 Found 104 hits with Last Name = 'liston' and Initial = 'dr'
Found 104 hits with Last Name = 'liston' and Initial = 'dr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
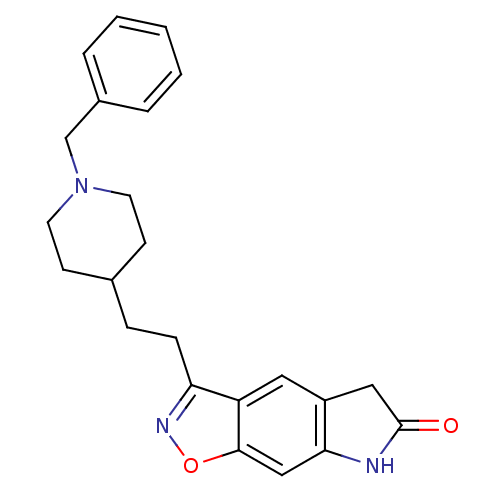
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032161
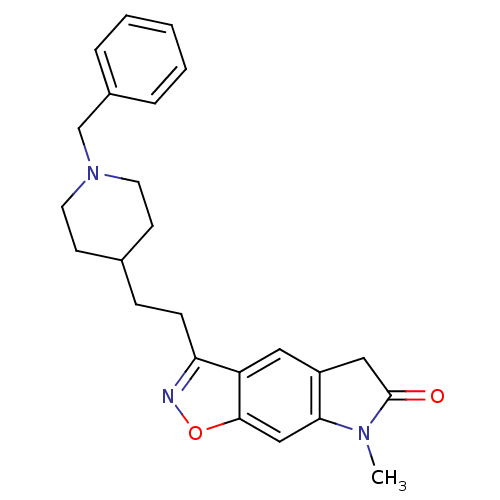
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-23-20(13-19(22)14-24(26)28)21(25-29-23)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
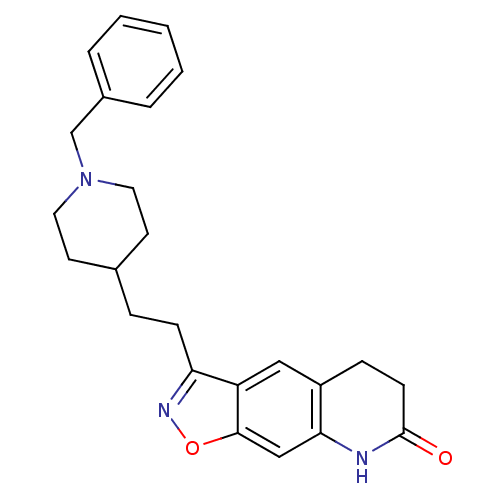
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039721
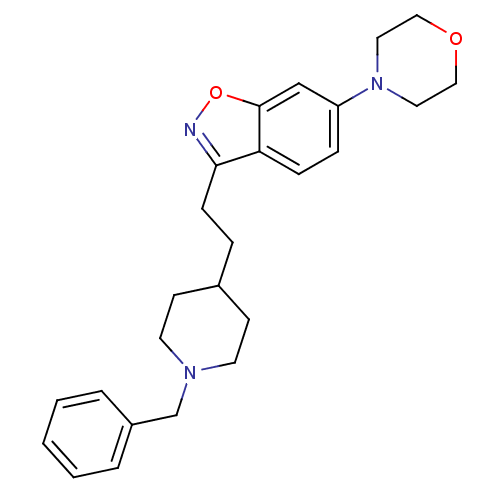
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...)Show SMILES C(Cc1noc2cc(ccc12)N1CCOCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O2/c1-2-4-21(5-3-1)19-27-12-10-20(11-13-27)6-9-24-23-8-7-22(18-25(23)30-26-24)28-14-16-29-17-15-28/h1-5,7-8,18,20H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
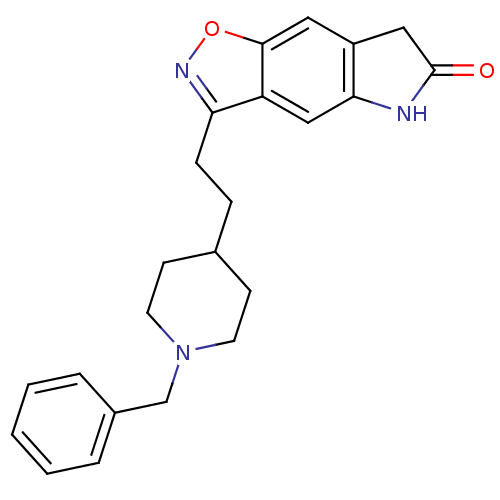
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
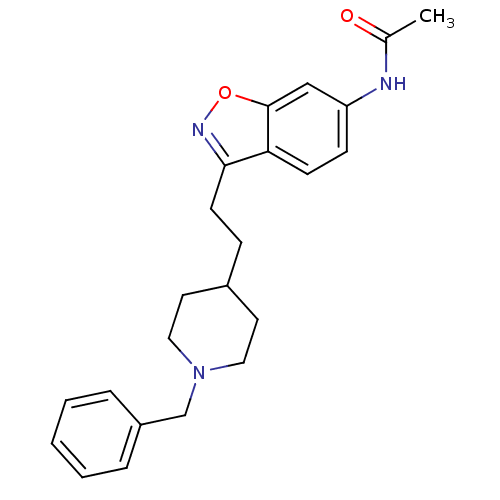
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165

(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
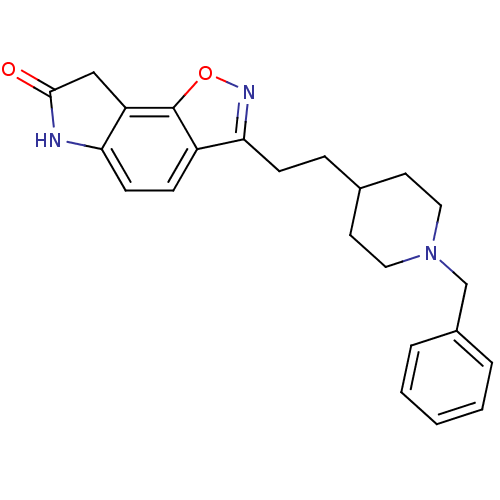
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034001
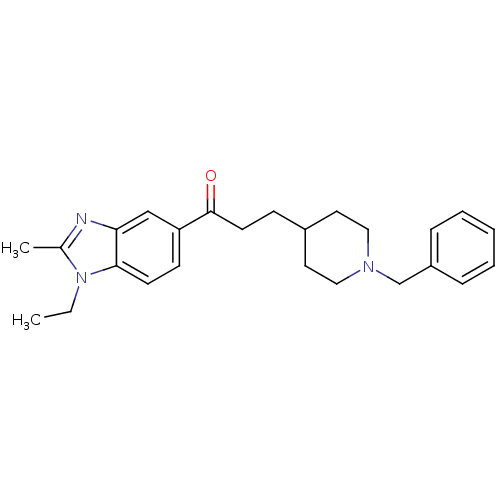
(3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...)Show SMILES CCn1c(C)nc2cc(ccc12)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O/c1-3-28-19(2)26-23-17-22(10-11-24(23)28)25(29)12-9-20-13-15-27(16-14-20)18-21-7-5-4-6-8-21/h4-8,10-11,17,20H,3,9,12-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039729
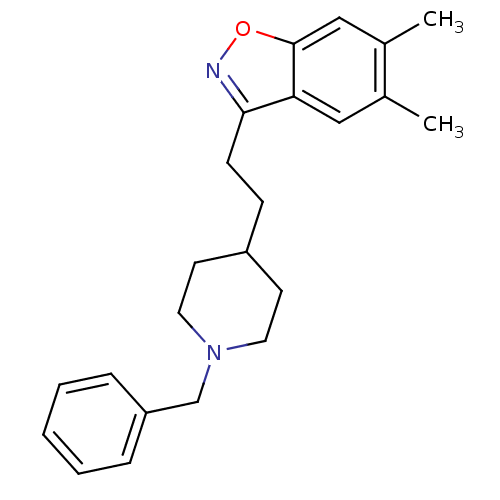
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...)Show InChI InChI=1S/C23H28N2O/c1-17-14-21-22(24-26-23(21)15-18(17)2)9-8-19-10-12-25(13-11-19)16-20-6-4-3-5-7-20/h3-7,14-15,19H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Butyrylcholinesterase obtained from human serum was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Butyrylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034002

(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-benzothiaz...)Show InChI InChI=1S/C23H26N2OS/c1-17-24-21-9-8-20(15-23(21)27-17)22(26)10-7-18-11-13-25(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
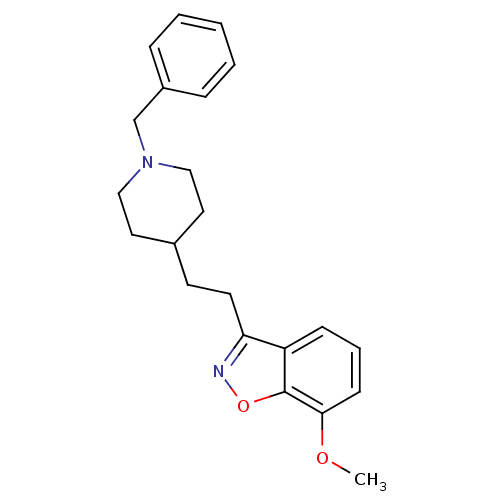
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039712
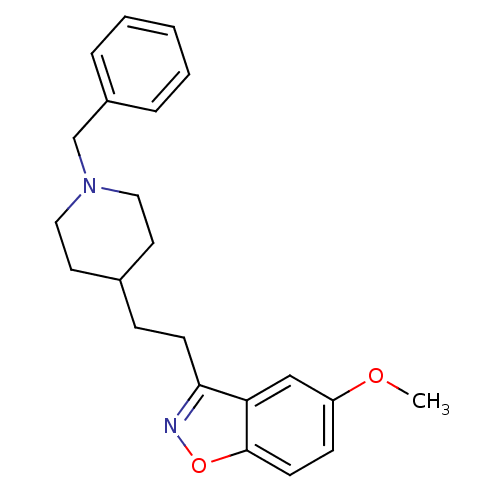
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-10-22-20(15-19)21(23-26-22)9-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8,10,15,17H,7,9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039728
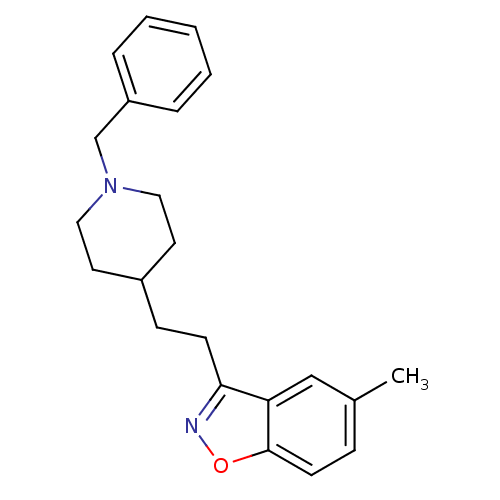
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...)Show InChI InChI=1S/C22H26N2O/c1-17-7-10-22-20(15-17)21(23-25-22)9-8-18-11-13-24(14-12-18)16-19-5-3-2-4-6-19/h2-7,10,15,18H,8-9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039717
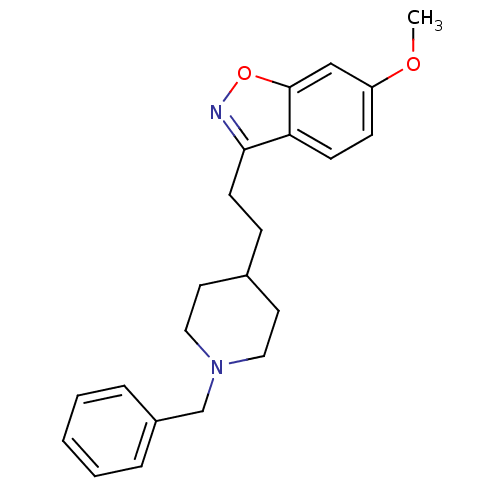
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-9-20-21(23-26-22(20)15-19)10-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039723
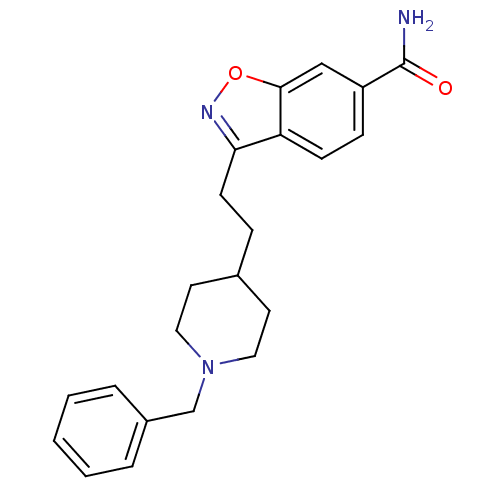
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H25N3O2/c23-22(26)18-7-8-19-20(24-27-21(19)14-18)9-6-16-10-12-25(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039727
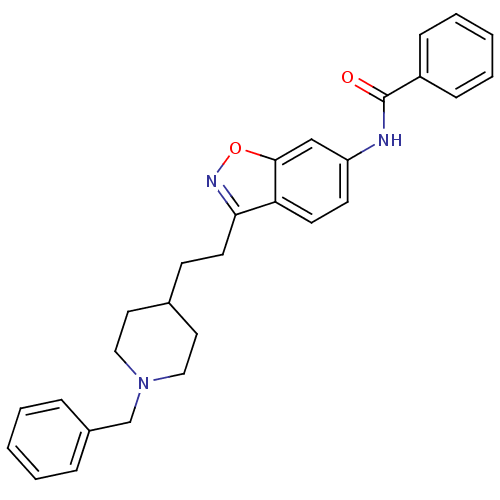
(CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=C(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c32-28(23-9-5-2-6-10-23)29-24-12-13-25-26(30-33-27(25)19-24)14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034003
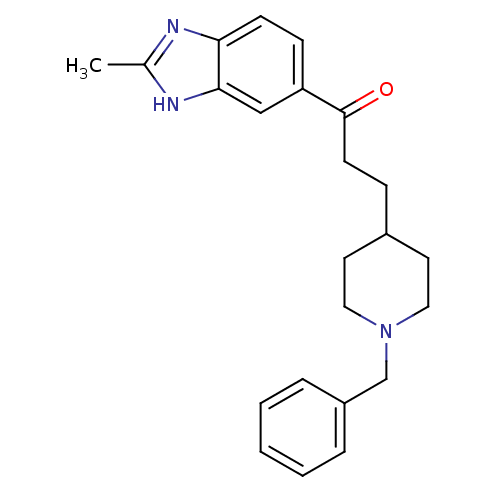
(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-1H-benzoim...)Show SMILES Cc1nc2ccc(cc2[nH]1)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H27N3O/c1-17-24-21-9-8-20(15-22(21)25-17)23(27)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039725
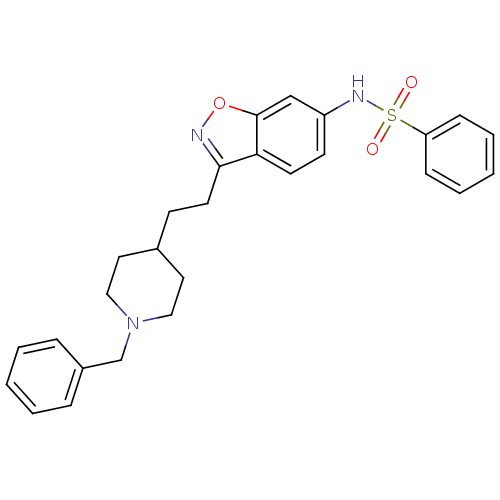
(CHEMBL92955 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=S(=O)(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C27H29N3O3S/c31-34(32,24-9-5-2-6-10-24)29-23-12-13-25-26(28-33-27(25)19-23)14-11-21-15-17-30(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21,29H,11,14-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039719

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H25N3O/c22-18-7-8-19-20(23-25-21(19)14-18)9-6-16-10-12-24(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039731

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O2/c24-18-7-8-19-20(22-25-21(19)14-18)9-6-16-10-12-23(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16,24H,6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034000
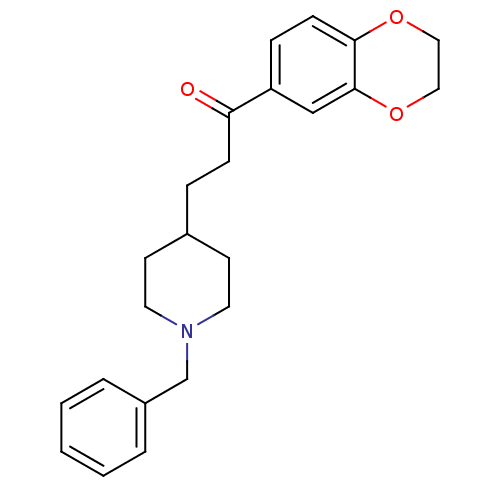
(3-(1-Benzyl-piperidin-4-yl)-1-(2,3-dihydro-benzo[1...)Show InChI InChI=1S/C23H27NO3/c25-21(20-7-9-22-23(16-20)27-15-14-26-22)8-6-18-10-12-24(13-11-18)17-19-4-2-1-3-5-19/h1-5,7,9,16,18H,6,8,10-15,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034004
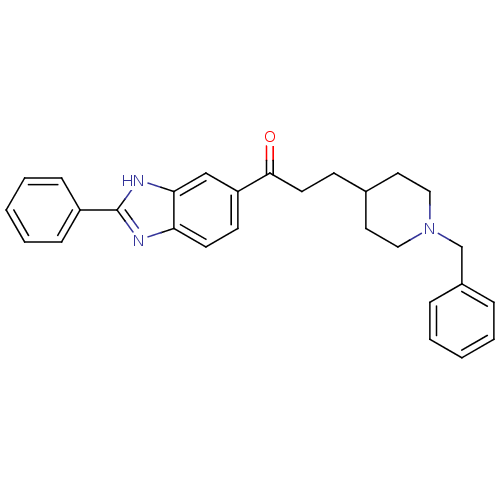
(3-(1-Benzyl-piperidin-4-yl)-1-(2-phenyl-1H-benzoim...)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc2nc([nH]c2c1)-c1ccccc1 Show InChI InChI=1S/C28H29N3O/c32-27(14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22)24-12-13-25-26(19-24)30-28(29-25)23-9-5-2-6-10-23/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279984

(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of acetylcholinesterase isolated from human erythrocytes. |
Bioorg Med Chem Lett 1: 411-414 (1991)
Article DOI: 10.1016/S0960-894X(00)80267-X
BindingDB Entry DOI: 10.7270/Q2G73DN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279990
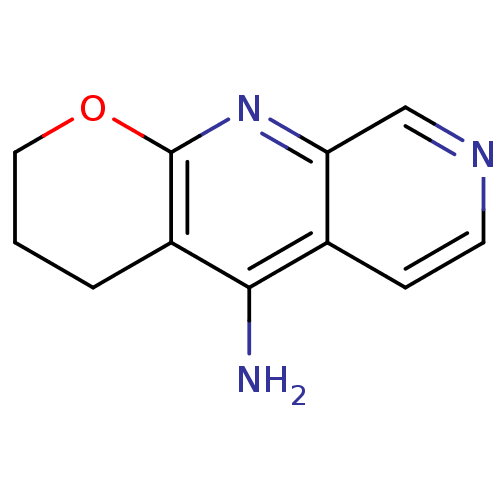
(10-Amino-3,4-dihydro-2H-1-oxa-7-aza-9-azonia-anthr...)Show InChI InChI=1S/C11H11N3O/c12-10-7-3-4-13-6-9(7)14-11-8(10)2-1-5-15-11/h3-4,6H,1-2,5H2,(H2,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of acetylcholinesterase isolated from human erythrocytes. |
Bioorg Med Chem Lett 1: 411-414 (1991)
Article DOI: 10.1016/S0960-894X(00)80267-X
BindingDB Entry DOI: 10.7270/Q2G73DN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039734

(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-bromobenzo[d...)Show InChI InChI=1S/C21H23BrN2O/c22-18-7-8-19-20(23-25-21(19)14-18)9-6-16-10-12-24(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150885
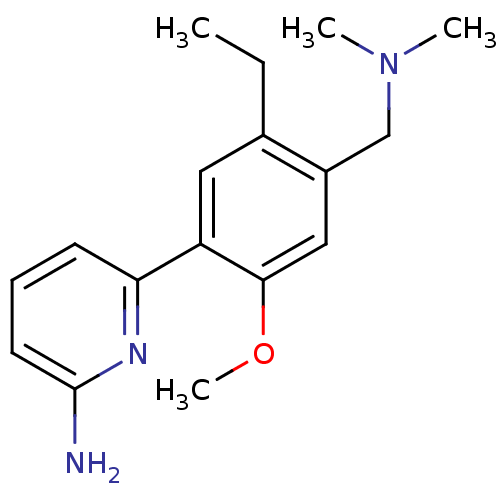
(6-(4-Dimethylaminomethyl-5-ethyl-2-methoxy-phenyl)...)Show InChI InChI=1S/C17H23N3O/c1-5-12-9-14(15-7-6-8-17(18)19-15)16(21-4)10-13(12)11-20(2)3/h6-10H,5,11H2,1-4H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039716
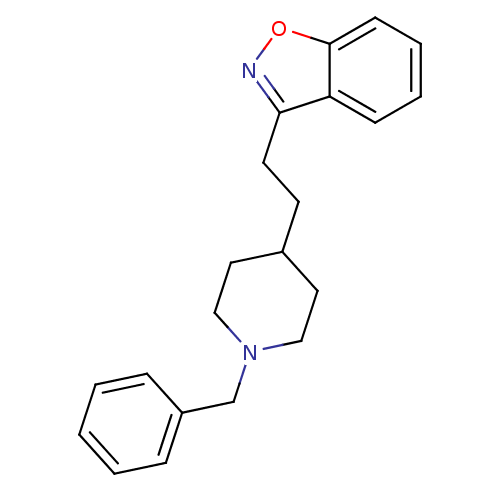
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O/c1-2-6-18(7-3-1)16-23-14-12-17(13-15-23)10-11-20-19-8-4-5-9-21(19)24-22-20/h1-9,17H,10-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150887
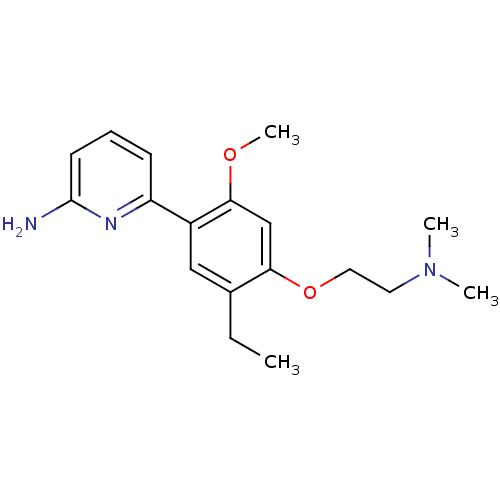
(6-[4-(2-Dimethylamino-ethoxy)-5-ethyl-2-methoxy-ph...)Show InChI InChI=1S/C18H25N3O2/c1-5-13-11-14(15-7-6-8-18(19)20-15)17(22-4)12-16(13)23-10-9-21(2)3/h6-8,11-12H,5,9-10H2,1-4H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Butyrylcholinesterase obtained from human serum was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Butyrylcholinesterase from human serum |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150890
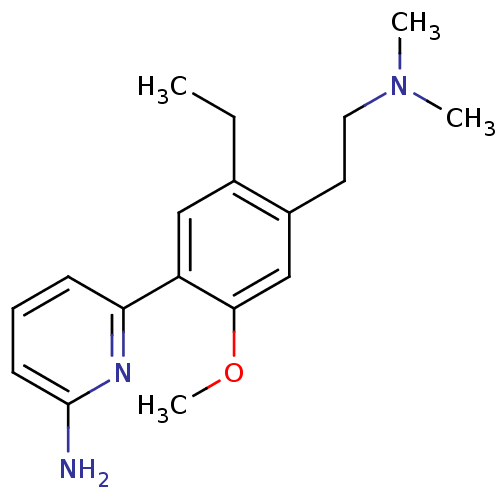
(6-[4-(2-Dimethylamino-ethyl)-5-ethyl-2-methoxy-phe...)Show InChI InChI=1S/C18H25N3O/c1-5-13-11-15(16-7-6-8-18(19)20-16)17(22-4)12-14(13)9-10-21(2)3/h6-8,11-12H,5,9-10H2,1-4H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150884

(6-[4-(3-Dimethylamino-propyl)-5-ethyl-2-methoxy-ph...)Show InChI InChI=1S/C19H27N3O/c1-5-14-12-16(17-9-6-10-19(20)21-17)18(23-4)13-15(14)8-7-11-22(2)3/h6,9-10,12-13H,5,7-8,11H2,1-4H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279995
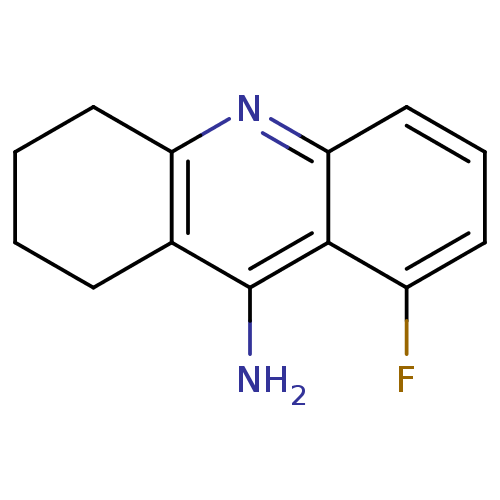
(8-Fluoro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13FN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of acetylcholinesterase isolated from human erythrocytes. |
Bioorg Med Chem Lett 1: 411-414 (1991)
Article DOI: 10.1016/S0960-894X(00)80267-X
BindingDB Entry DOI: 10.7270/Q2G73DN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279983

(8-Methyl-1,2,3,4-tetrahydro-acridin-9-ylamine | CH...)Show InChI InChI=1S/C14H16N2/c1-9-5-4-8-12-13(9)14(15)10-6-2-3-7-11(10)16-12/h4-5,8H,2-3,6-7H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of acetylcholinesterase isolated from human erythrocytes. |
Bioorg Med Chem Lett 1: 411-414 (1991)
Article DOI: 10.1016/S0960-894X(00)80267-X
BindingDB Entry DOI: 10.7270/Q2G73DN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039715
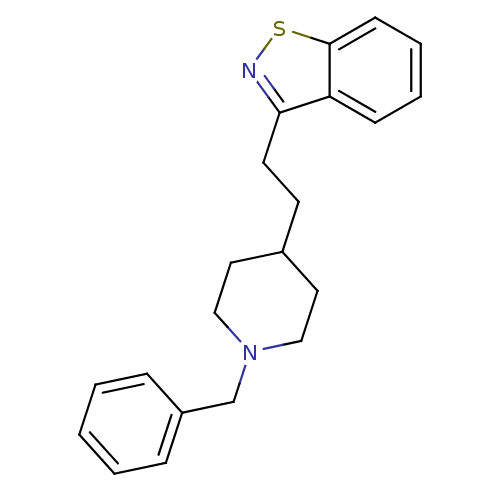
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-benzo[d]isot...)Show InChI InChI=1S/C21H24N2S/c1-2-6-18(7-3-1)16-23-14-12-17(13-15-23)10-11-20-19-8-4-5-9-21(19)24-22-20/h1-9,17H,10-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039718

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H23N3O/c23-15-19-6-8-20-21(24-26-22(20)14-19)9-7-17-10-12-25(13-11-17)16-18-4-2-1-3-5-18/h1-6,8,14,17H,7,9-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150883
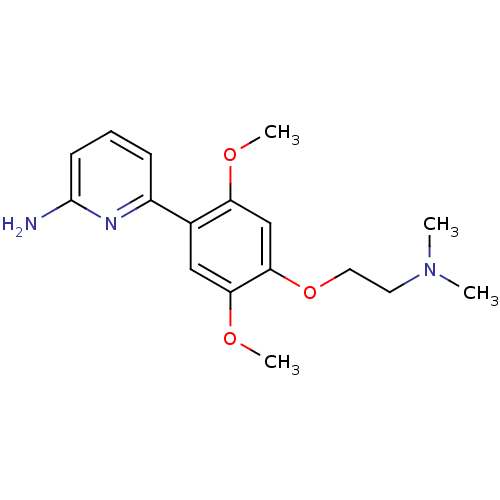
(6-[4-(2-Dimethylamino-ethoxy)-2,5-dimethoxy-phenyl...)Show InChI InChI=1S/C17H23N3O3/c1-20(2)8-9-23-16-11-14(21-3)12(10-15(16)22-4)13-6-5-7-17(18)19-13/h5-7,10-11H,8-9H2,1-4H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039720

(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1H-indazole ...)Show InChI InChI=1S/C21H25N3/c1-2-6-18(7-3-1)16-24-14-12-17(13-15-24)10-11-21-19-8-4-5-9-20(19)22-23-21/h1-9,17H,10-16H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081082
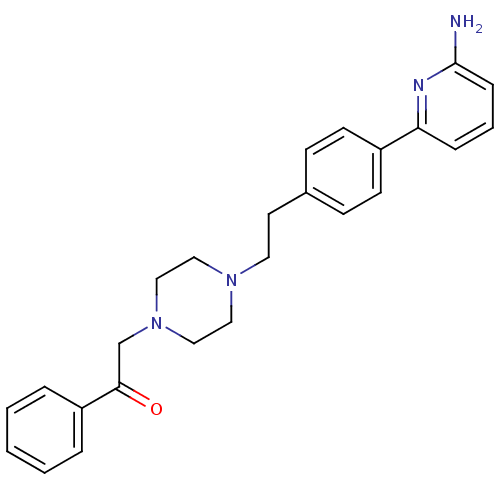
(2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...)Show SMILES Nc1cccc(n1)-c1ccc(CCN2CCN(CC(=O)c3ccccc3)CC2)cc1 Show InChI InChI=1S/C25H28N4O/c26-25-8-4-7-23(27-25)21-11-9-20(10-12-21)13-14-28-15-17-29(18-16-28)19-24(30)22-5-2-1-3-6-22/h1-12H,13-19H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase in human |
Bioorg Med Chem Lett 14: 4511-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.043
BindingDB Entry DOI: 10.7270/Q28S4QP6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of acetylcholinesterase isolated from human erythrocytes. |
Bioorg Med Chem Lett 1: 411-414 (1991)
Article DOI: 10.1016/S0960-894X(00)80267-X
BindingDB Entry DOI: 10.7270/Q2G73DN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data