

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546246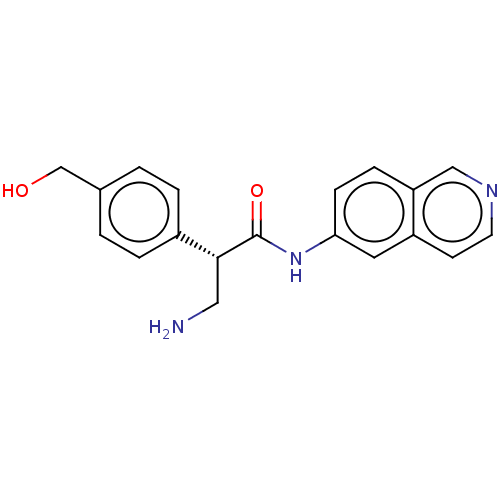 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ROCK1 expressed in baculovirus expression system by Kinase-Glo luminescent Assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b01033 BindingDB Entry DOI: 10.7270/Q2ZK5M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546246 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ROCK2 expressed in baculovirus expression system by Kinase-Glo luminescent Assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b01033 BindingDB Entry DOI: 10.7270/Q2ZK5M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM108255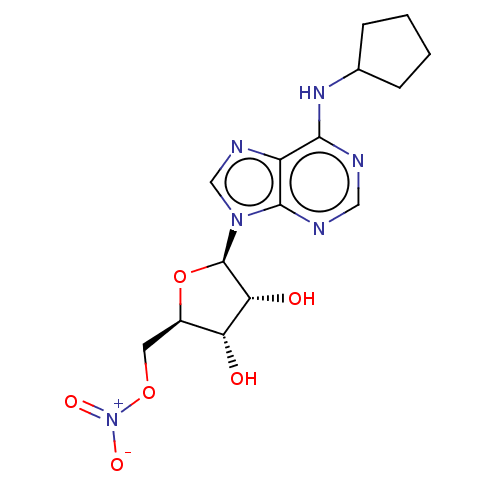 (US8609833, 86) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to A1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b01033 BindingDB Entry DOI: 10.7270/Q2ZK5M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to A3 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b01033 BindingDB Entry DOI: 10.7270/Q2ZK5M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50598611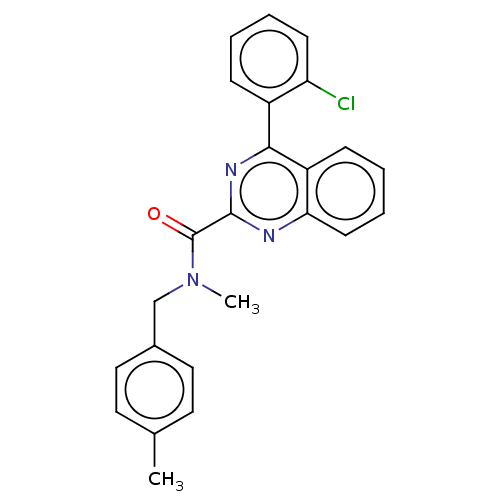 (CHEMBL5187502) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01946 BindingDB Entry DOI: 10.7270/Q2D79GFS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ROCK2 by Kinase-Glo luminescent assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.9b01033 BindingDB Entry DOI: 10.7270/Q2ZK5M83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070947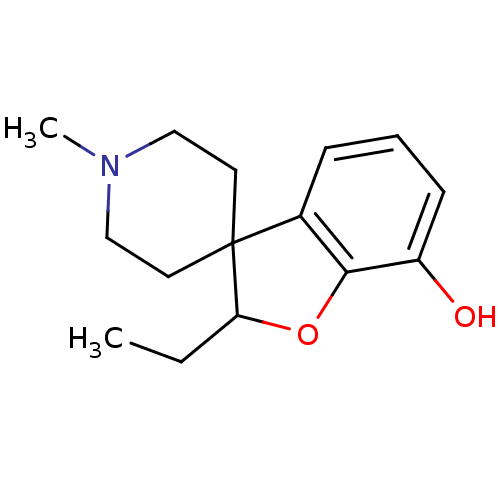 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070948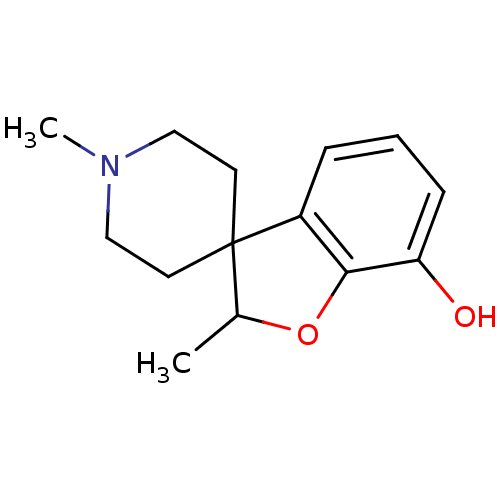 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50026752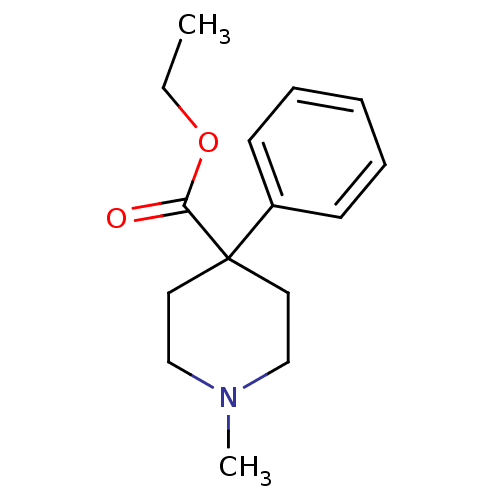 (1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070946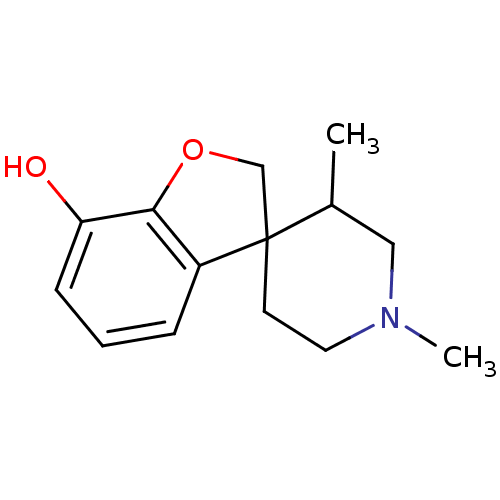 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070947 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070946 (1',3'-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50070949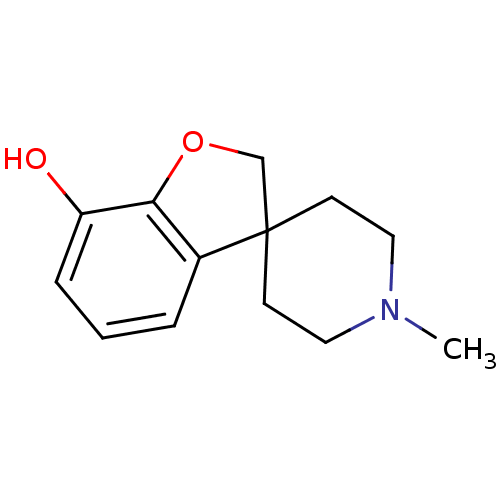 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against mu opioid receptor was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50548039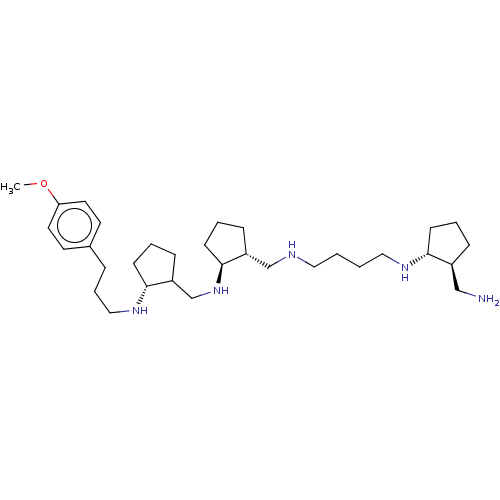 (CHEMBL4798513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LSD1 preincubated for 10 mins followed by H3(1-20)K4-dimethylated (K4me2) peptide substrate addition and measured after 30 mins b... | Citation and Details Article DOI: 10.1039/d0md00141d BindingDB Entry DOI: 10.7270/Q23R0XGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070948 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026752 (1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070948 (1',2-dimethylspiro[2,3-dihydrobenzo[b]furan-3,4'-(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50070949 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070949 (1'-methylspiro[2,3-dihydrobenzo[b]furan-3,4'-(hexa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50070947 (2-ethyl-1'-methylspiro[2,3-dihydrobenzo[b]furan-3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor kappa 1 was determined in brain membrane preparations from male Hartley guinea-pigs | Bioorg Med Chem Lett 8: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2K073D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50603177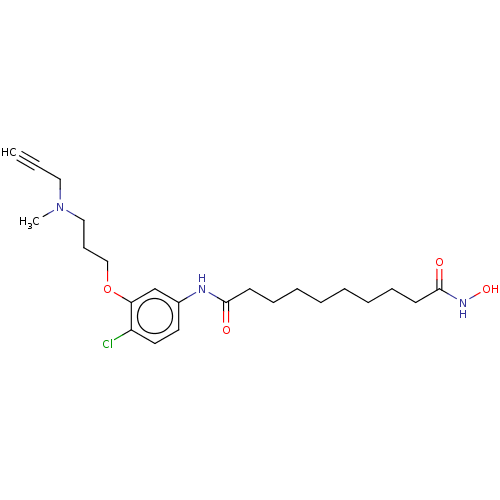 (CHEMBL5200566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01726 BindingDB Entry DOI: 10.7270/Q2FJ2MVK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50245492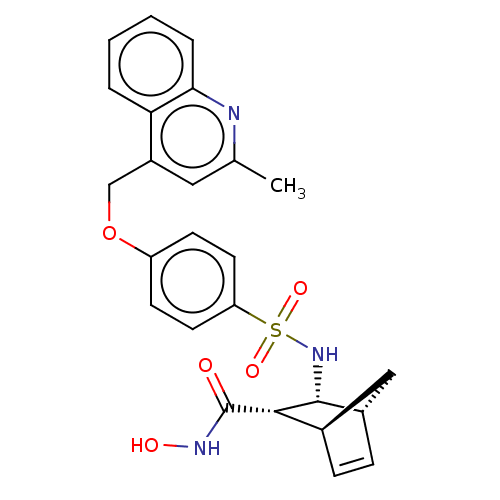 (CHEMBL4082554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant TACE catalytic domain (unknown origin) using pro-TNFalpha peptide Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins ... | J Med Chem 60: 527-553 (2017) Article DOI: 10.1021/acs.jmedchem.6b00935 BindingDB Entry DOI: 10.7270/Q29C70V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241905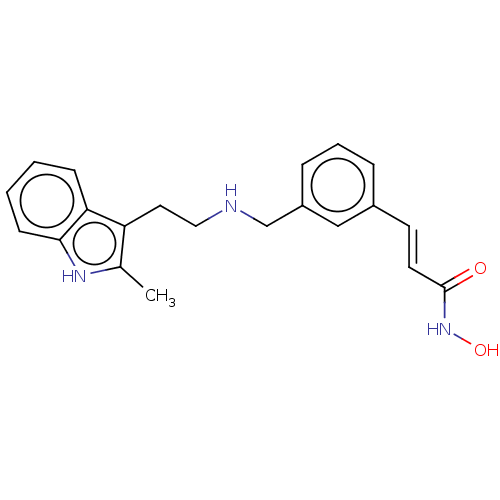 (CHEMBL4080164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505038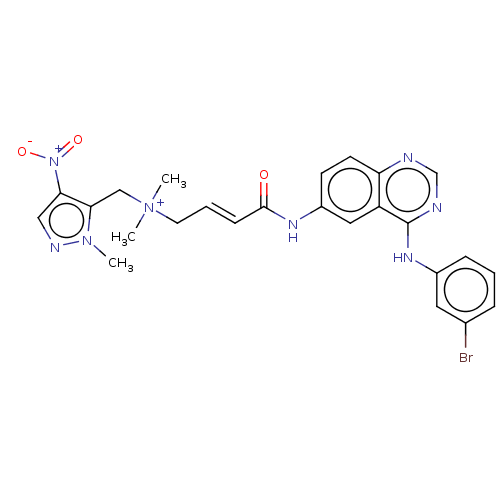 (CHEMBL4516232) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505039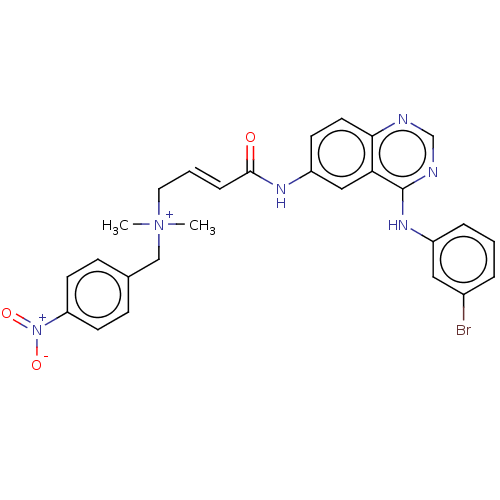 (CHEMBL4439982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505037 (CHEMBL4473277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505036 (CHEMBL4540138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505035 (CHEMBL4527121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50505040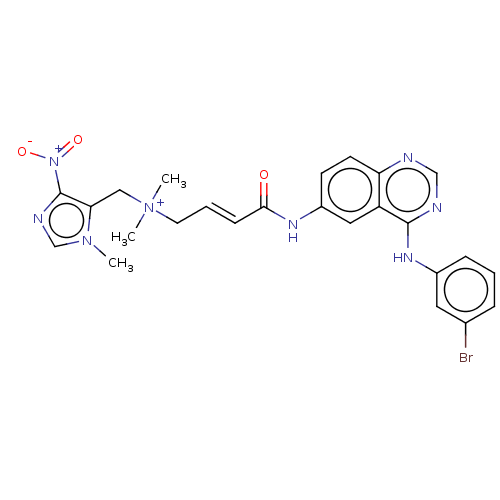 (CHEMBL4560430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of erbB1 (unknown origin) using FRET-capable peptide substrate by FRET assay | J Med Chem 62: 2851-2893 (2019) Article DOI: 10.1021/acs.jmedchem.8b00147 BindingDB Entry DOI: 10.7270/Q2ZP49CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50247556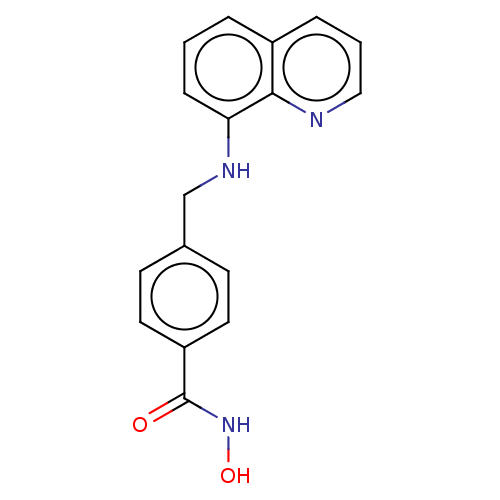 (CHEMBL4104117) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.291 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged HDAC6 expressed in baculovirus infected insect cells using RHK-K(Ac)-AMC as substra... | J Med Chem 61: 905-917 (2018) Article DOI: 10.1021/acs.jmedchem.7b01404 BindingDB Entry DOI: 10.7270/Q2Q81GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50603180 (CHEMBL5178014) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01726 BindingDB Entry DOI: 10.7270/Q2FJ2MVK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241901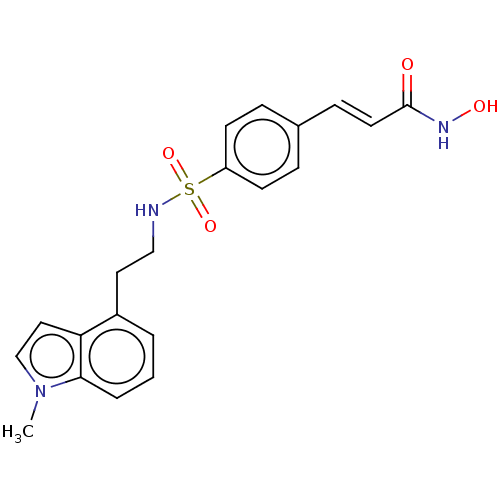 (CHEMBL4065026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50236621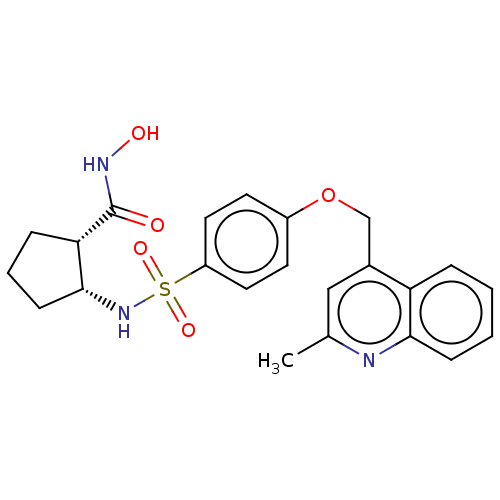 (CHEMBL4078142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant TACE catalytic domain (unknown origin) using pro-TNFalpha peptide Abz-LAQAVRSSSR-Dpa as substrate preincubated for 10 mins ... | J Med Chem 60: 527-553 (2017) Article DOI: 10.1021/acs.jmedchem.6b00935 BindingDB Entry DOI: 10.7270/Q29C70V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00966 BindingDB Entry DOI: 10.7270/Q2X352D4 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM50245498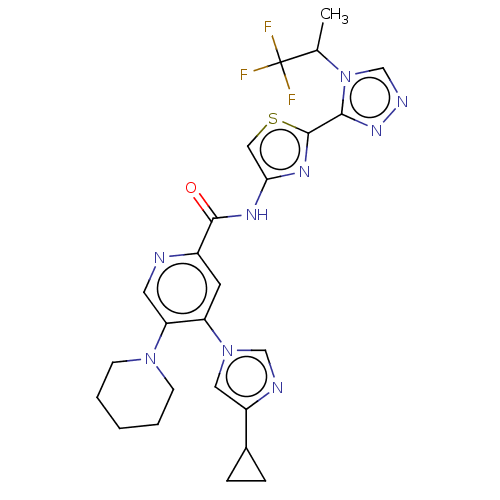 (CHEMBL4083562) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human ASK1 using STK3 peptide as substrate incubated for 30 mins followed by ATP addition measured for 3 hrs by TR-FRET ass... | J Med Chem 60: 527-553 (2017) Article DOI: 10.1021/acs.jmedchem.6b00935 BindingDB Entry DOI: 10.7270/Q29C70V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50247553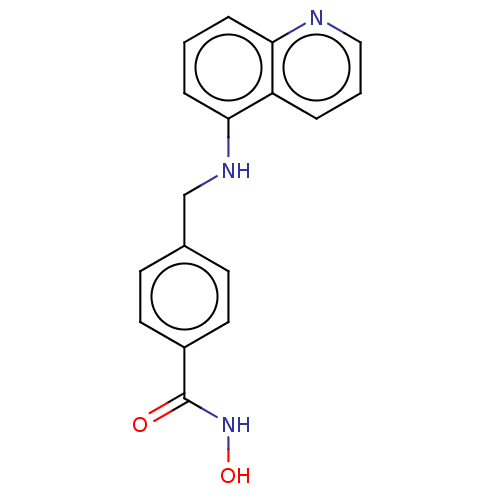 (CHEMBL4095667) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.795 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged HDAC6 expressed in baculovirus infected insect cells using RHK-K(Ac)-AMC as substra... | J Med Chem 61: 905-917 (2018) Article DOI: 10.1021/acs.jmedchem.7b01404 BindingDB Entry DOI: 10.7270/Q2Q81GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50241906 (CHEMBL4069853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC2 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50386657 (CHEMBL2048746) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 after 30 mins by fluorometric assay | J Med Chem 55: 3777-91 (2012) Article DOI: 10.1021/jm300197a BindingDB Entry DOI: 10.7270/Q2CR5VD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01726 BindingDB Entry DOI: 10.7270/Q2FJ2MVK | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241905 (CHEMBL4080164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114602 BindingDB Entry DOI: 10.7270/Q2CJ8JF1 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241906 (CHEMBL4069853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50241901 (CHEMBL4065026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University (TMU) Curated by ChEMBL | Assay Description Inhibition of recombinant human full length HDAC1 using Fluor-de-Lys as substrate after 60 mins by spectrofluorimetric analysis | Eur J Med Chem 134: 13-23 (2017) Article DOI: 10.1016/j.ejmech.2017.03.079 BindingDB Entry DOI: 10.7270/Q2154K5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 after 30 mins by fluorometric assay | J Med Chem 55: 3777-91 (2012) Article DOI: 10.1021/jm300197a BindingDB Entry DOI: 10.7270/Q2CR5VD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24346 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111725 BindingDB Entry DOI: 10.7270/Q2V1286G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 971 total ) | Next | Last >> |