

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536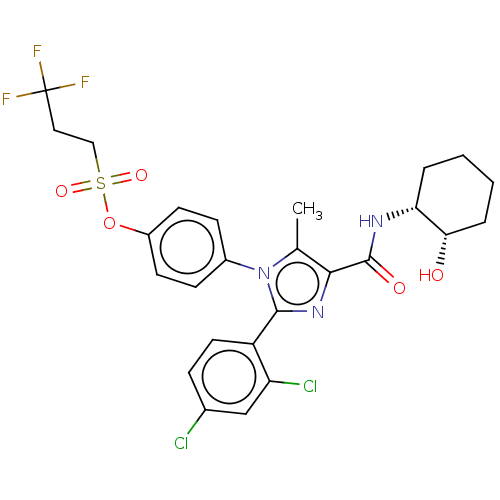 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243536 (CHEMBL4062749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004808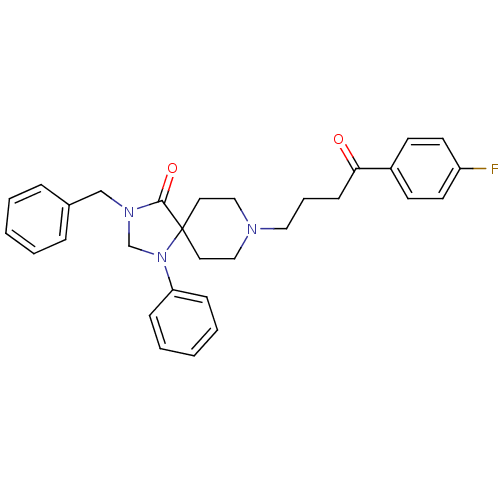 (3-Benzyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004801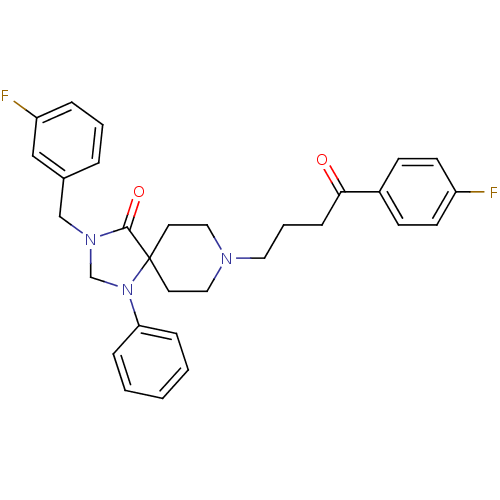 (3-(3-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004819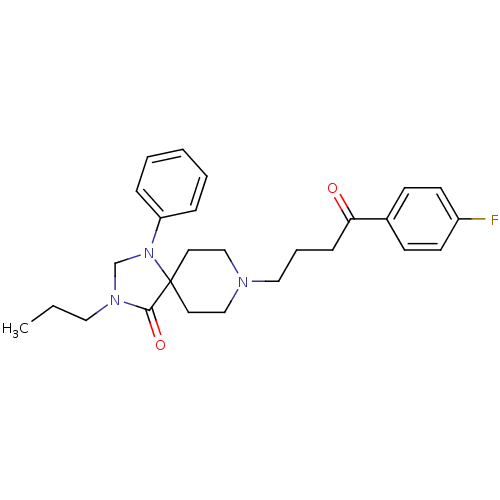 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-1-phenyl-3-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004817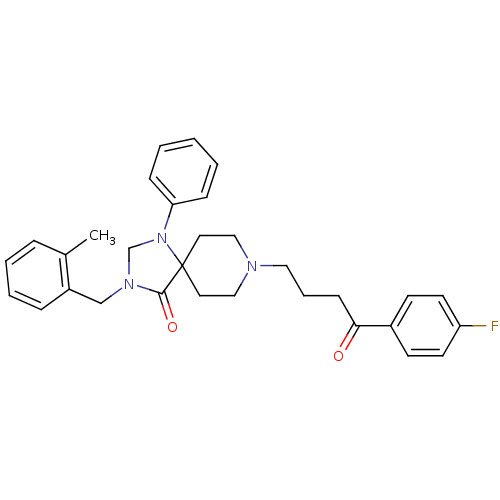 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-methyl-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004803 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-phenethyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004805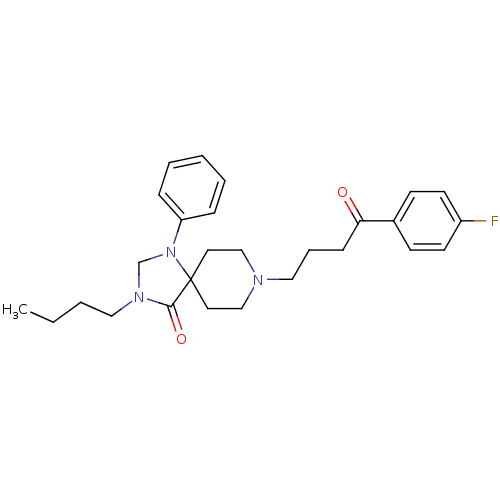 (3-Butyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004807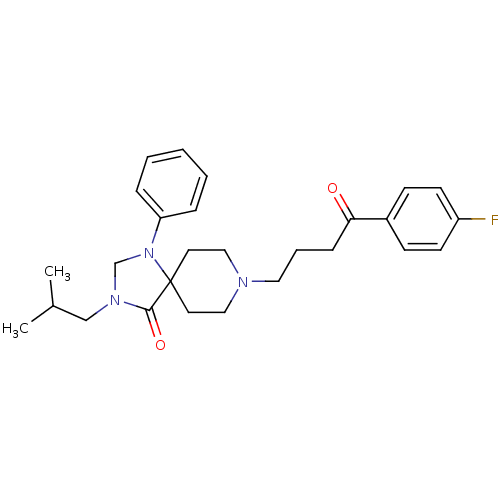 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-isobutyl-1-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50459886 (CHEMBL261010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from human 5-HT3A receptor expressed in HEK293 cells by scintillation counting method | Bioorg Med Chem Lett 27: 3207-3218 (2017) Article DOI: 10.1016/j.bmcl.2017.04.073 BindingDB Entry DOI: 10.7270/Q2MC92NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004816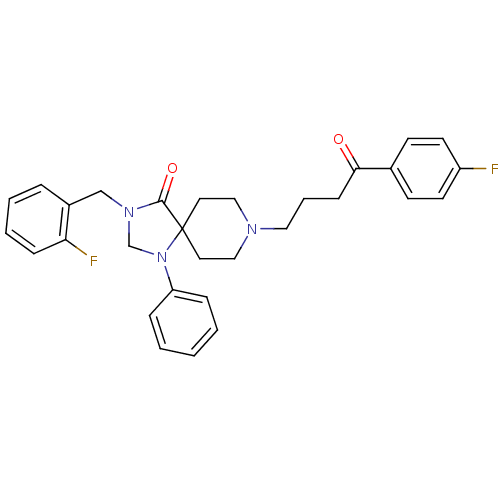 (3-(2-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004810 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-methoxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004818 (3-Ethyl-8-[4-(4-fluoro-phenyl)-4-oxo-butyl]-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004802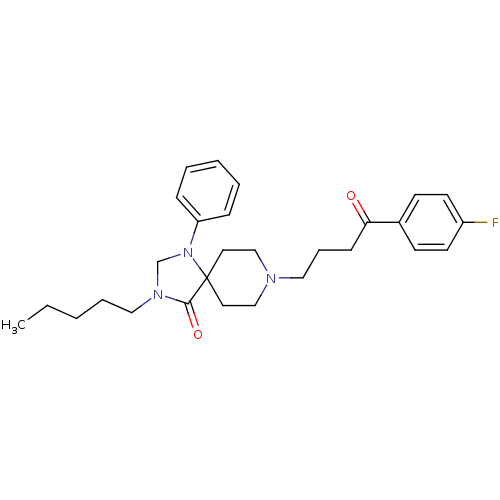 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-pentyl-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004804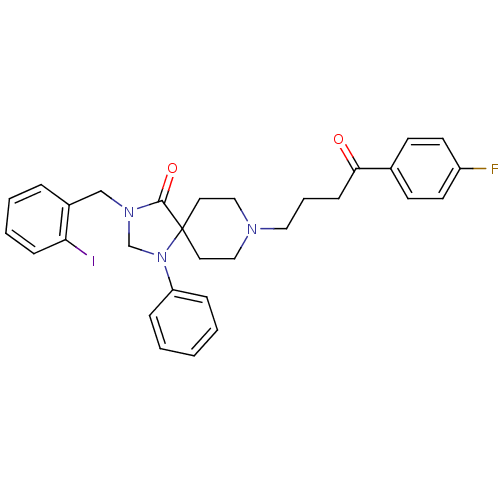 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-iodo-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004815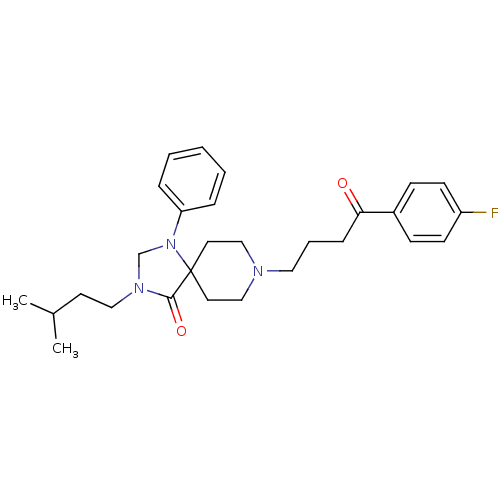 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(3-methyl-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004806 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(4-methyl-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004800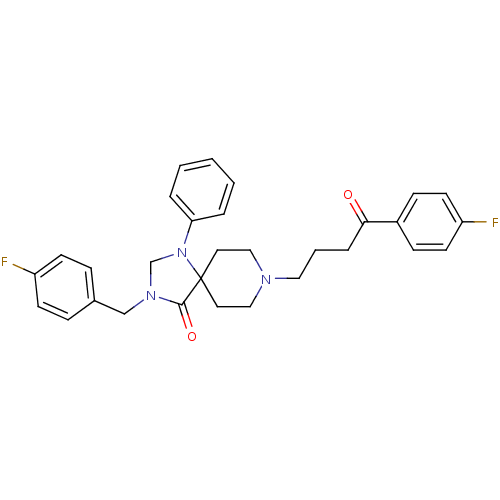 (3-(4-Fluoro-benzyl)-8-[4-(4-fluoro-phenyl)-4-oxo-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine receptor D2 | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8336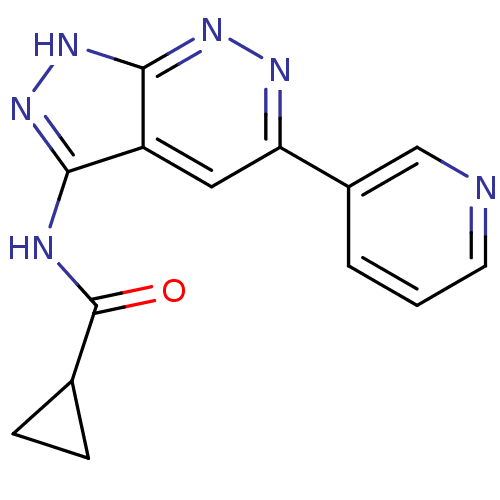 (N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536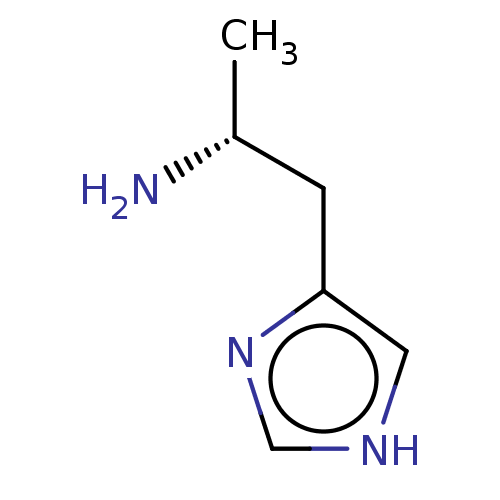 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004812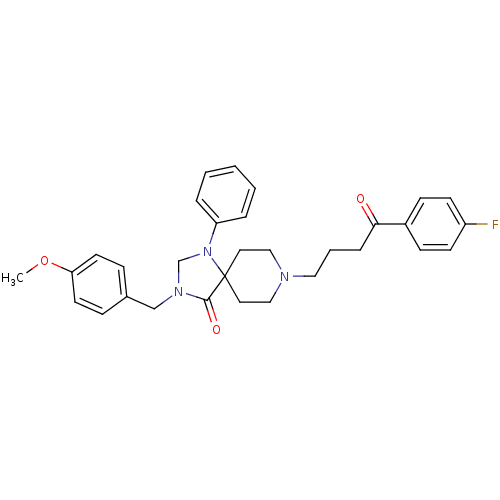 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(4-methoxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50004819 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-1-phenyl-3-pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity towards 5-hydroxytryptamine 2 receptor by displacing [125]I-LSD radioligand | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243545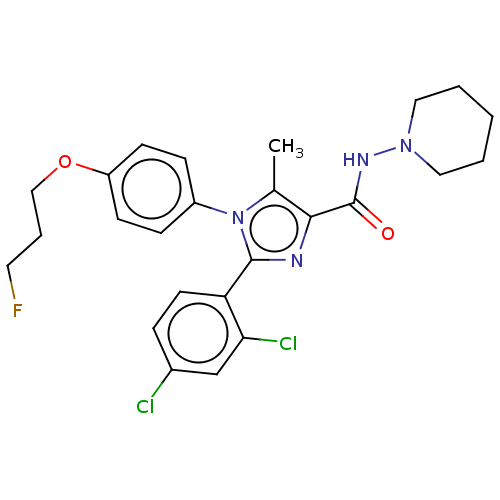 (CHEMBL4078689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243589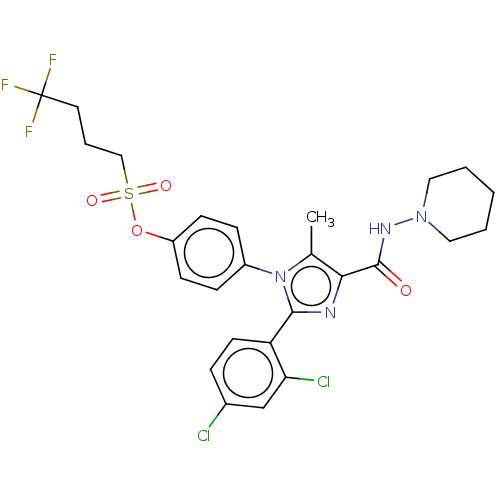 (CHEMBL4095223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243608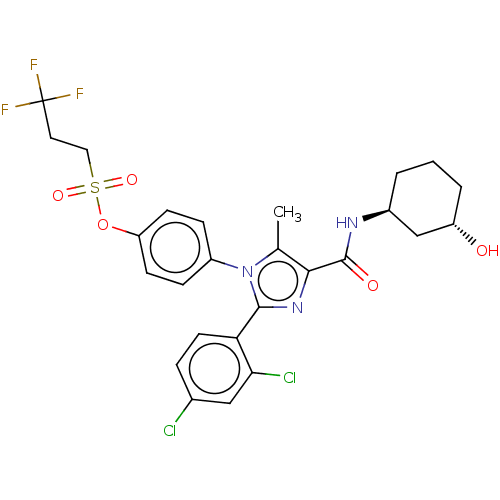 (CHEMBL4100882) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243625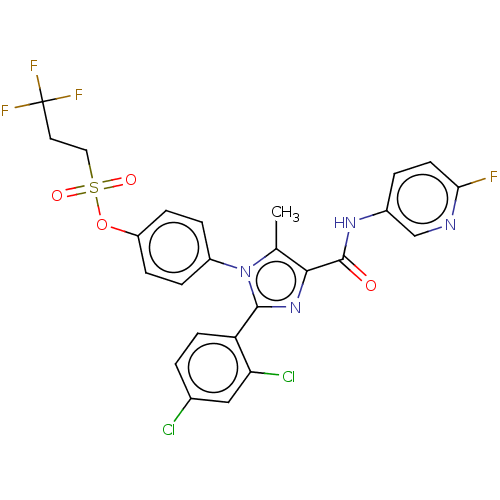 (CHEMBL4094098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243545 (CHEMBL4078689) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243589 (CHEMBL4095223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243608 (CHEMBL4100882) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243625 (CHEMBL4094098) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411339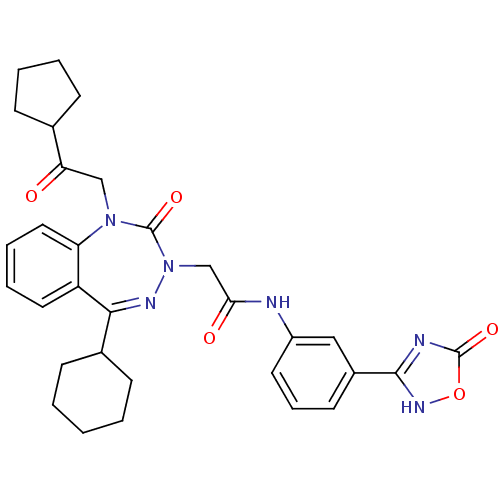 (CHEMBL227276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM8337 (N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... | Bioorg Med Chem Lett 13: 1581-4 (2003) Article DOI: 10.1016/s0960-894x(03)00135-5 BindingDB Entry DOI: 10.7270/Q2BK19JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004813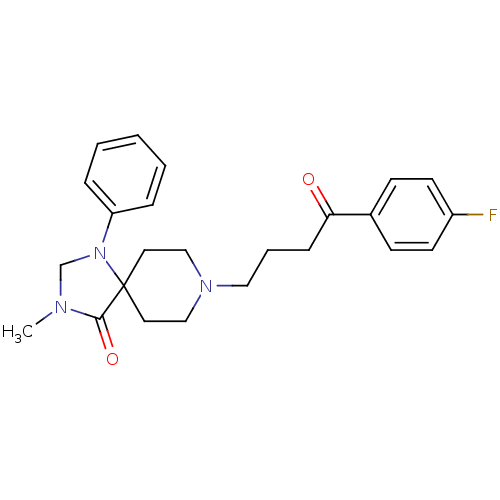 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-methyl-1-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004809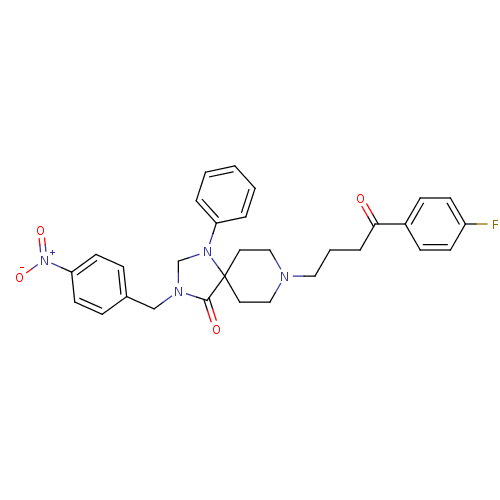 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(4-nitro-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50004814 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-(2-nitro-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 by displacing [3H]-spiperone | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243626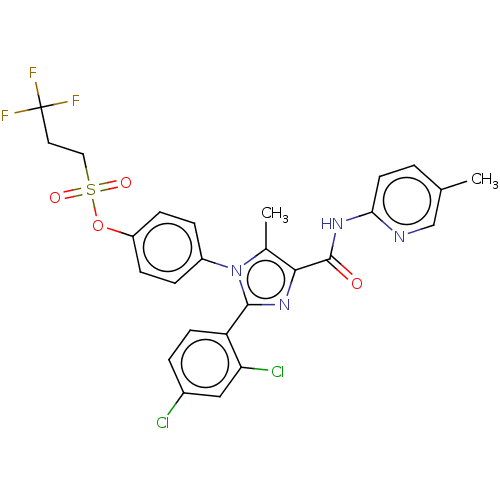 (CHEMBL4089821) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243588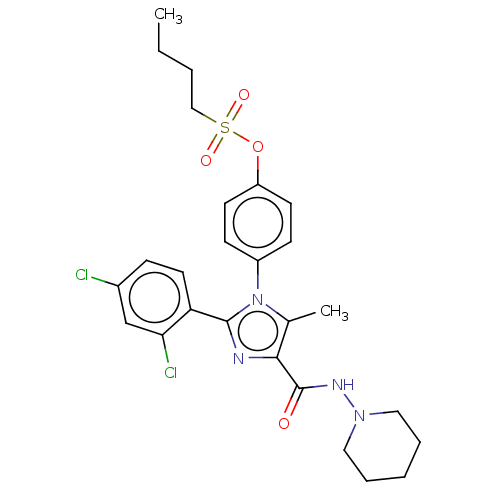 (CHEMBL4067800) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243645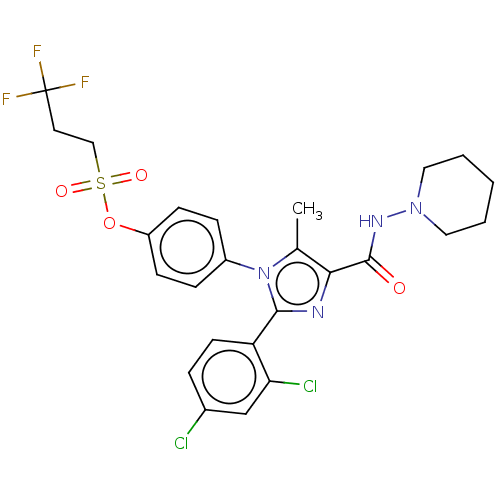 (CHEMBL4087520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243627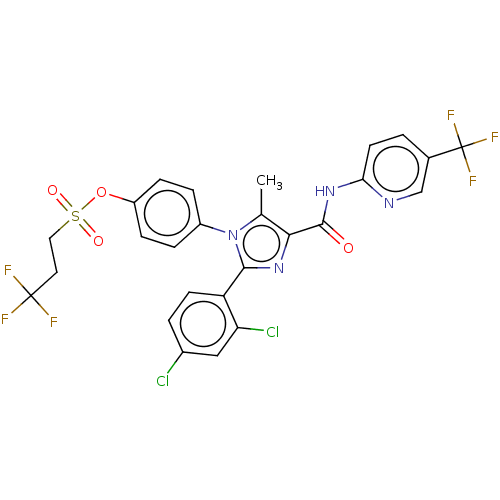 (CHEMBL4068708) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243627 (CHEMBL4068708) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243626 (CHEMBL4089821) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243588 (CHEMBL4067800) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50243645 (CHEMBL4087520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... | J Med Chem 60: 9545-9564 (2017) Article DOI: 10.1021/acs.jmedchem.7b00861 BindingDB Entry DOI: 10.7270/Q2X92DQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50004807 (8-[4-(4-Fluoro-phenyl)-4-oxo-butyl]-3-isobutyl-1-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity towards 5-hydroxytryptamine 2 receptor by displacing [125]I-LSD radioligand | J Med Chem 35: 423-30 (1992) BindingDB Entry DOI: 10.7270/Q2B85725 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50056102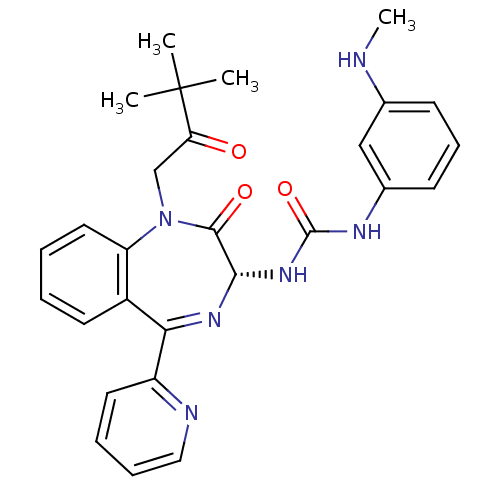 ((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig ileum LMMP homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305851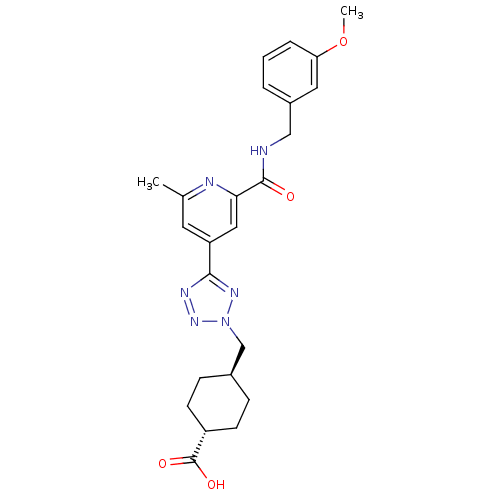 (CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305851 (CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length human recombinant MMP13 using MCA-Arg-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-Glu-Arg-NH2 as substrate preincubated for 60 mins followe... | J Med Chem 59: 313-27 (2016) Article DOI: 10.1021/acs.jmedchem.5b01434 BindingDB Entry DOI: 10.7270/Q20G3N0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 12682 total ) | Next | Last >> |