

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM435577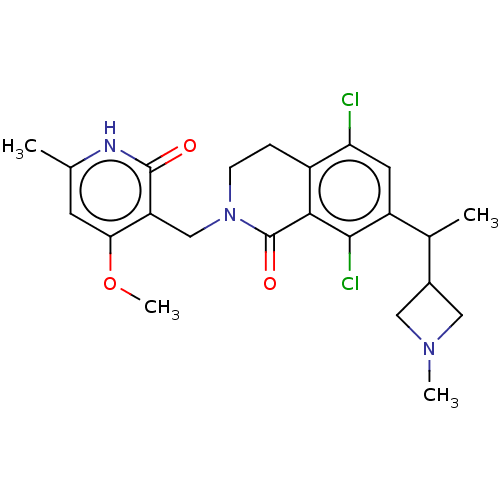 (US10570121, Example 87) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299749 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM159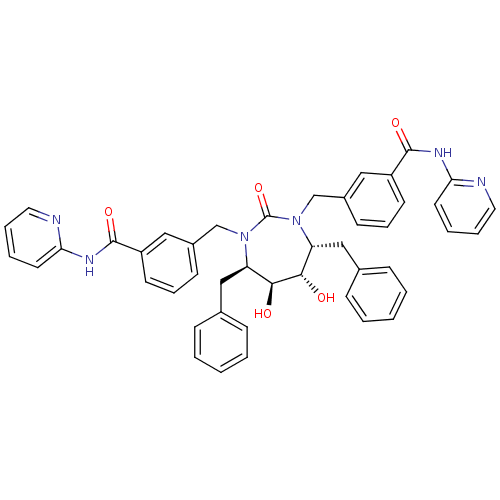 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054159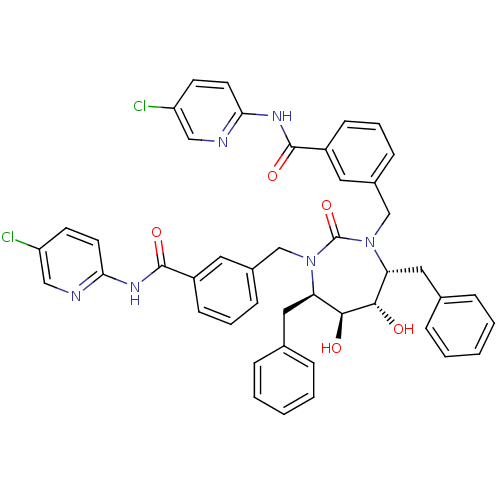 (5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054156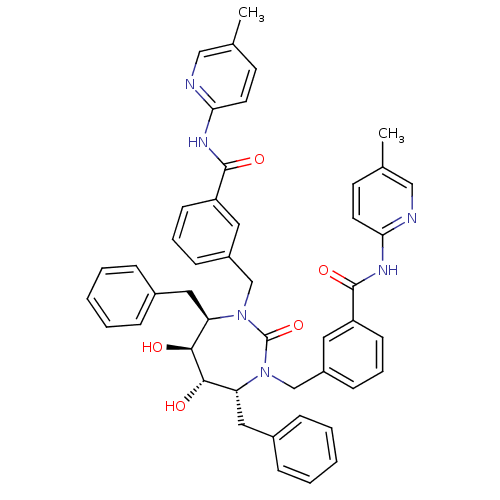 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM435691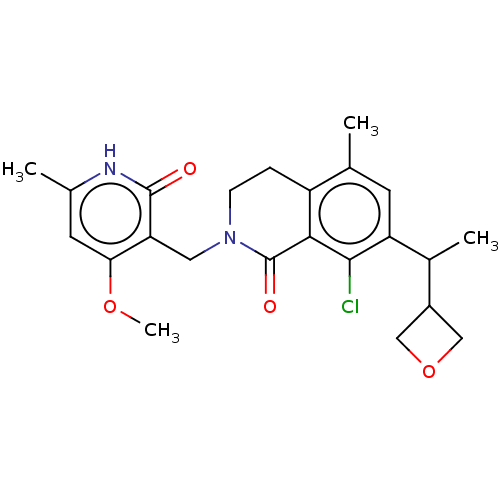 (US10570121, Example 197 | US10570121, Example 198) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054156 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054159 (5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299975 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299902 (6-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300079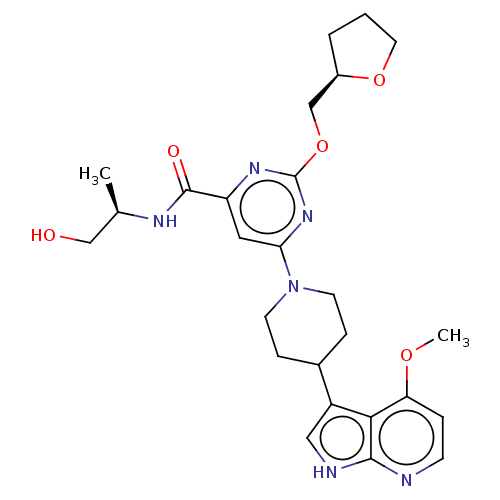 (N-[(2R)-1- hydroxypropan-2- yl]-6-[4-(4- methoxy-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300077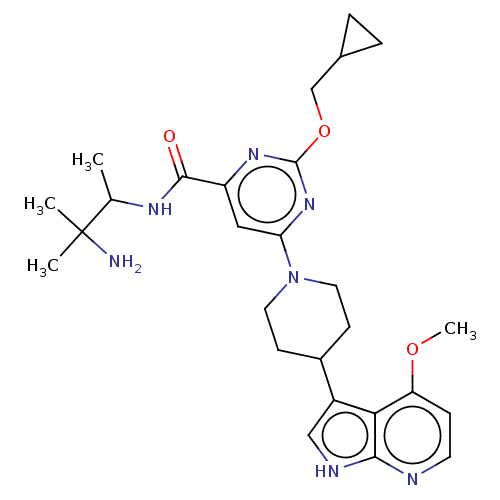 (N-(3-amino-3- methylbutan- 2-yl)-2- (cyclo- propyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300035 (2-(benzyloxy)-N- [(2R)-1- hydroxypropan-2- yl]-6-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300011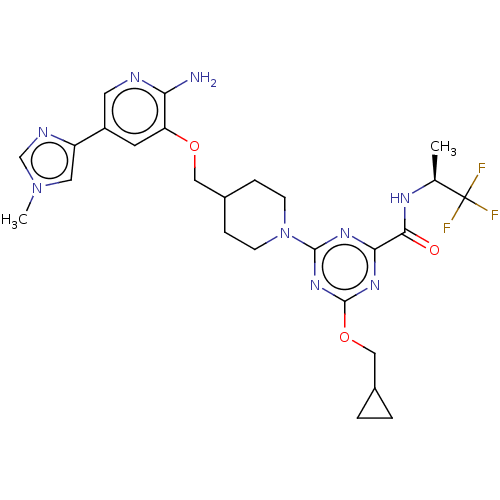 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054184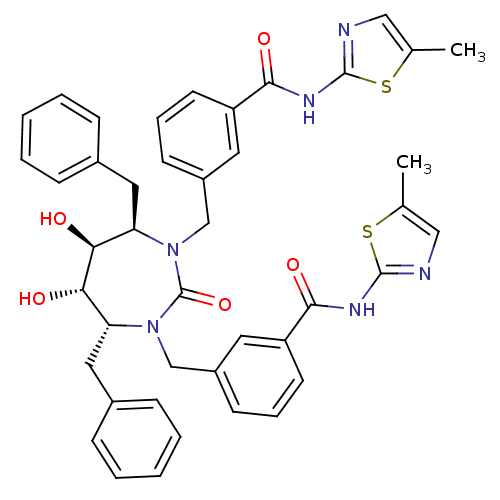 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(5-methyl-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300088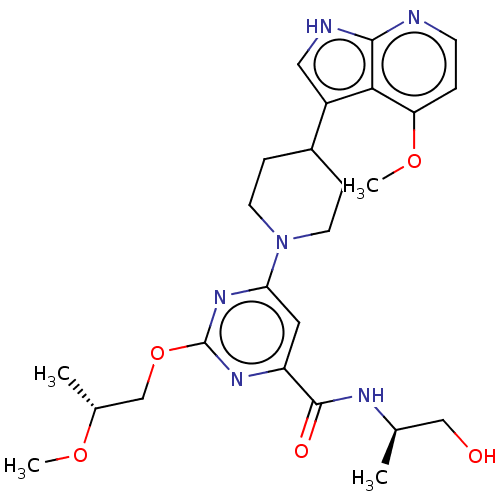 (N-[(2R)-1- hydroxypropan-2- yl]-2-[(2R)-2- methoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160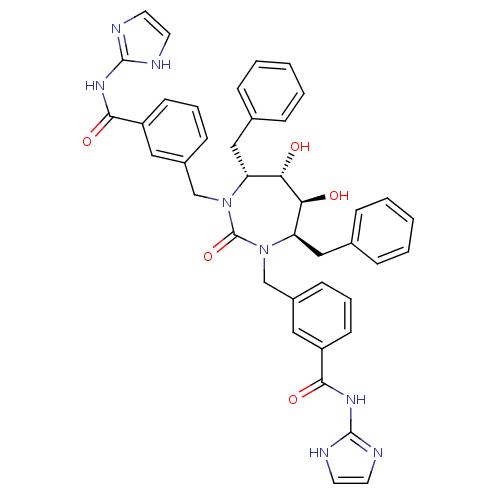 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299998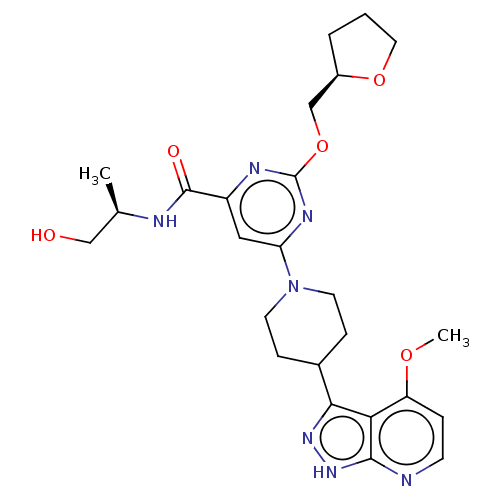 (N-[(2R)-1- hydroxypropan-2- yl]-6-[4-(4- methoxy-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526620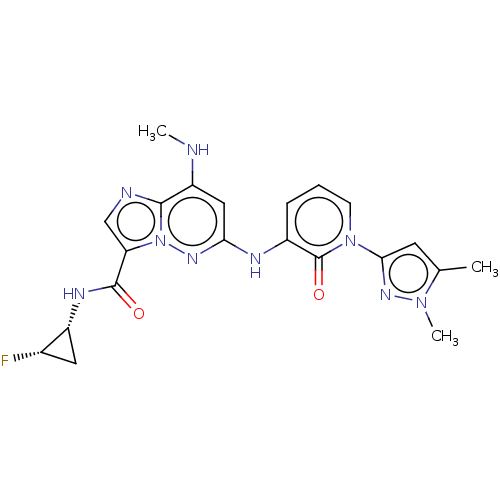 (CHEMBL4460368) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299931 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299744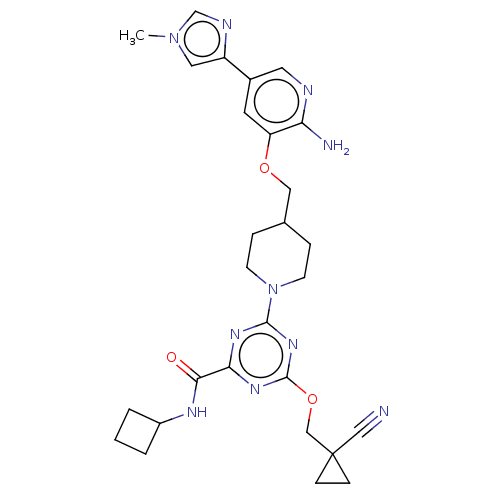 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054178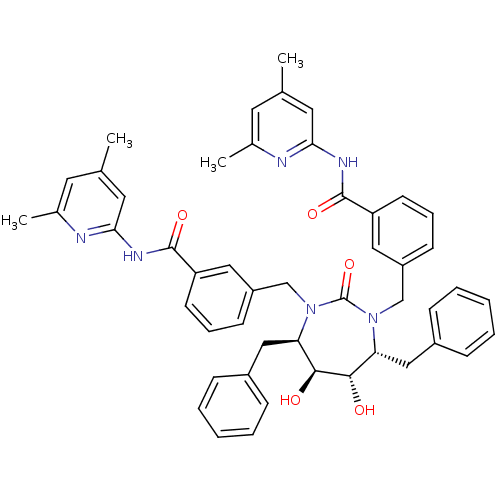 (2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299909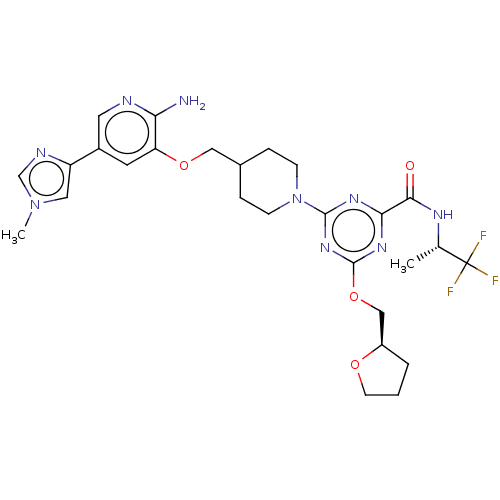 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300012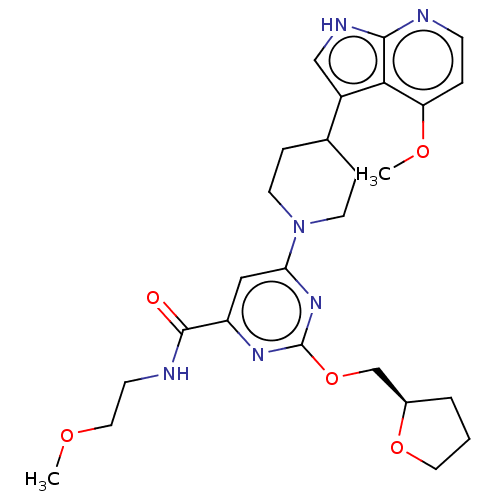 (N-(2- methoxyethyl)-6- [4-(4-methoxy-1H- pyrrolo[2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300049 ((3,3- difluoroazetidin-1- yl)(2-{[(2R)-1- methoxyp...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300007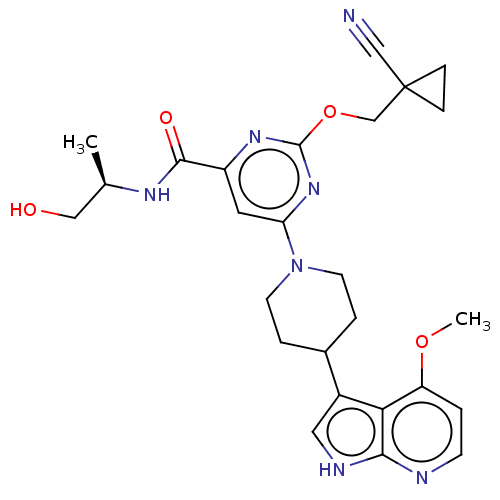 (2-[(1- cyanocyclopropyl) methoxy]-N-[(2R)- 1-hydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054174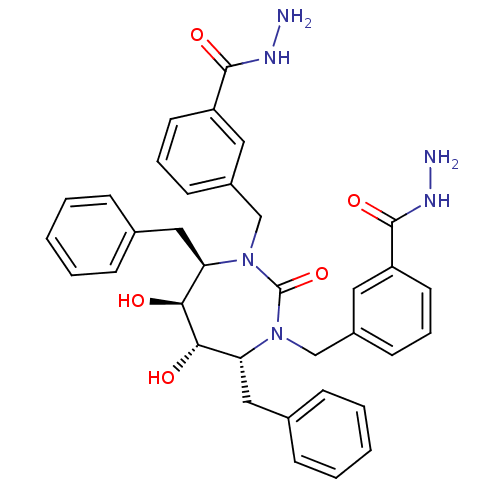 (3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054179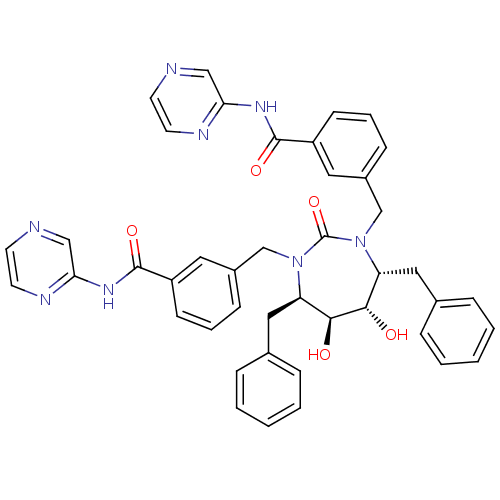 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054174 (3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526622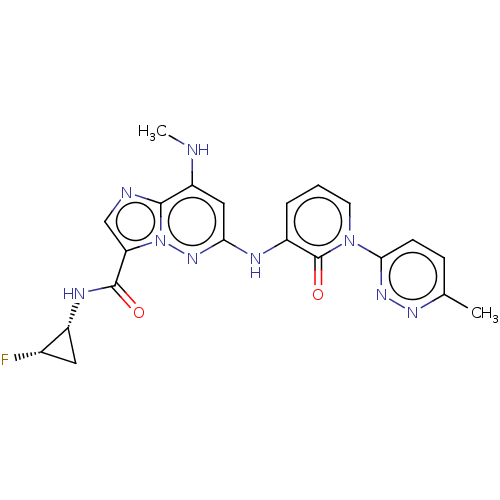 (CHEMBL4442827) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299893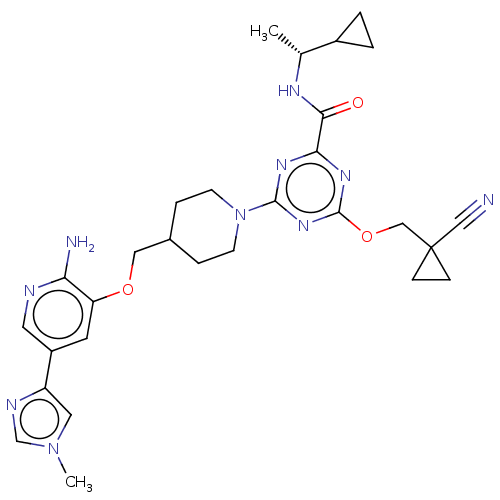 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054180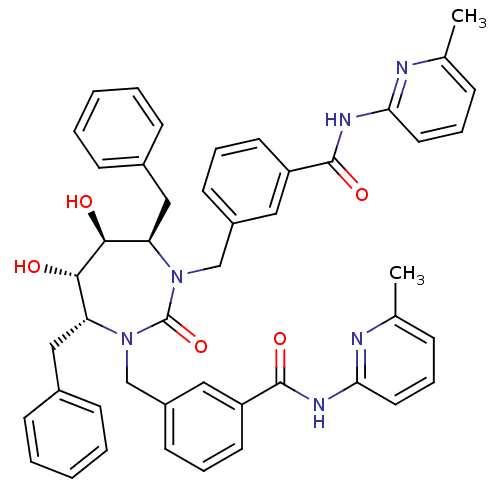 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50507816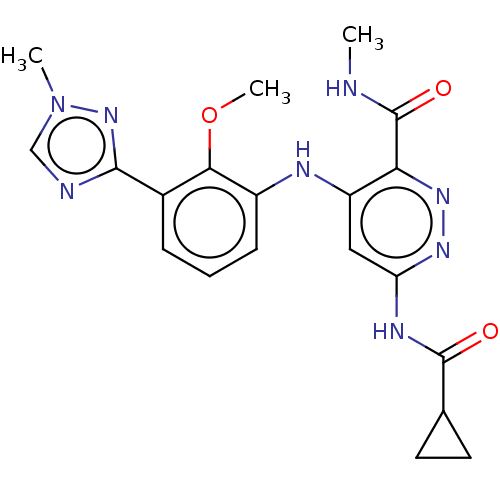 (Bms-986165 | Deucravacitinib) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... | J Med Chem 62: 8973-8995 (2019) Article DOI: 10.1021/acs.jmedchem.9b00444 BindingDB Entry DOI: 10.7270/Q2930XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300070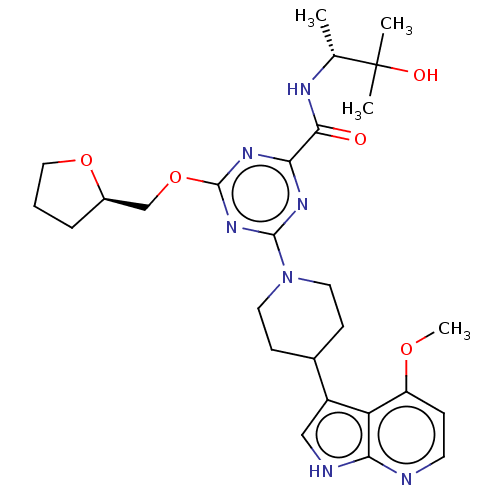 (N-[(2R)-3- hydroxy-3- methylbutan-2-yl]- 4-[4-(4-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300090 (N-[(2R)-1- hydroxypropan-2- yl]-2-[(2S)-2- methoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299917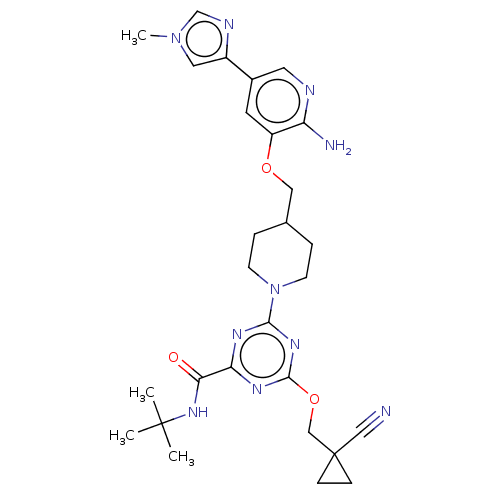 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50018830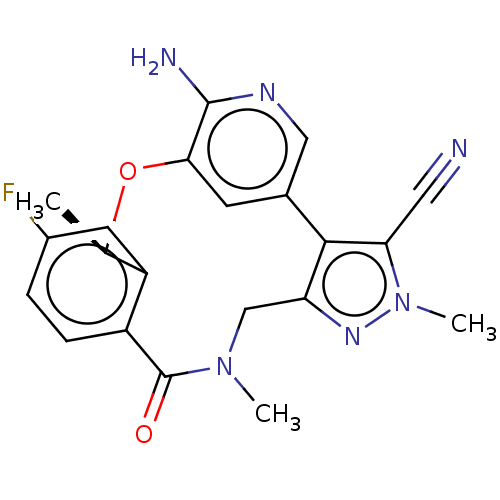 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by off-chip mobility shift assay | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054179 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054168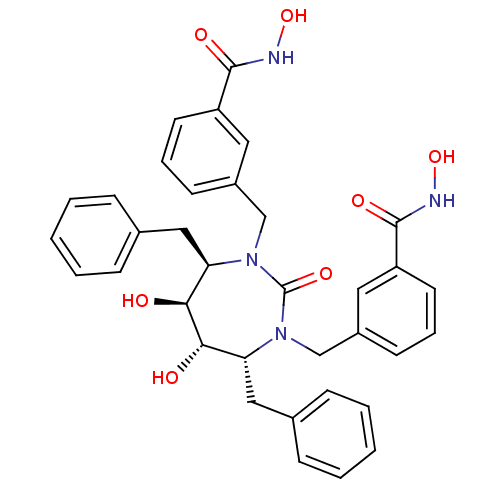 ((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836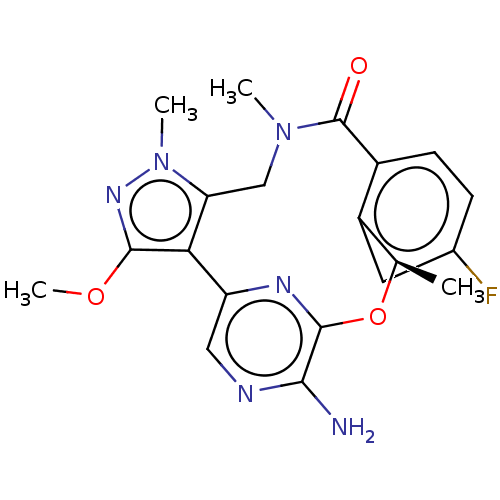 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054168 ((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054180 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054178 (2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246932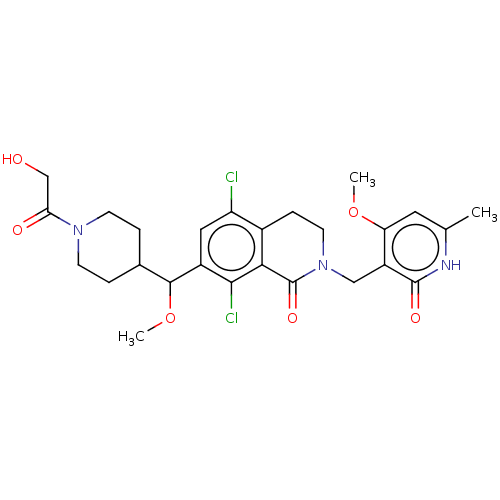 (CHEMBL4073864 | US10570121, Example 112) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299901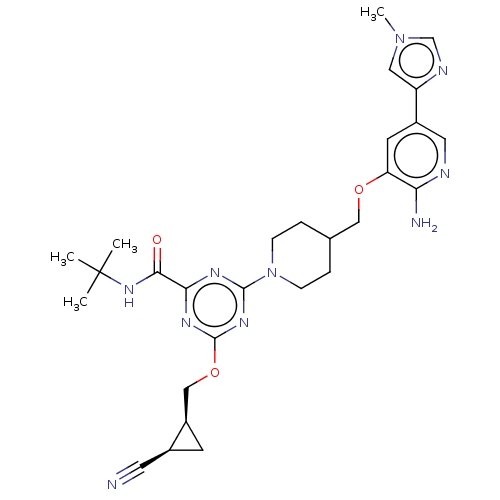 (4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM300125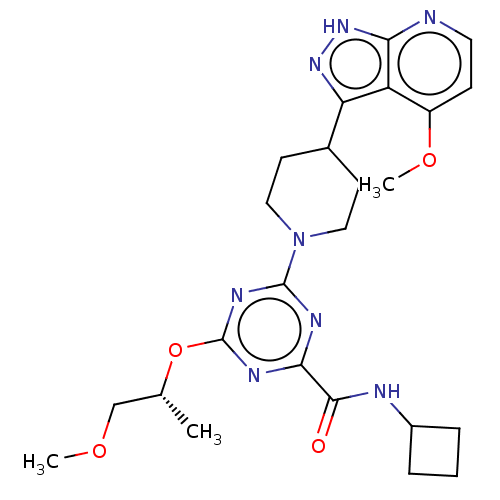 (N-cyclobutyl-4- {[(2R)-1- methoxypropan-2- yl]oxy}...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM299996 (4-[(1- cyanocyclopropyl) methoxy]-6-[4-(4- methoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... | US Patent US9593097 (2017) BindingDB Entry DOI: 10.7270/Q2GM89BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155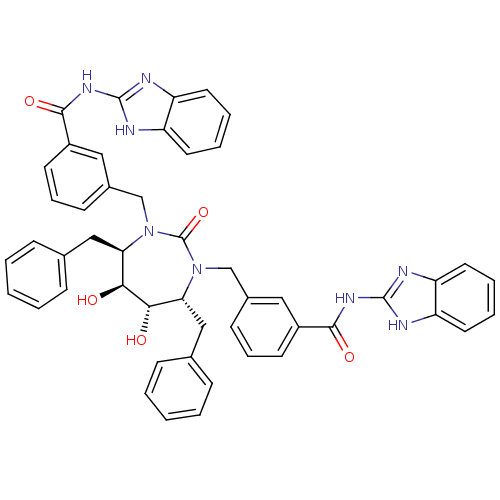 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50526613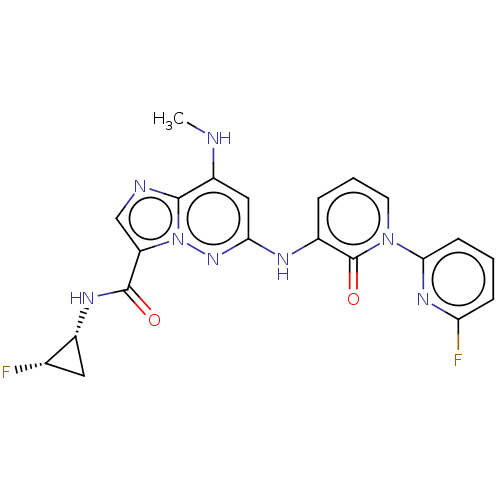 (CHEMBL4571920) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... | ACS Med Chem Lett 10: 383-388 (2019) Article DOI: 10.1021/acsmedchemlett.9b00035 BindingDB Entry DOI: 10.7270/Q2TM7FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 28443 total ) | Next | Last >> |