 Found 176 hits with Last Name = 'little' and Initial = 'e'
Found 176 hits with Last Name = 'little' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Human rhinovirus B) | BDBM50065621

(CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)\C=C1/CCCOC1=O Show InChI InChI=1S/C33H42N4O7/c1-22(2)18-27(37-33(42)44-21-24-12-7-4-8-13-24)31(40)36-28(19-23-10-5-3-6-11-23)30(39)35-26(15-16-29(34)38)20-25-14-9-17-43-32(25)41/h3-8,10-13,20,22,26-28H,9,14-19,21H2,1-2H3,(H2,34,38)(H,35,39)(H,36,40)(H,37,42)/b25-20+/t26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) |
J Med Chem 41: 2806-18 (1998)
Article DOI: 10.1021/jm980068d
BindingDB Entry DOI: 10.7270/Q29G5KZQ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288762
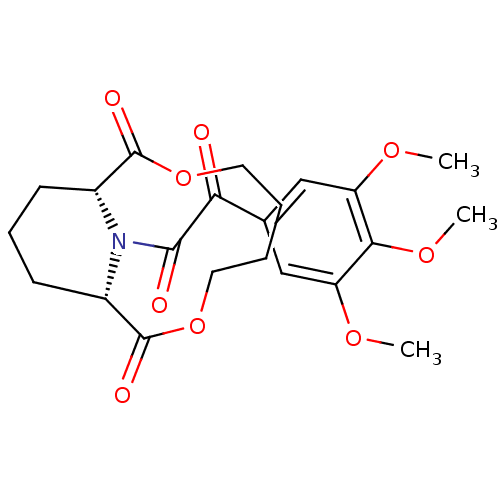
((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCCOC2=O Show InChI InChI=1S/C22H27NO9/c1-28-16-11-13(12-17(29-2)19(16)30-3)18(24)20(25)23-14-7-6-8-15(23)22(27)32-10-5-4-9-31-21(14)26/h11-12,14-15H,4-10H2,1-3H3/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288763

((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(COCc1ccccc1)COC2=O Show InChI InChI=1S/C29H33NO10/c1-35-23-12-20(13-24(36-2)26(23)37-3)25(31)27(32)30-21-10-7-11-22(30)29(34)40-17-19(16-39-28(21)33)15-38-14-18-8-5-4-6-9-18/h4-6,8-9,12-13,19,21-22H,7,10-11,14-17H2,1-3H3/t19?,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288765

((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO[Si](C)(C)C(C)(C)C)COC2=O Show InChI InChI=1S/C28H41NO10Si/c1-28(2,3)40(7,8)39-16-17-14-37-26(32)19-10-9-11-20(27(33)38-15-17)29(19)25(31)23(30)18-12-21(34-4)24(36-6)22(13-18)35-5/h12-13,17,19-20H,9-11,14-16H2,1-8H3/t17?,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288764
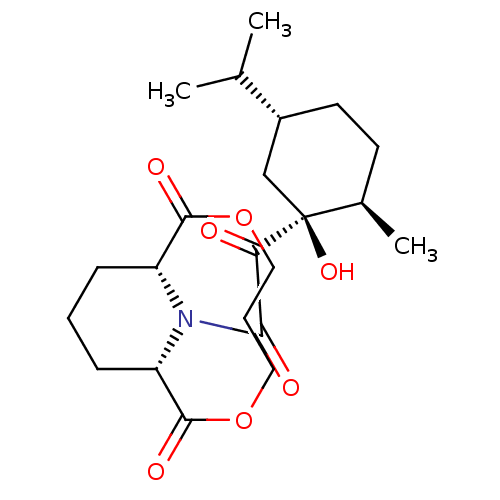
((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C22H33NO7/c1-13(2)15-9-8-14(3)22(28,12-15)18(24)19(25)23-16-6-4-7-17(23)21(27)30-11-5-10-29-20(16)26/h13-17,28H,4-12H2,1-3H3/t14-,15-,16-,17+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288768
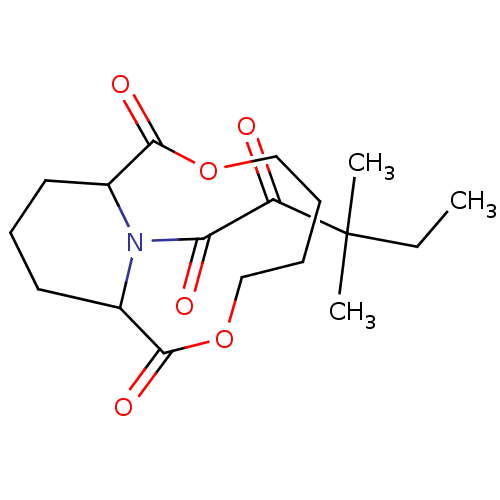
((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...)Show SMILES CCC(C)(C)C(=O)C(=O)N1C2CCCC1C(=O)OCCCCOC2=O Show InChI InChI=1S/C18H27NO6/c1-4-18(2,3)14(20)15(21)19-12-8-7-9-13(19)17(23)25-11-6-5-10-24-16(12)22/h12-13H,4-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288767
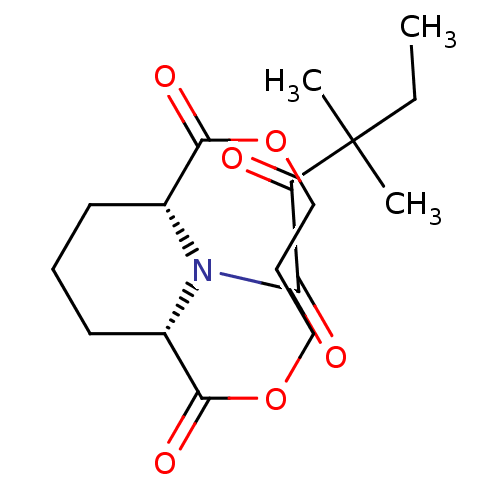
((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...)Show SMILES CCC(C)(C)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C17H25NO6/c1-4-17(2,3)13(19)14(20)18-11-7-5-8-12(18)16(22)24-10-6-9-23-15(11)21/h11-12H,4-10H2,1-3H3/t11-,12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288766
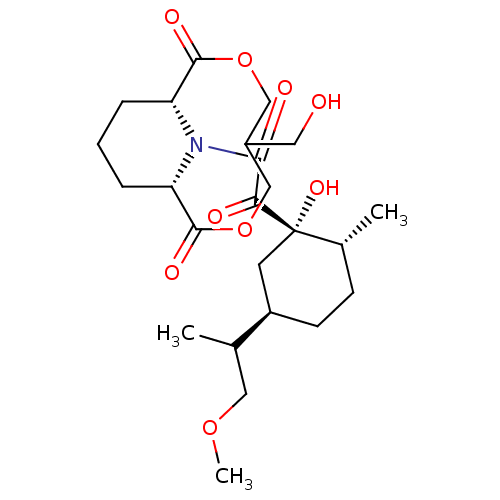
((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...)Show SMILES COCC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO)COC2=O Show InChI InChI=1S/C24H37NO9/c1-14(11-32-3)17-8-7-15(2)24(31,9-17)20(27)21(28)25-18-5-4-6-19(25)23(30)34-13-16(10-26)12-33-22(18)29/h14-19,26,31H,4-13H2,1-3H3/t14?,15-,16?,17-,18-,19+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288769
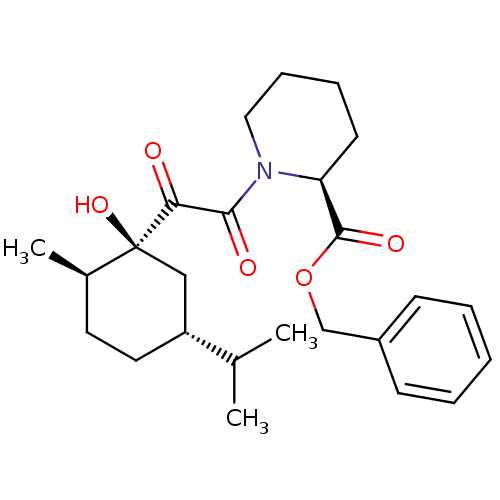
((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C25H35NO5/c1-17(2)20-13-12-18(3)25(30,15-20)22(27)23(28)26-14-8-7-11-21(26)24(29)31-16-19-9-5-4-6-10-19/h4-6,9-10,17-18,20-21,30H,7-8,11-16H2,1-3H3/t18-,20-,21+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572440

(CHEMBL4469438)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572444
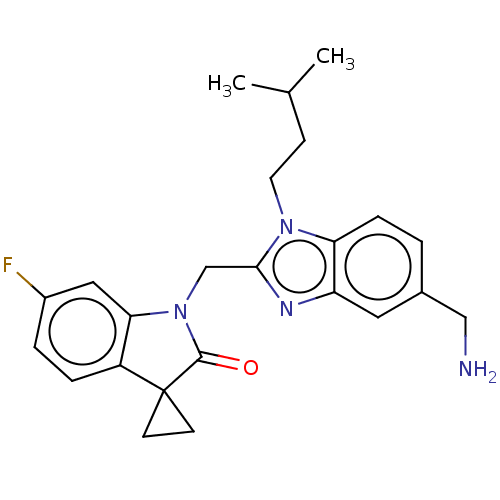
(CHEMBL4878302)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(F)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572466

(CHEMBL4876200)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CCOCC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572442

(CHEMBL4872095)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cc(F)ccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572450
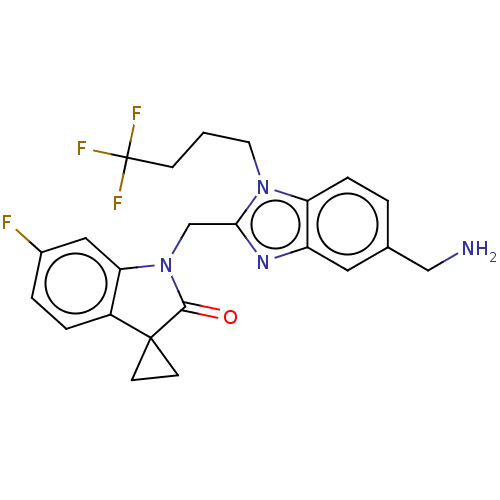
(Rv-521 | Rv521 | Sisunatovir)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572463

(CHEMBL4871101)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccnc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572464

(CHEMBL4858040)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccncc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572451
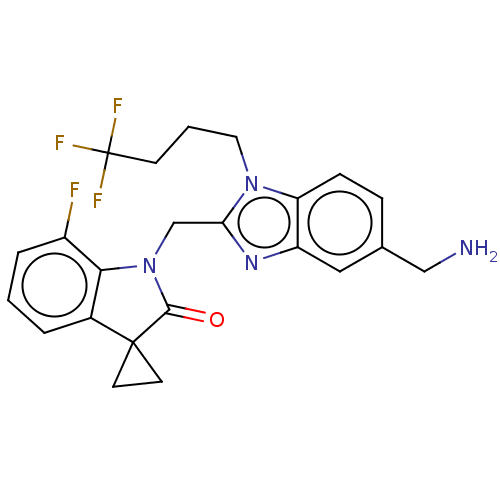
(CHEMBL4867108)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4cccc(F)c34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572465
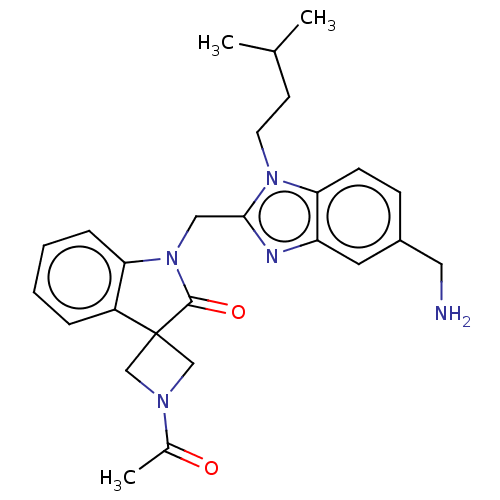
(CHEMBL4858949)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CN(C3)C(C)=O)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572445

(CHEMBL4865906)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(Cl)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572453

(CHEMBL4874709)Show SMILES NCc1ccc2n(C3CCOCC3)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572447
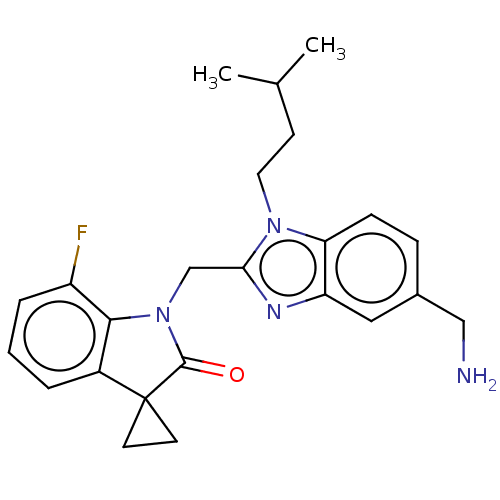
(CHEMBL4860271)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccc(F)c23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572449

(CHEMBL4853602)Show SMILES NCc1ccc2n(CCCCO)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572452
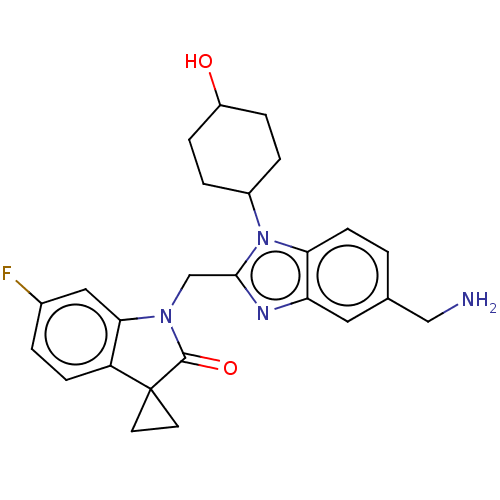
(CHEMBL4873528)Show SMILES NCc1ccc2n(C3CCC(O)CC3)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 |(23.93,-12.4,;22.59,-11.64,;21.26,-12.41,;21.26,-13.96,;19.93,-14.74,;18.59,-13.97,;17.13,-14.45,;16.66,-15.91,;15.16,-16.23,;14.69,-17.69,;15.72,-18.84,;15.24,-20.3,;17.23,-18.52,;17.7,-17.05,;16.22,-13.21,;14.68,-13.21,;13.91,-11.88,;14.52,-10.48,;16.02,-10.15,;13.36,-9.45,;12.58,-8.11,;14.13,-8.11,;12.04,-10.23,;10.58,-9.76,;9.44,-10.79,;9.77,-12.3,;8.64,-13.34,;11.23,-12.76,;12.37,-11.73,;17.12,-11.96,;18.59,-12.43,;19.92,-11.65,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572448
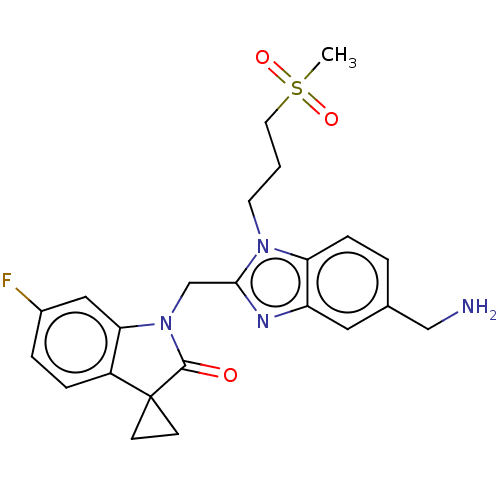
(CHEMBL4863172)Show SMILES CS(=O)(=O)CCCn1c(CN2C(=O)C3(CC3)c3ccc(F)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572454

(CHEMBL4855358)Show SMILES NC(=N)c1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054585
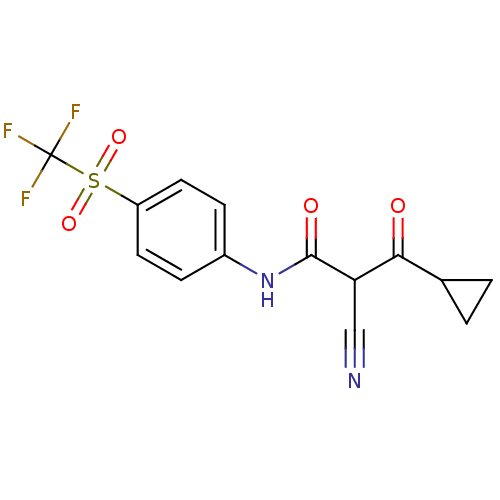
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-trifluoro...)Show SMILES FC(F)(F)S(=O)(=O)c1ccc(NC(=O)C(C#N)C(=O)C2CC2)cc1 Show InChI InChI=1S/C14H11F3N2O4S/c15-14(16,17)24(22,23)10-5-3-9(4-6-10)19-13(21)11(7-18)12(20)8-1-2-8/h3-6,8,11H,1-2H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572443
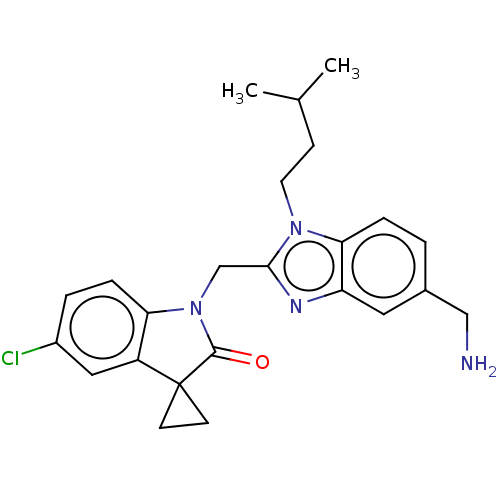
(CHEMBL4872263)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cc(Cl)ccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054556
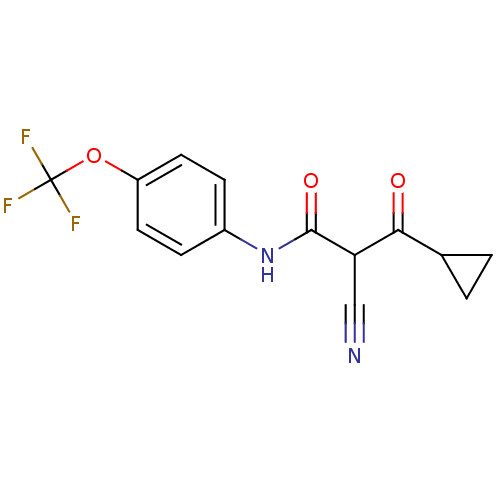
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-trifluoro...)Show InChI InChI=1S/C14H11F3N2O3/c15-14(16,17)22-10-5-3-9(4-6-10)19-13(21)11(7-18)12(20)8-1-2-8/h3-6,8,11H,1-2H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054573
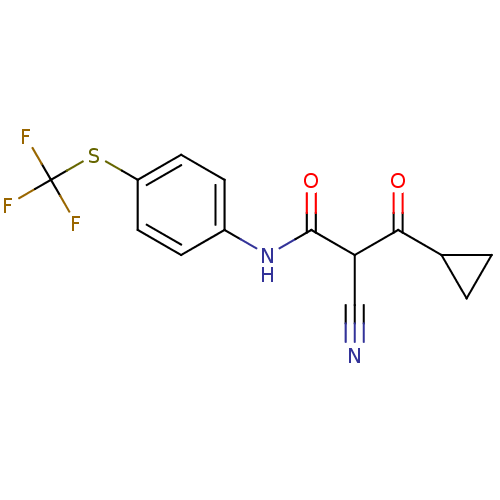
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-trifluoro...)Show InChI InChI=1S/C14H11F3N2O2S/c15-14(16,17)22-10-5-3-9(4-6-10)19-13(21)11(7-18)12(20)8-1-2-8/h3-6,8,11H,1-2H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572462
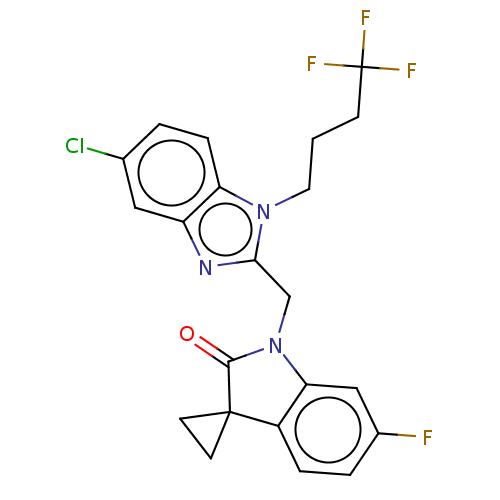
(CHEMBL4876843)Show SMILES Fc1ccc2c(c1)N(Cc1nc3cc(Cl)ccc3n1CCCC(F)(F)F)C(=O)C21CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054601
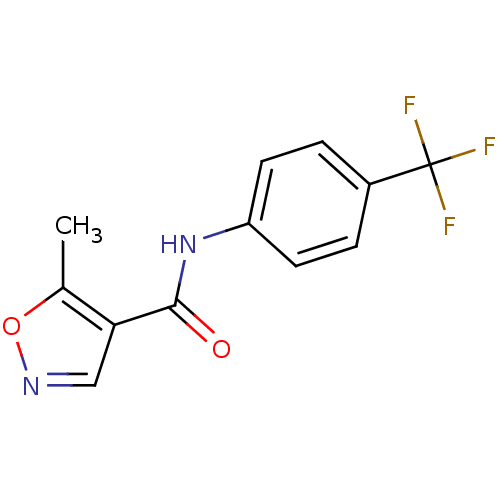
(5-Methyl-N-(4-(trifluoromethyl)phenyl)-4-isoxazole...)Show InChI InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572441

(CHEMBL4862291)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3c2cccc3Cl)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054590
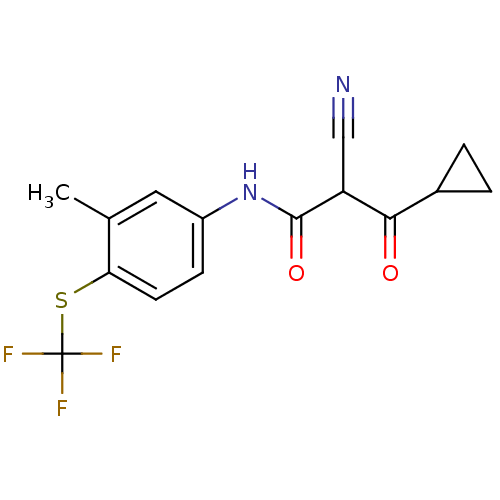
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(3-methyl-4-...)Show InChI InChI=1S/C15H13F3N2O2S/c1-8-6-10(4-5-12(8)23-15(16,17)18)20-14(22)11(7-19)13(21)9-2-3-9/h4-6,9,11H,2-3H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054541

((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(3-methyl-4-...)Show SMILES Cc1cc(NC(=O)C(C#N)C(=O)C2CC2)ccc1C(F)(F)C(F)(F)F Show InChI InChI=1S/C16H13F5N2O2/c1-8-6-10(4-5-12(8)15(17,18)16(19,20)21)23-14(25)11(7-22)13(24)9-2-3-9/h4-6,9,11H,2-3H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM14712
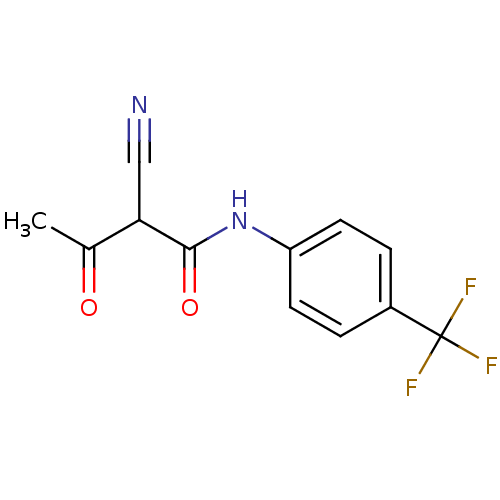
((2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)pheny...)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,10H,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054552

((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-methylsul...)Show InChI InChI=1S/C14H14N2O2S/c1-19-11-6-4-10(5-7-11)16-14(18)12(8-15)13(17)9-2-3-9/h4-7,9,12H,2-3H2,1H3,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054560
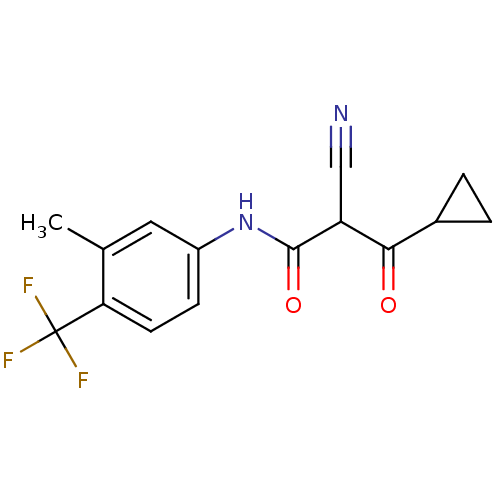
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(3-methyl-4-...)Show InChI InChI=1S/C15H13F3N2O2/c1-8-6-10(4-5-12(8)15(16,17)18)20-14(22)11(7-19)13(21)9-2-3-9/h4-6,9,11H,2-3H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054581
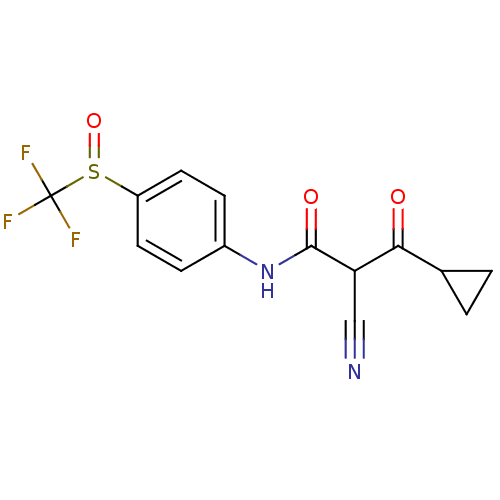
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-trifluoro...)Show SMILES FC(F)(F)S(=O)c1ccc(NC(=O)C(C#N)C(=O)C2CC2)cc1 Show InChI InChI=1S/C14H11F3N2O3S/c15-14(16,17)23(22)10-5-3-9(4-6-10)19-13(21)11(7-18)12(20)8-1-2-8/h3-6,8,11H,1-2H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054597

((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(5-trifluoro...)Show InChI InChI=1S/C13H10F3N3O2/c14-13(15,16)8-3-4-10(18-6-8)19-12(21)9(5-17)11(20)7-1-2-7/h3-4,6-7,9H,1-2H2,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054574
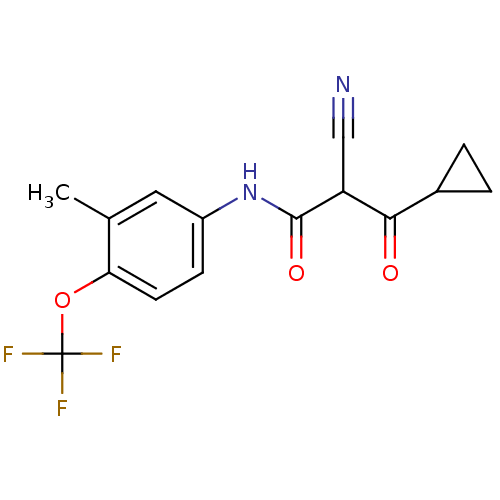
((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(3-methyl-4-...)Show InChI InChI=1S/C15H13F3N2O3/c1-8-6-10(4-5-12(8)23-15(16,17)18)20-14(22)11(7-19)13(21)9-2-3-9/h4-6,9,11H,2-3H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054557

((Z)-2-Cyano-3-cyclopropyl-3-hydroxy-N-(4-nitro-phe...)Show SMILES [O-][N+](=O)c1ccc(NC(=O)C(=C=[N-])C(=[OH+])C2CC2)cc1 Show InChI InChI=1S/C13H10N3O4/c14-7-11(12(17)8-1-2-8)13(18)15-9-3-5-10(6-4-9)16(19)20/h3-6,8H,1-2H2,(H,15,18)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM29024

(CHEMBL141732 | cyanocyclopropylpropenamide, 4)Show InChI InChI=1S/C14H11F3N2O2/c15-14(16,17)9-3-5-10(6-4-9)19-13(21)11(7-18)12(20)8-1-2-8/h3-6,8,11H,1-2H2,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50407985

(CHEMBL2111978)Show SMILES Clc1ccc(\C=C/c2ccc(NC(=O)C(C#N)C(=O)C3CC3)cc2)cc1 Show InChI InChI=1S/C21H17ClN2O2/c22-17-9-3-14(4-10-17)1-2-15-5-11-18(12-6-15)24-21(26)19(13-23)20(25)16-7-8-16/h1-6,9-12,16,19H,7-8H2,(H,24,26)/b2-1- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested on enzyme dihydroorotate dehydrogenase in mouse |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054600

((Z)-2-Cyano-3-hydroxy-penta-2,4-dienoic acid (4-tr...)Show InChI InChI=1S/C13H9F3N2O2/c1-2-11(19)10(7-17)12(20)18-9-5-3-8(4-6-9)13(14,15)16/h2-6,10H,1H2,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054553
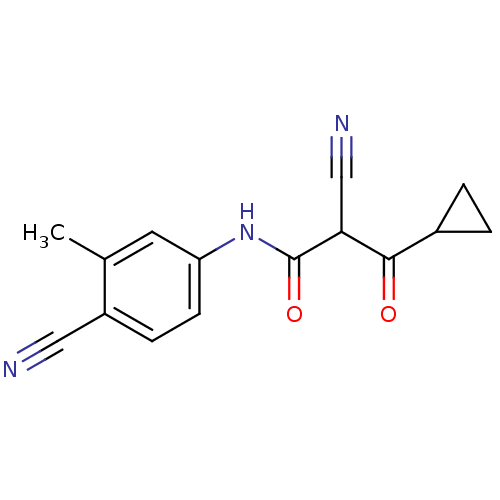
((Z)-2-Cyano-N-(4-cyano-3-methyl-phenyl)-3-cyclopro...)Show InChI InChI=1S/C15H13N3O2/c1-9-6-12(5-4-11(9)7-16)18-15(20)13(8-17)14(19)10-2-3-10/h4-6,10,13H,2-3H2,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50054601

(5-Methyl-N-(4-(trifluoromethyl)phenyl)-4-isoxazole...)Show InChI InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested on enzyme dihydroorotate dehydrogenase in mouse |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Rattus norvegicus (rat)) | BDBM50054594

((Z)-N-(4-Chloro-3-methyl-phenyl)-2-cyano-3-cyclopr...)Show InChI InChI=1S/C14H13ClN2O2/c1-8-6-10(4-5-12(8)15)17-14(19)11(7-16)13(18)9-2-3-9/h4-6,9,11H,2-3H2,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against enzyme dihydroorotate dehydrogenase in rat |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50054553

((Z)-2-Cyano-N-(4-cyano-3-methyl-phenyl)-3-cyclopro...)Show InChI InChI=1S/C15H13N3O2/c1-9-6-12(5-4-11(9)7-16)18-15(20)13(8-17)14(19)10-2-3-10/h4-6,10,13H,2-3H2,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested on enzyme dihydroorotate dehydrogenase in mouse |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50054584
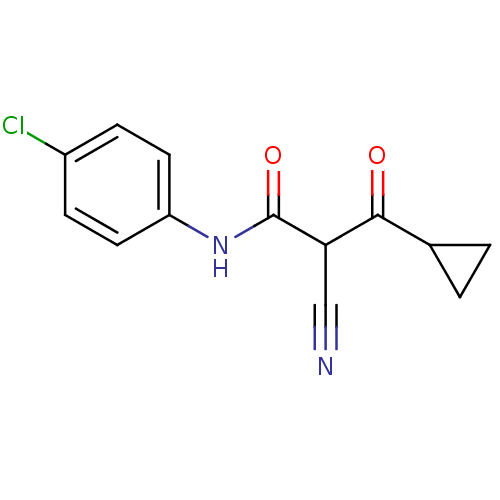
((Z)-N-(4-Chloro-phenyl)-2-cyano-3-cyclopropyl-3-hy...)Show InChI InChI=1S/C13H11ClN2O2/c14-9-3-5-10(6-4-9)16-13(18)11(7-15)12(17)8-1-2-8/h3-6,8,11H,1-2H2,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested on enzyme dihydroorotate dehydrogenase in mouse |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50054548

((Z)-N-(4-Bromo-phenyl)-2-cyano-3-cyclopropyl-3-hyd...)Show InChI InChI=1S/C13H11BrN2O2/c14-9-3-5-10(6-4-9)16-13(18)11(7-15)12(17)8-1-2-8/h3-6,8,11H,1-2H2,(H,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested on enzyme dihydroorotate dehydrogenase in mouse |
J Med Chem 39: 4608-21 (1996)
Article DOI: 10.1021/jm9604437
BindingDB Entry DOI: 10.7270/Q20G3KSX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data