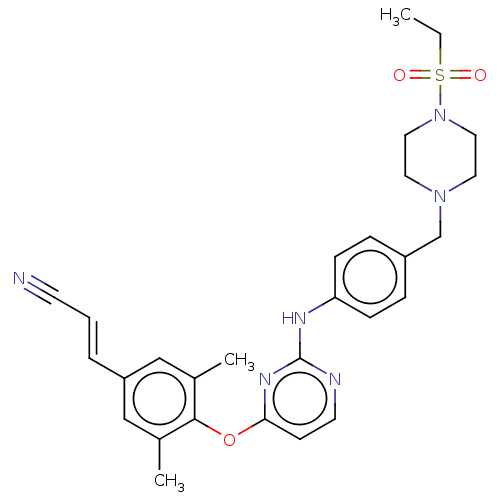 (CHEMBL4745950) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562583 (CHEMBL4745950) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562579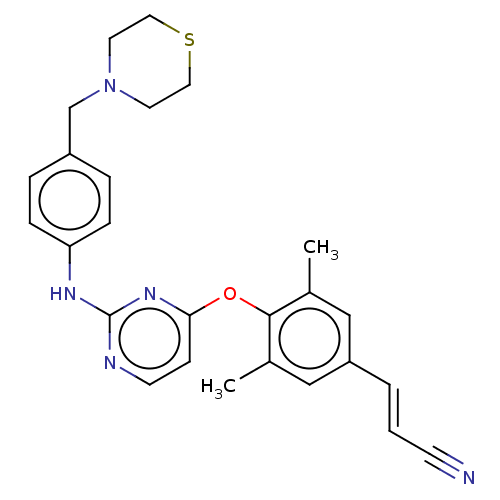 (CHEMBL4740496) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562579 (CHEMBL4740496) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562584 (CHEMBL4740036) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562584 (CHEMBL4740036) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562577 (CHEMBL4756531) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562577 (CHEMBL4756531) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562575 (CHEMBL4764884) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562575 (CHEMBL4764884) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504275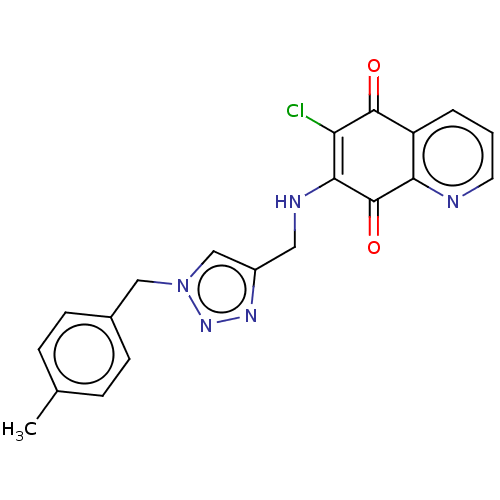 (CHEMBL4521092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562582 (CHEMBL4750593) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562582 (CHEMBL4750593) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562578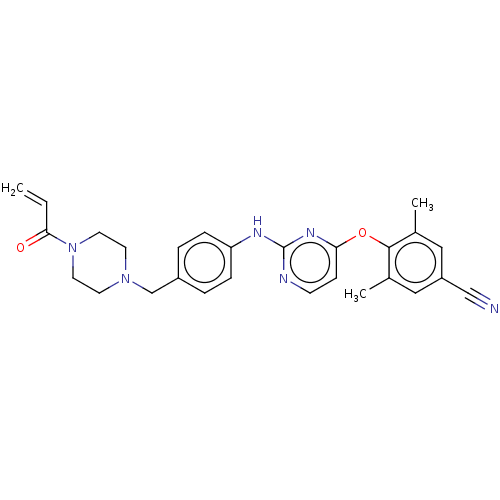 (CHEMBL4741136) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562578 (CHEMBL4741136) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562576 (CHEMBL4748138) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50562576 (CHEMBL4748138) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504277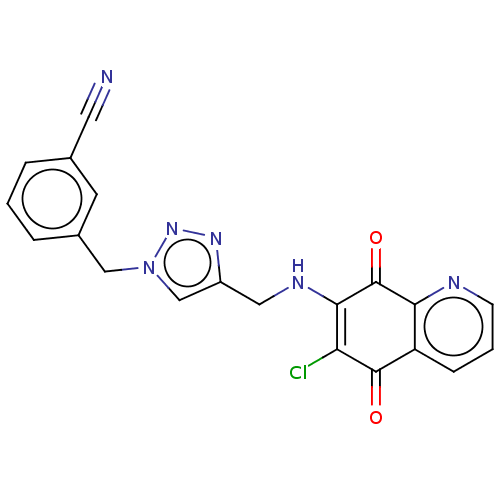 (CHEMBL4566962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504276 (CHEMBL4466461) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50504275 (CHEMBL4521092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25A using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50504277 (CHEMBL4566962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25A using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase assessed as reduction in biotin-dUTP incorporation into template incubated for 1 hr by ELISA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113051 BindingDB Entry DOI: 10.7270/Q2C53QK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25C using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50504277 (CHEMBL4566962) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25B using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50504276 (CHEMBL4466461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25A using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25A using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25B using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50504275 (CHEMBL4521092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25B using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50504276 (CHEMBL4466461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of recombinant human Cdc25B using OMFP as substrate preincubated for 5 to 8 mins and measured every 5 mins 60 mins by fluorescence based a... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111696 BindingDB Entry DOI: 10.7270/Q21R6TS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308256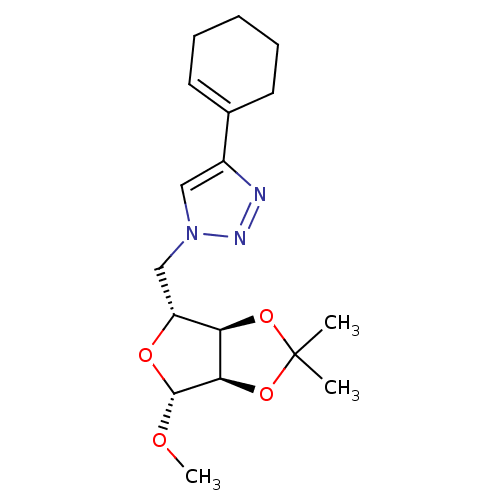 (1-O-Methyl-2,3-O-isopropylidene-5-(4-cyclohexene-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308259 (1-O-Methyl-2,3-O-isopropylidene-5-[4-(1-hydroxycyc...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308258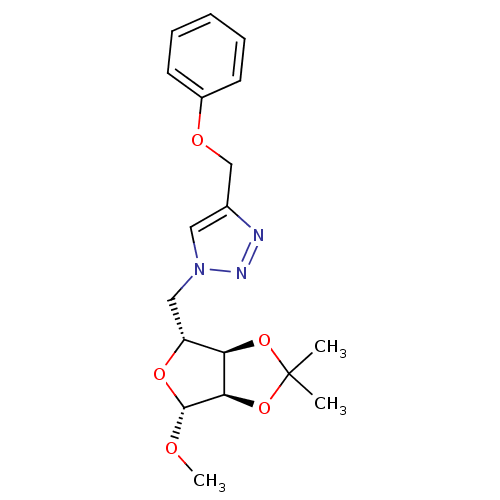 (1-O-Methyl-2,3-O-isopropylidene-5-(4-phenoxymethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308255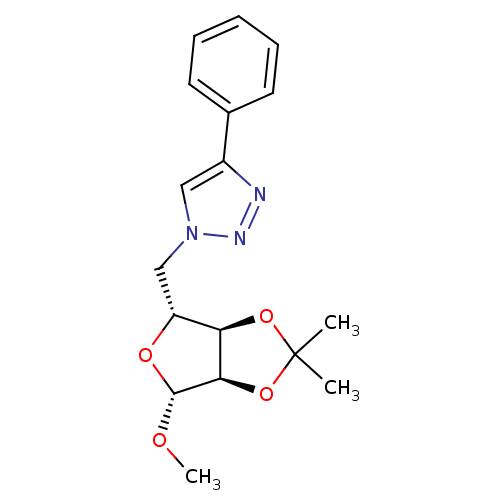 (1-O-Methyl-2,3-O-isopropylidene-5-(4-phenyl-1H-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308257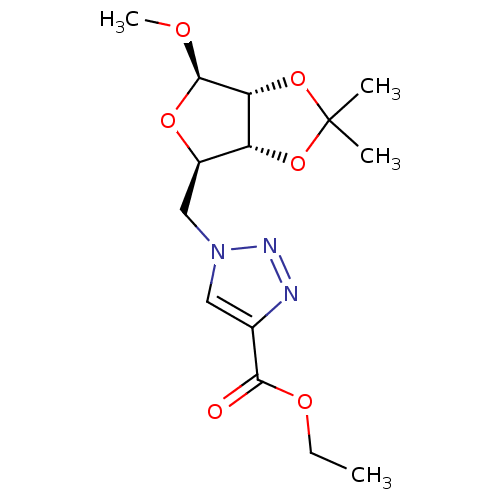 (1-O-Methyl-2,3-O-isopropylidene-5-(4-carboxylate e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50308260 (1-O-Methyl-2,3-O-isopropylidene-5-[4-(tetrahydro-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-glucosidase MAL12 (Saccharomyces cerevisiae) | BDBM50333465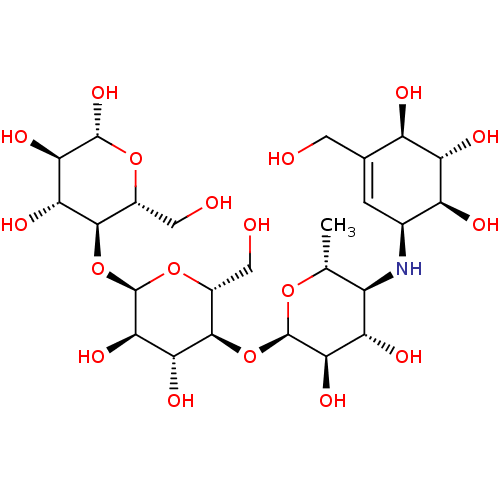 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of yeast alpha-glucosidase MAL12 assessed as inhibition of p-nitrophenol release by spectrophotometry | J Med Chem 53: 2364-75 (2010) Article DOI: 10.1021/jm901265h BindingDB Entry DOI: 10.7270/Q20R9QC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||