

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139046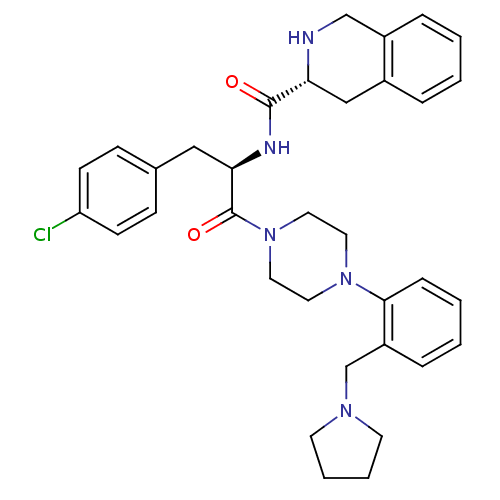 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139032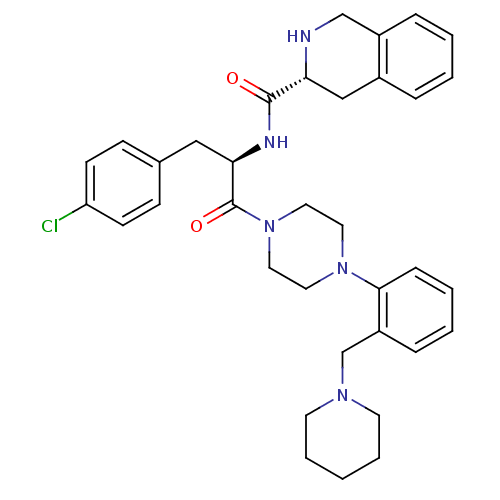 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139027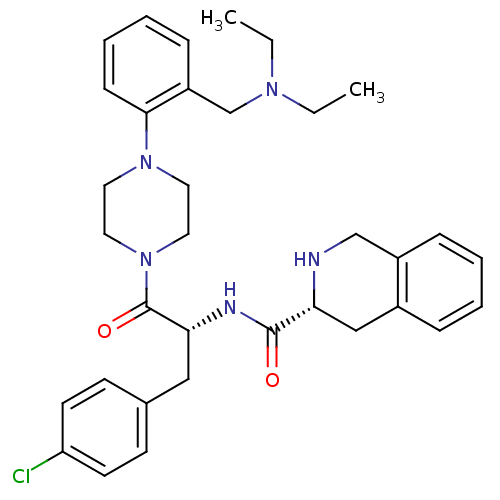 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139043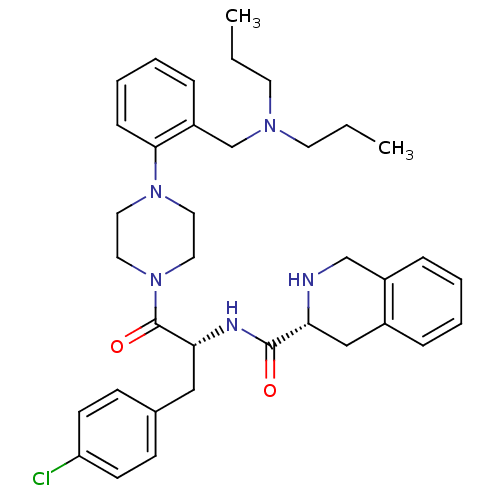 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139028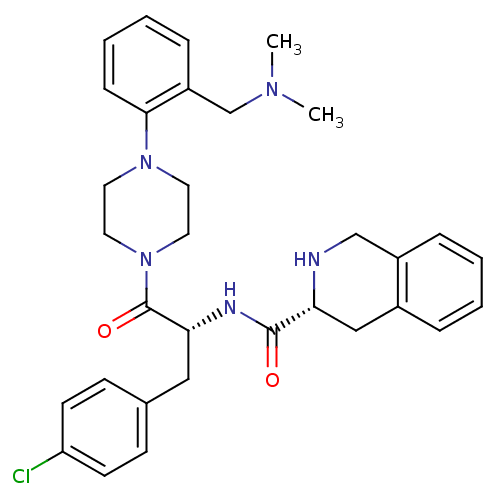 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139023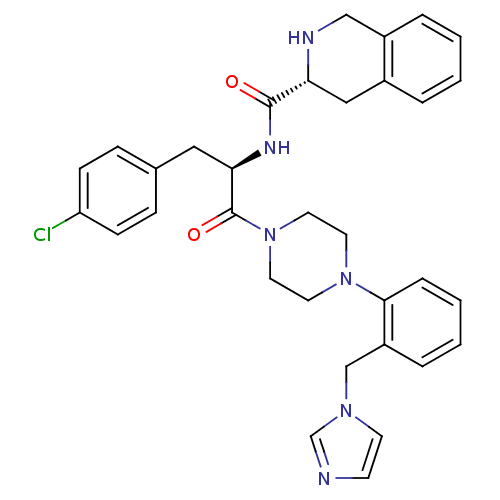 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139029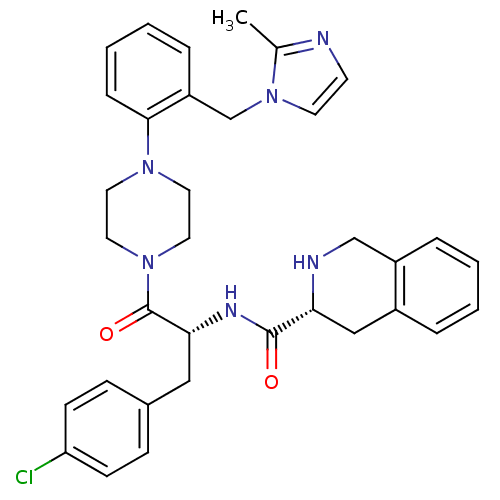 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139036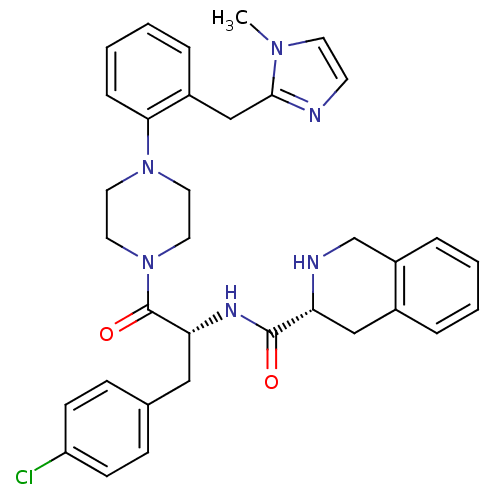 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139045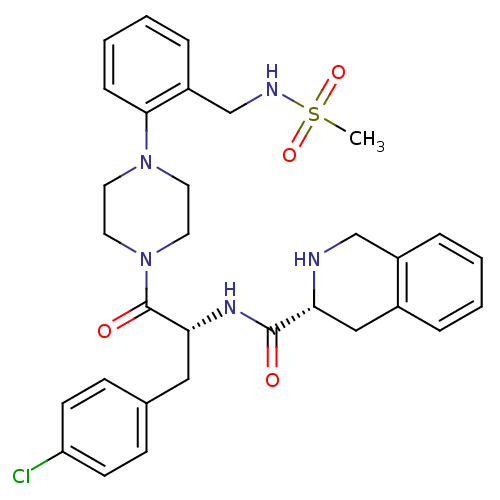 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50134496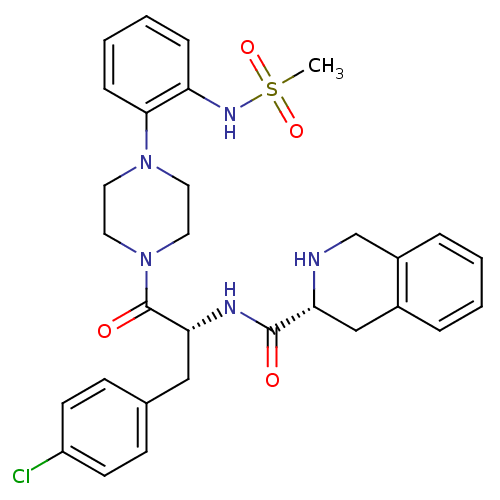 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139047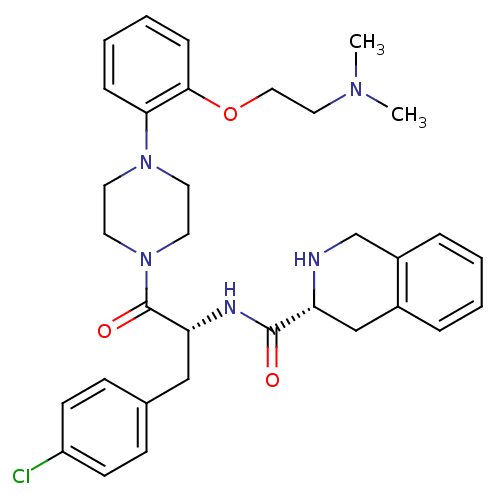 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139048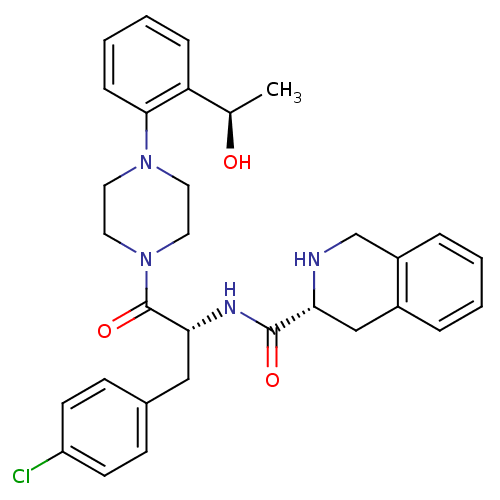 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139044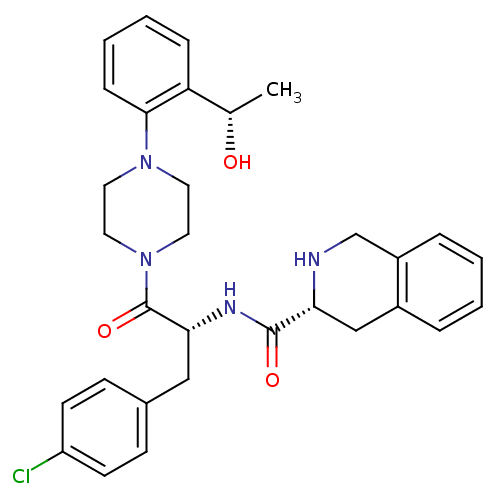 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139040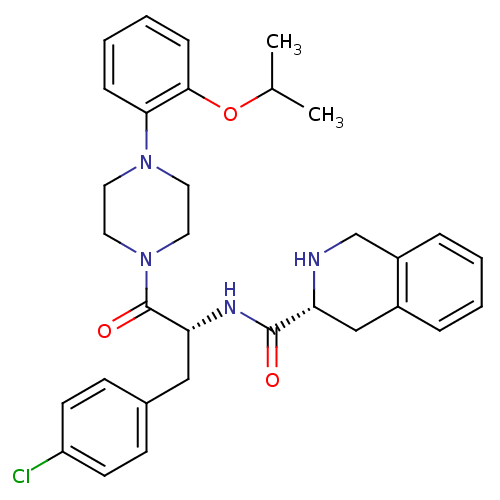 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139025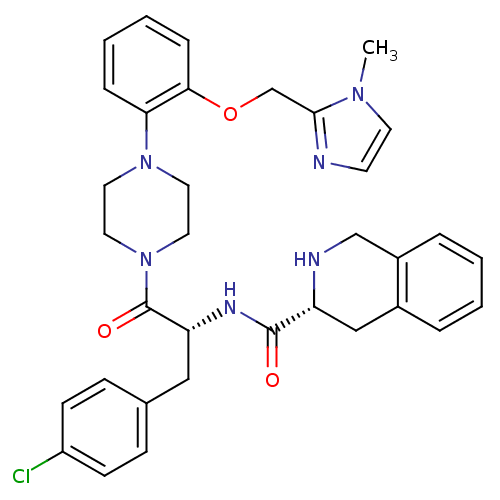 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139022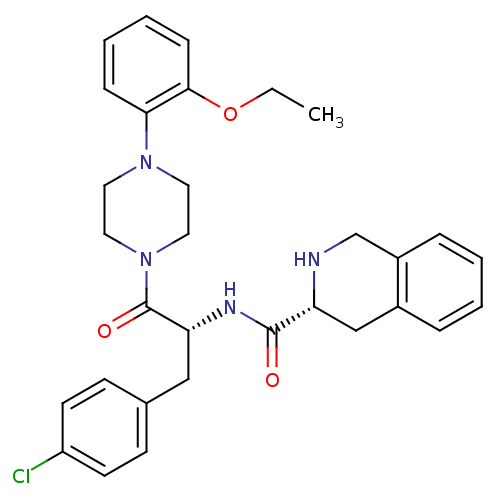 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139038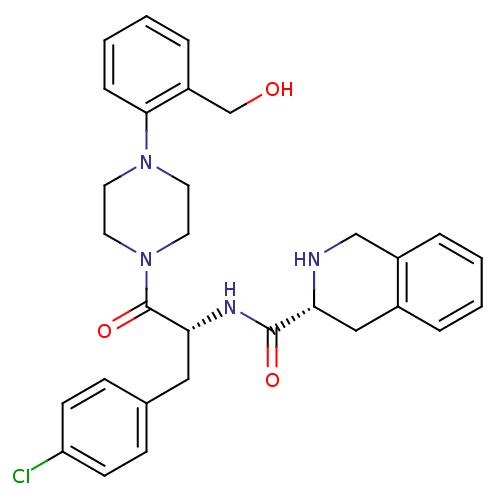 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139031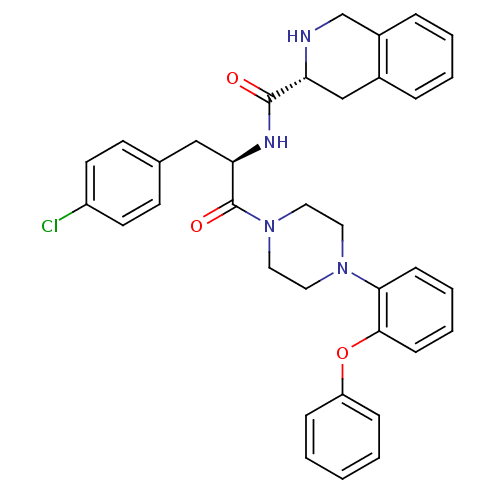 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139030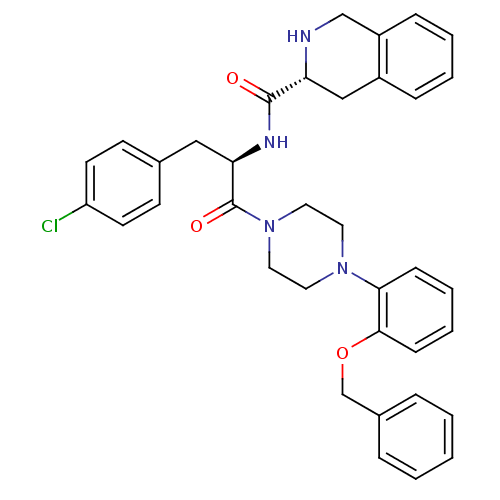 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139039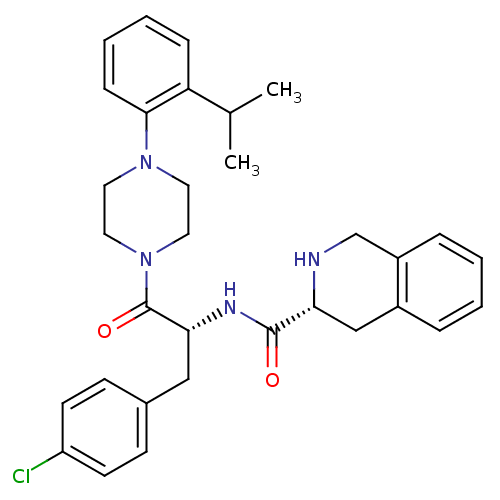 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139041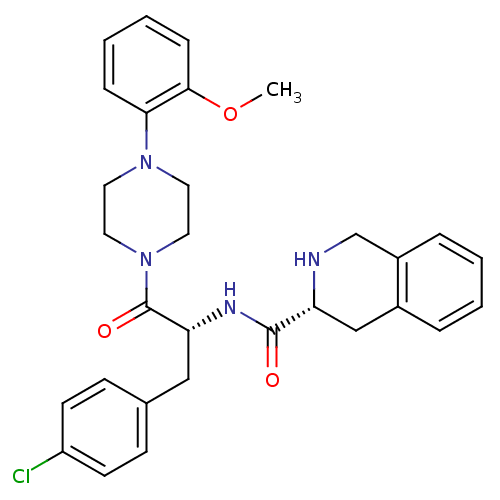 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139042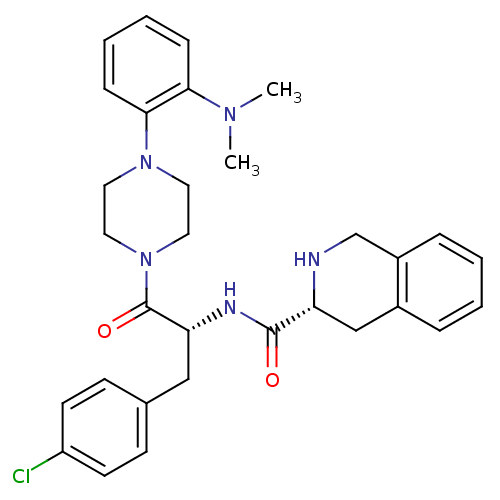 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139034 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 5 receptor (MC5R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139033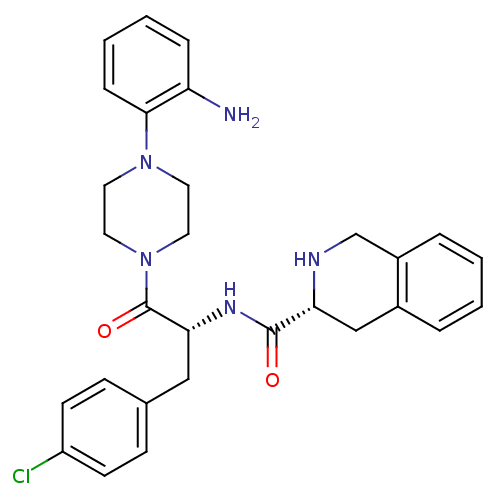 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139035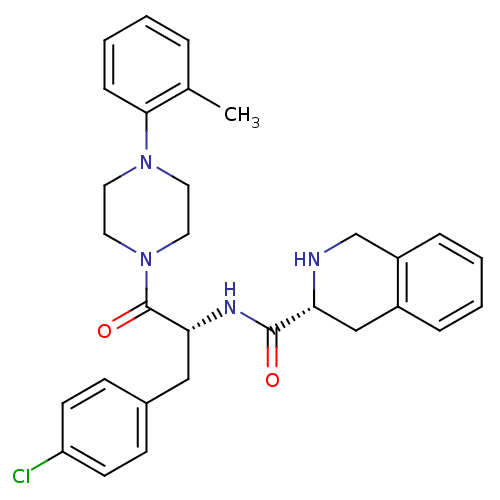 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139026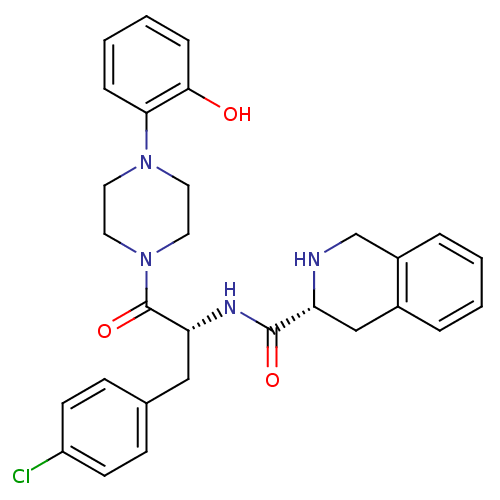 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139037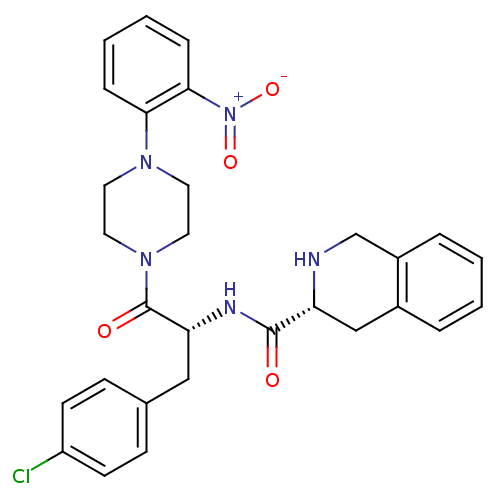 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP release | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50139023 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50139024 (1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor using [125I]NDP-alpha-MSH as a radioligand in HEK293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (MC3R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50134496 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50139028 ((R)-1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (MC1R) using [125I]NDP-alpha-MSH as a radioligand in membranes of HEK 293 cells | J Med Chem 47: 744-55 (2004) Article DOI: 10.1021/jm0304109 BindingDB Entry DOI: 10.7270/Q2DJ5F21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150699 (3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150699 (3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150700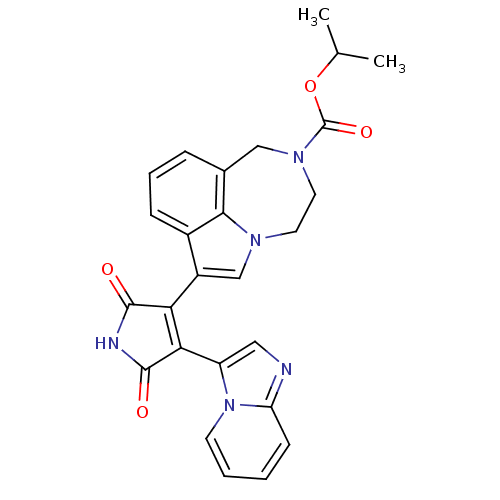 (7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150698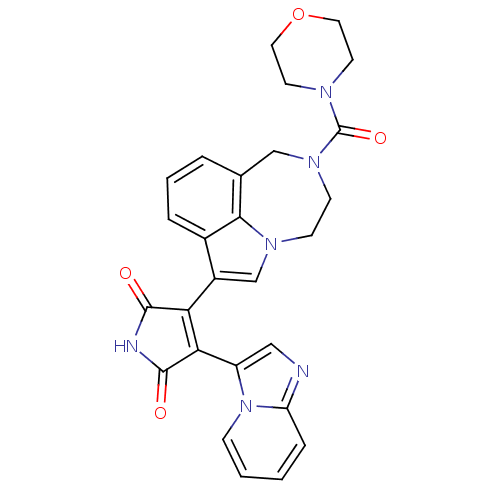 (3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150701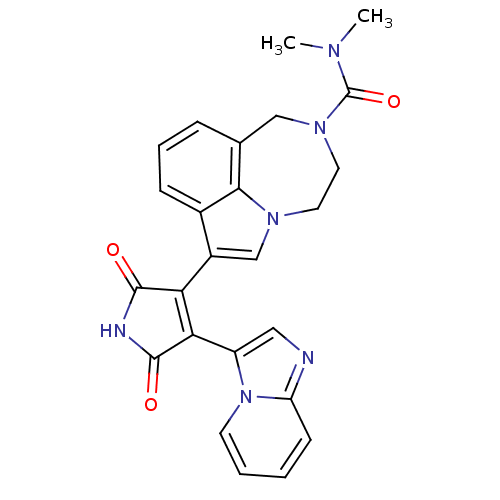 (7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150702 (3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150702 (3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50082933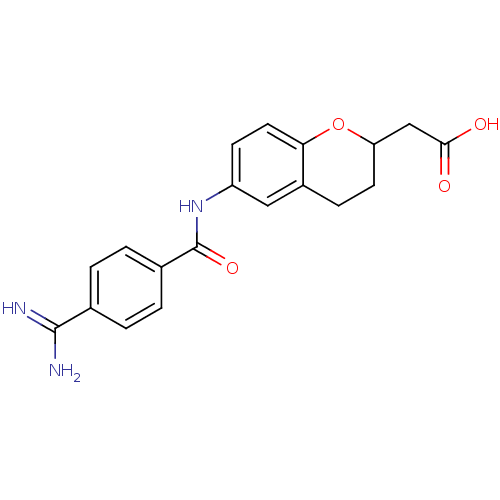 (CHEMBL323720 | [6-(4-Carbamimidoyl-benzoylamino)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) | J Med Chem 42: 4875-89 (1999) BindingDB Entry DOI: 10.7270/Q2NC60DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50082930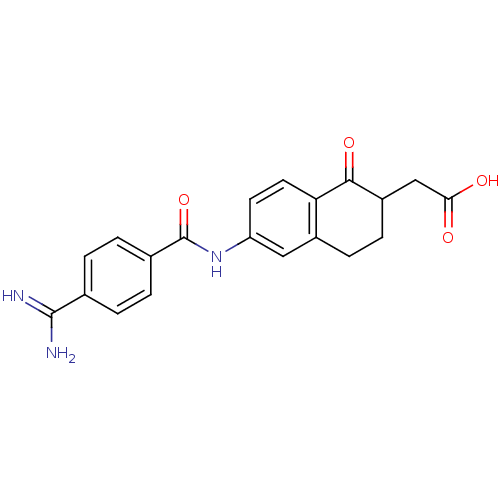 (CHEMBL117662 | [6-(4-Carbamimidoyl-benzoylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of fibrinogen binding to purified human GPIIb-IIIa in ELISA | Bioorg Med Chem Lett 10: 385-9 (2000) BindingDB Entry DOI: 10.7270/Q2FB5252 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50082930 (CHEMBL117662 | [6-(4-Carbamimidoyl-benzoylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of fibrinogen binding to purified human alpha IIb beta3 integrin (GP IIb-IIIa) | J Med Chem 42: 4875-89 (1999) BindingDB Entry DOI: 10.7270/Q2NC60DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50082933 (CHEMBL323720 | [6-(4-Carbamimidoyl-benzoylamino)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of fibrinogen binding to purified human GPIIb-IIIa in ELISA | Bioorg Med Chem Lett 10: 385-9 (2000) BindingDB Entry DOI: 10.7270/Q2FB5252 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50150698 (3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells | J Med Chem 47: 3934-7 (2004) Article DOI: 10.1021/jm049768a BindingDB Entry DOI: 10.7270/Q2B56KG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50085605 (CHEMBL126411 | [6-(4-Carbamimidoyl-2-fluoro-benzoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of fibrinogen binding to purified human GPIIb-IIIa in ELISA | Bioorg Med Chem Lett 10: 385-9 (2000) BindingDB Entry DOI: 10.7270/Q2FB5252 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 167 total ) | Next | Last >> |