 Found 360 hits with Last Name = 'liu' and Initial = 'jo'
Found 360 hits with Last Name = 'liu' and Initial = 'jo' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112356
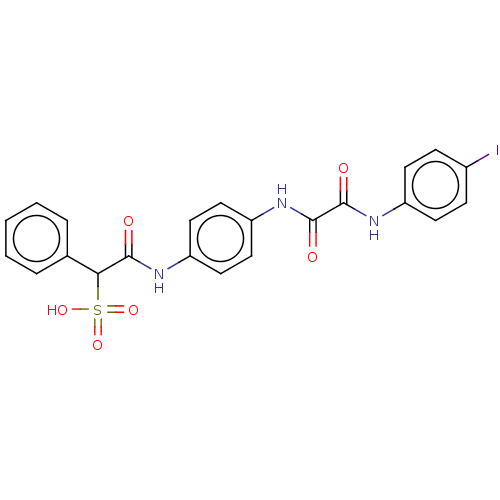
(CHEMBL3609373)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(I)cc2)cc1)c1ccccc1 Show InChI InChI=1S/C22H18IN3O6S/c23-15-6-8-16(9-7-15)25-21(28)22(29)26-18-12-10-17(11-13-18)24-20(27)19(33(30,31)32)14-4-2-1-3-5-14/h1-13,19H,(H,24,27)(H,25,28)(H,26,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50420258

(CEFSULODIN)Show SMILES NC(=O)c1cc[n+](CC2=C(N3[C@H](SC2)[C@H](NC(=O)C(c2ccccc2)S(O)(=O)=O)C3=O)C(O)=O)cc1 |t:8| Show InChI InChI=1S/C22H20N4O8S2/c23-18(27)13-6-8-25(9-7-13)10-14-11-35-21-15(20(29)26(21)16(14)22(30)31)24-19(28)17(36(32,33)34)12-4-2-1-3-5-12/h1-9,15,17,21H,10-11H2,(H4-,23,24,27,28,30,31,32,33,34)/p+1/t15-,17?,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate Lineweaver-Burk plot analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36597
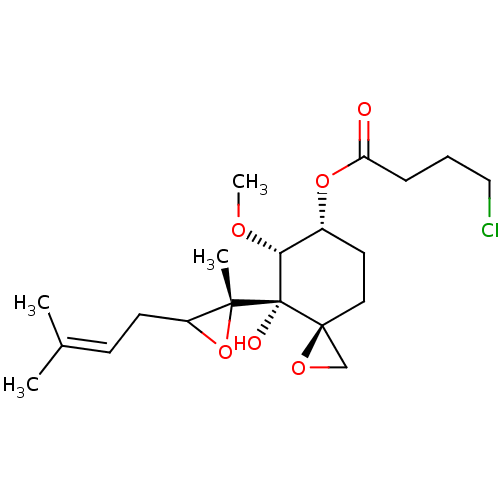
(FOS-69)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#6]-[#6]-[#6]Cl |r| Show InChI InChI=1S/C20H31ClO6/c1-13(2)7-8-15-18(3,27-15)20(23)17(24-4)14(9-10-19(20)12-25-19)26-16(22)6-5-11-21/h7,14-15,17,23H,5-6,8-12H2,1-4H3/t14-,15?,17-,18-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36589
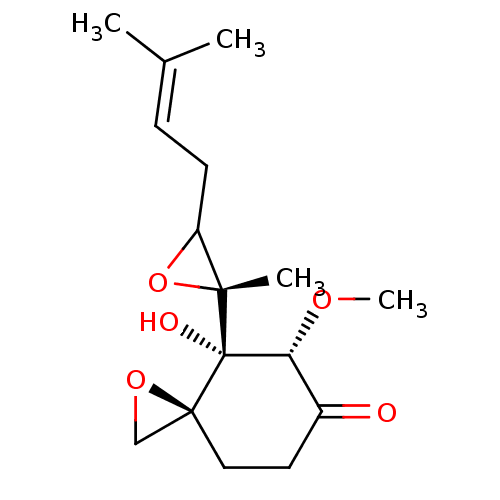
(Ovalicin)Show SMILES [#6]-[#8]-[#6@@H]1-[#6](=O)-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H24O5/c1-10(2)5-6-12-14(3,21-12)16(18)13(19-4)11(17)7-8-15(16)9-20-15/h5,12-13,18H,6-9H2,1-4H3/t12?,13-,14-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17446

((3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-m...)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C19H28ClNO6/c1-11(2)5-6-13-18(3,27-13)16-15(24-4)12(7-8-19(16)10-25-19)26-17(23)21-14(22)9-20/h5,12-13,15-16H,6-10H2,1-4H3,(H,21,22,23)/t12-,13-,15-,16-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human MetAP2 |
J Med Chem 49: 5645-8 (2006)
Article DOI: 10.1021/jm060559v
BindingDB Entry DOI: 10.7270/Q2PN959W |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50131562
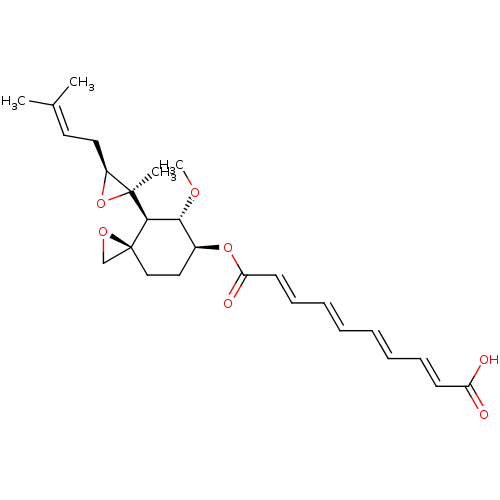
(CHEMBL117603 | Deca-2,4,6,8-tetraenedioic acid mon...)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6@H]-1[C@@]1([#6])[#8]-[#6@H]1-[#6]\[#6]=[#6](/[#6])-[#6])-[#8]-[#6](=O)\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]\[#6]=[#6]\[#6](-[#8])=O Show InChI InChI=1S/C26H34O7/c1-18(2)13-14-20-25(3,33-20)24-23(30-4)19(15-16-26(24)17-31-26)32-22(29)12-10-8-6-5-7-9-11-21(27)28/h5-13,19-20,23-24H,14-17H2,1-4H3,(H,27,28)/b7-5+,8-6+,11-9+,12-10+/t19-,20-,23+,24+,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against purified recombinant human Methionine Aminopeptidase 2 (MetAP2) |
J Med Chem 46: 3452-4 (2003)
Article DOI: 10.1021/jm0341103
BindingDB Entry DOI: 10.7270/Q2NG4Q0J |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36588

(AGM-1470)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl |r| Show InChI InChI=1S/C19H28ClNO6/c1-11(2)5-6-13-18(3,27-13)16-15(24-4)12(7-8-19(16)10-25-19)26-17(23)21-14(22)9-20/h5,12-13,15-16H,6-10H2,1-4H3,(H,21,22,23)/t12-,13?,15-,16?,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36595
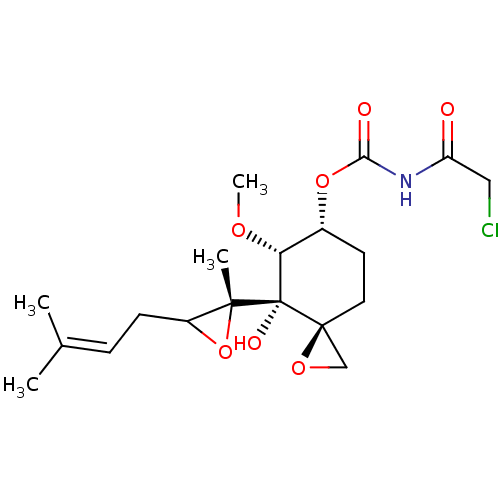
(FOS-68)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl |r| Show InChI InChI=1S/C19H28ClNO7/c1-11(2)5-6-13-17(3,28-13)19(24)15(25-4)12(7-8-18(19)10-26-18)27-16(23)21-14(22)9-20/h5,12-13,15,24H,6-10H2,1-4H3,(H,21,22,23)/t12-,13?,15-,17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36592
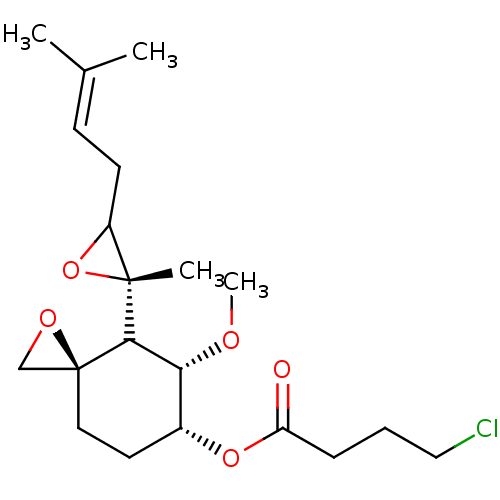
(FOS-70)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#6]-[#6]-[#6]Cl |r| Show InChI InChI=1S/C20H31ClO5/c1-13(2)7-8-15-19(3,26-15)18-17(23-4)14(9-10-20(18)12-24-20)25-16(22)6-5-11-21/h7,14-15,17-18H,5-6,8-12H2,1-4H3/t14-,15?,17-,18?,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088483
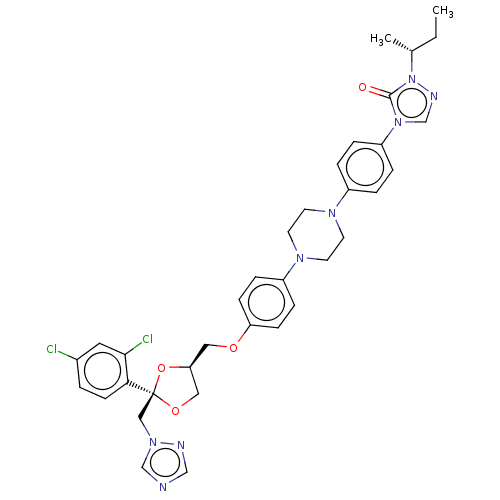
(CHEMBL1835951)Show SMILES CC[C@@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@H]3CO[C@@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088484

(CHEMBL1835950)Show SMILES CC[C@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@H]3CO[C@@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088485

(CHEMBL1835949)Show SMILES CC[C@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@@H]3CO[C@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088486
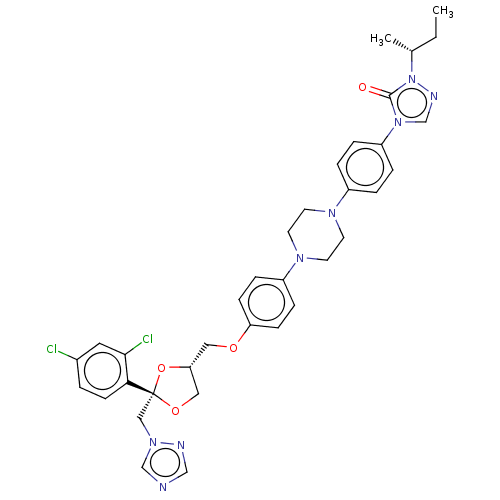
(CHEMBL454471)Show SMILES CC[C@@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@@H]3CO[C@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36596
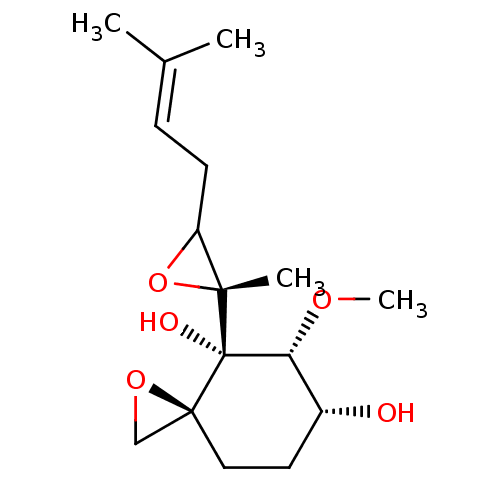
(FOS-34)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@H](-[#8])-[#6]-[#6][C@]2([#6]-[#8]2)[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H26O5/c1-10(2)5-6-12-14(3,21-12)16(18)13(19-4)11(17)7-8-15(16)9-20-15/h5,11-13,17-18H,6-9H2,1-4H3/t11-,12?,13-,14-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36590
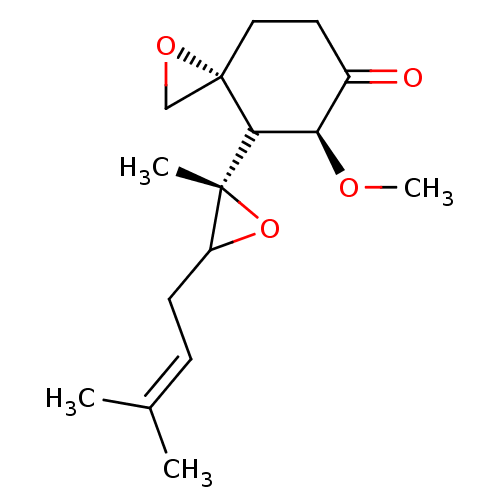
(FOS-72)Show SMILES [#6]-[#8]-[#6@H]-1-[#6]([C@]2([#6])[#8]-[#6]2-[#6]\[#6]=[#6](\[#6])-[#6])[C@@]2([#6]-[#8]2)[#6]-[#6]-[#6]-1=O |r| Show InChI InChI=1S/C16H24O4/c1-10(2)5-6-12-15(3,20-12)14-13(18-4)11(17)7-8-16(14)9-19-16/h5,12-14H,6-9H2,1-4H3/t12?,13-,14?,15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36591
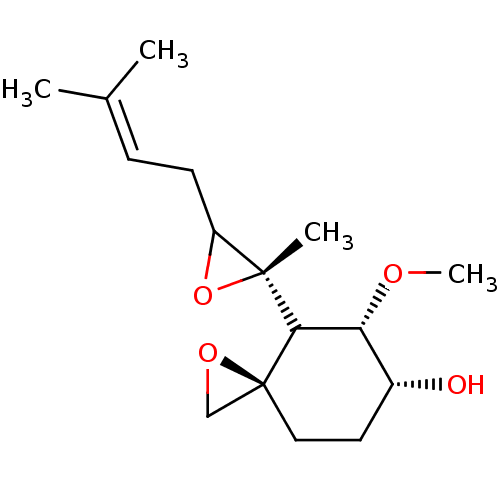
(FOS-37)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@H](-[#8])-[#6]-[#6][C@]2([#6]-[#8]2)[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H26O4/c1-10(2)5-6-12-15(3,20-12)14-13(18-4)11(17)7-8-16(14)9-19-16/h5,11-14,17H,6-9H2,1-4H3/t11-,12?,13-,14?,15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088480
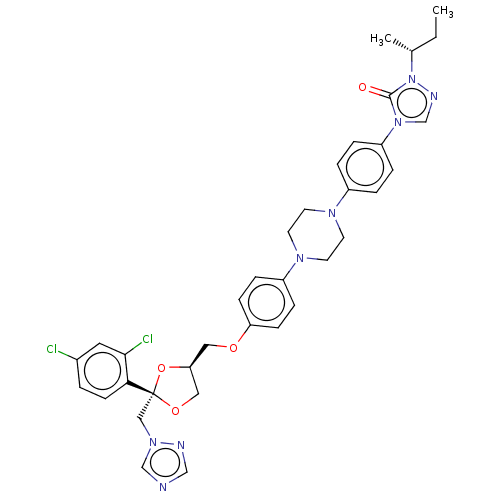
(CHEMBL1835953)Show SMILES CC[C@@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@H]3CO[C@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrates after 5 mins by LC/MS analysis in presence of NADPH |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088481
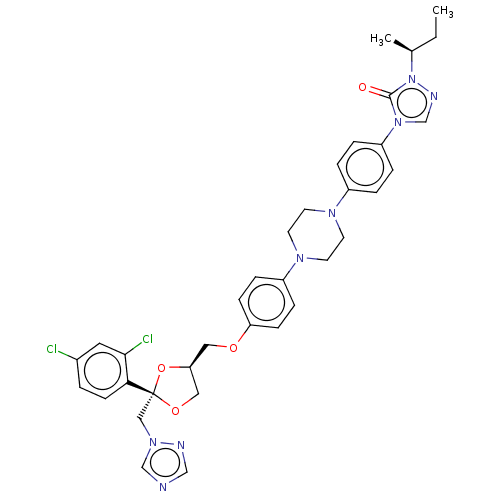
(CHEMBL1835952)Show SMILES CC[C@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@H]3CO[C@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrates after 5 mins by LC/MS analysis in presence of NADPH |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088479
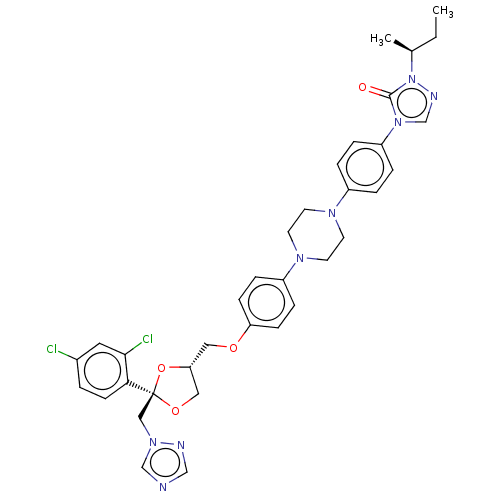
(CHEMBL1835954)Show SMILES CC[C@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@@H]3CO[C@@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrates after 5 mins by LC/MS analysis in presence of NADPH |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088455
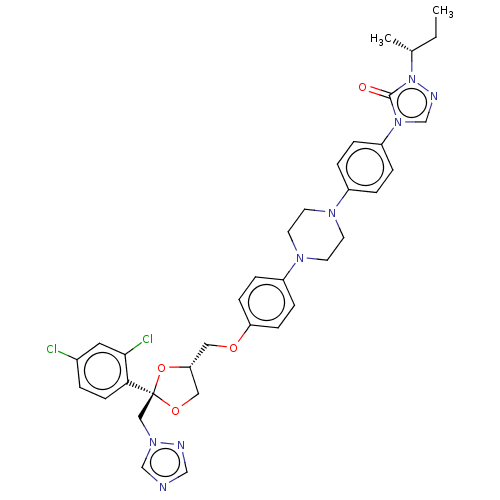
(CHEMBL1835955)Show SMILES CC[C@@H](C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@@H]3CO[C@@](Cn4cncn4)(O3)c3ccc(Cl)cc3Cl)cc2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25-,31-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrates after 5 mins by LC/MS analysis in presence of NADPH |
Drug Metab Dispos 40: 426-35 (2012)
Article DOI: 10.1124/dmd.111.042739
BindingDB Entry DOI: 10.7270/Q2B859TV |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36599
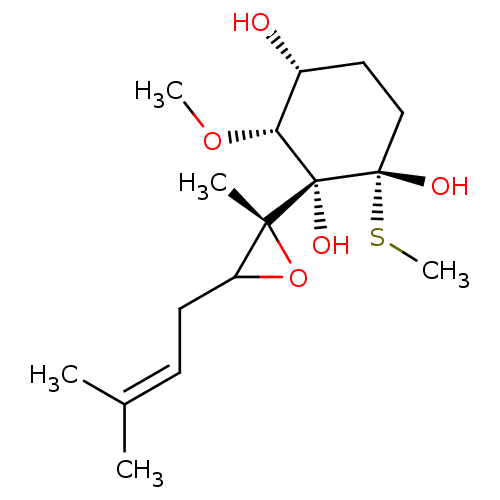
(FOS-201)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@H](-[#8])-[#6]-[#6][C@]([#8])([#16]-[#6])[C@@]1([#8])[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C16H28O5S/c1-10(2)6-7-12-14(3,21-12)16(19)13(20-4)11(17)8-9-15(16,18)22-5/h6,11-13,17-19H,7-9H2,1-5H3/t11-,12?,13-,14-,15+,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435227
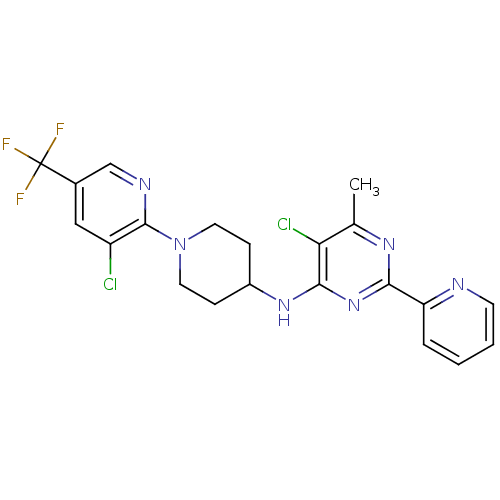
(CHEMBL2392772)Show SMILES Cc1nc(nc(NC2CCN(CC2)c2ncc(cc2Cl)C(F)(F)F)c1Cl)-c1ccccn1 Show InChI InChI=1S/C21H19Cl2F3N6/c1-12-17(23)19(31-18(29-12)16-4-2-3-7-27-16)30-14-5-8-32(9-6-14)20-15(22)10-13(11-28-20)21(24,25)26/h2-4,7,10-11,14H,5-6,8-9H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50052428

(2-(2,6-Dioxo-piperidin-3-yl)-4,5,6,7-tetrafluoro-i...)Show SMILES Fc1c2C(=O)N(C3CCC(=O)NC3=O)C(=O)c2c(F)c(F)c1F Show InChI InChI=1S/C13H6F4N2O4/c14-7-5-6(8(15)10(17)9(7)16)13(23)19(12(5)22)3-1-2-4(20)18-11(3)21/h3H,1-2H2,(H,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibitory activity against TNF-alpha production |
J Med Chem 39: 3044-5 (1996)
Article DOI: 10.1021/jm960284r
BindingDB Entry DOI: 10.7270/Q2RB73PR |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435224
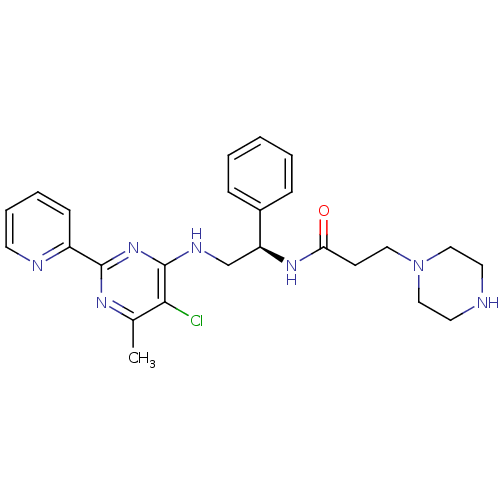
(CHEMBL2392893)Show SMILES Cc1nc(nc(NC[C@H](NC(=O)CCN2CCNCC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C25H30ClN7O/c1-18-23(26)25(32-24(30-18)20-9-5-6-11-28-20)29-17-21(19-7-3-2-4-8-19)31-22(34)10-14-33-15-12-27-13-16-33/h2-9,11,21,27H,10,12-17H2,1H3,(H,31,34)(H,29,30,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435217
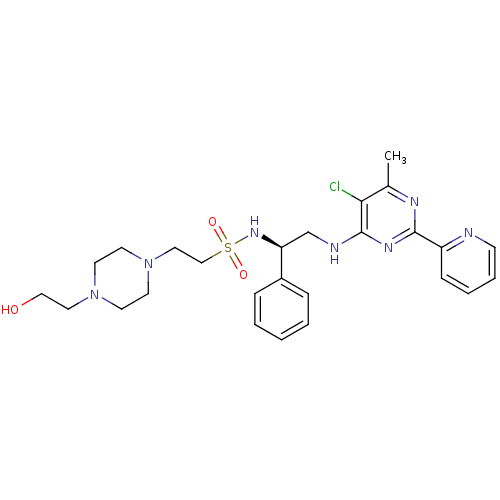
(CHEMBL2392900)Show SMILES Cc1nc(nc(NC[C@H](NS(=O)(=O)CCN2CCN(CCO)CC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C26H34ClN7O3S/c1-20-24(27)26(31-25(30-20)22-9-5-6-10-28-22)29-19-23(21-7-3-2-4-8-21)32-38(36,37)18-16-34-13-11-33(12-14-34)15-17-35/h2-10,23,32,35H,11-19H2,1H3,(H,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435226
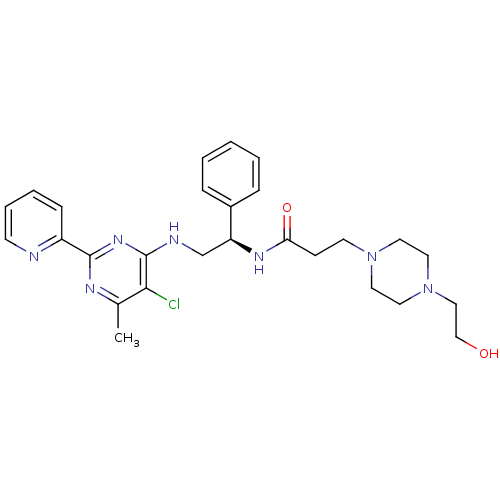
(CHEMBL2392891)Show SMILES Cc1nc(nc(NC[C@H](NC(=O)CCN2CCN(CCO)CC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C27H34ClN7O2/c1-20-25(28)27(33-26(31-20)22-9-5-6-11-29-22)30-19-23(21-7-3-2-4-8-21)32-24(37)10-12-34-13-15-35(16-14-34)17-18-36/h2-9,11,23,36H,10,12-19H2,1H3,(H,32,37)(H,30,31,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435219
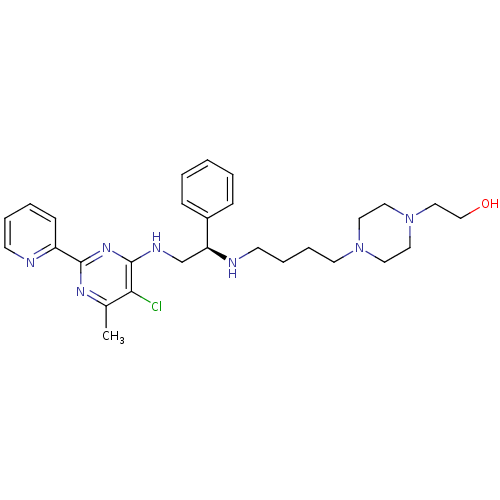
(CHEMBL2392898)Show SMILES Cc1nc(nc(NC[C@H](NCCCCN2CCN(CCO)CC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C28H38ClN7O/c1-22-26(29)28(34-27(33-22)24-11-5-6-12-30-24)32-21-25(23-9-3-2-4-10-23)31-13-7-8-14-35-15-17-36(18-16-35)19-20-37/h2-6,9-12,25,31,37H,7-8,13-21H2,1H3,(H,32,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435218
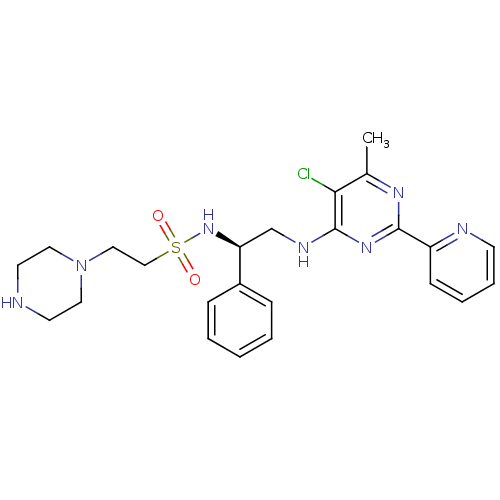
(CHEMBL2392899)Show SMILES Cc1nc(nc(NC[C@H](NS(=O)(=O)CCN2CCNCC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C24H30ClN7O2S/c1-18-22(25)24(30-23(29-18)20-9-5-6-10-27-20)28-17-21(19-7-3-2-4-8-19)31-35(33,34)16-15-32-13-11-26-12-14-32/h2-10,21,26,31H,11-17H2,1H3,(H,28,29,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50433375
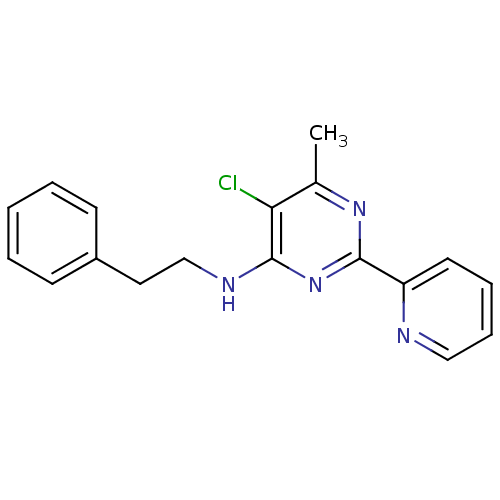
(CHEMBL472879 | GNF-Pf-359)Show InChI InChI=1S/C18H17ClN4/c1-13-16(19)18(21-12-10-14-7-3-2-4-8-14)23-17(22-13)15-9-5-6-11-20-15/h2-9,11H,10,12H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435229
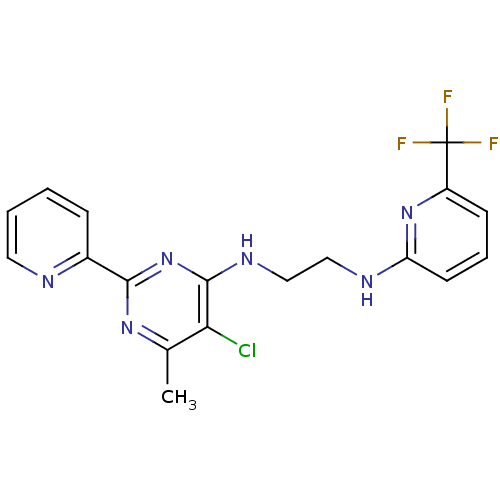
(CHEMBL2392771)Show SMILES Cc1nc(nc(NCCNc2cccc(n2)C(F)(F)F)c1Cl)-c1ccccn1 Show InChI InChI=1S/C18H16ClF3N6/c1-11-15(19)17(28-16(26-11)12-5-2-3-8-23-12)25-10-9-24-14-7-4-6-13(27-14)18(20,21)22/h2-8H,9-10H2,1H3,(H,24,27)(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435228
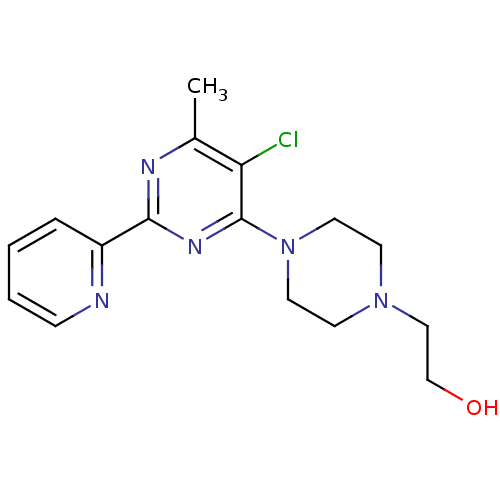
(CHEMBL460432)Show InChI InChI=1S/C16H20ClN5O/c1-12-14(17)16(22-8-6-21(7-9-22)10-11-23)20-15(19-12)13-4-2-3-5-18-13/h2-5,23H,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435225
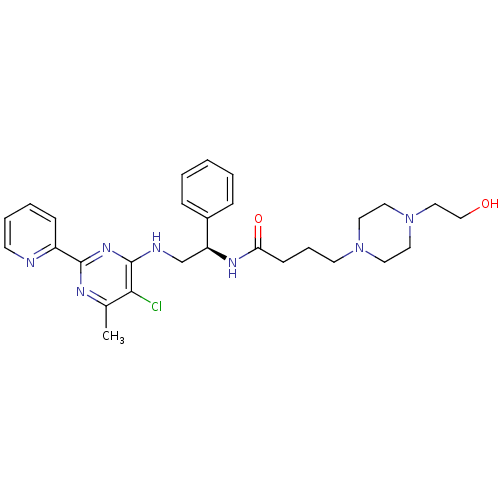
(CHEMBL2392892)Show SMILES Cc1nc(nc(NC[C@H](NC(=O)CCCN2CCN(CCO)CC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C28H36ClN7O2/c1-21-26(29)28(34-27(32-21)23-10-5-6-12-30-23)31-20-24(22-8-3-2-4-9-22)33-25(38)11-7-13-35-14-16-36(17-15-35)18-19-37/h2-6,8-10,12,24,37H,7,11,13-20H2,1H3,(H,33,38)(H,31,32,34)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435223
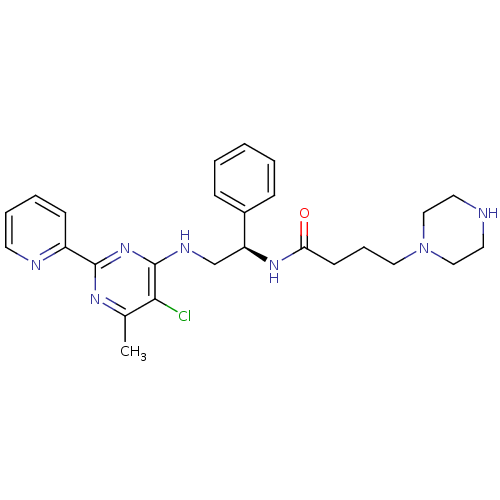
(CHEMBL2392894)Show SMILES Cc1nc(nc(NC[C@H](NC(=O)CCCN2CCNCC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C26H32ClN7O/c1-19-24(27)26(33-25(31-19)21-10-5-6-12-29-21)30-18-22(20-8-3-2-4-9-20)32-23(35)11-7-15-34-16-13-28-14-17-34/h2-6,8-10,12,22,28H,7,11,13-18H2,1H3,(H,32,35)(H,30,31,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435220
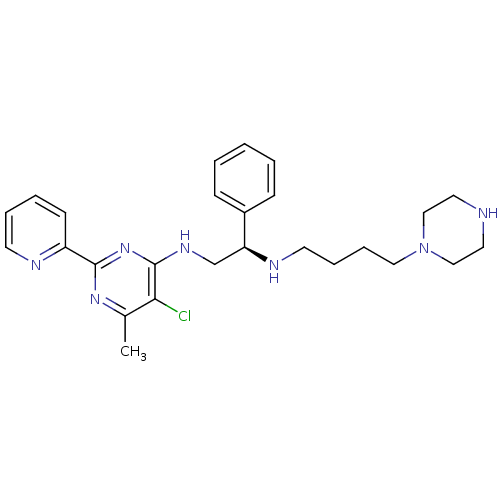
(CHEMBL2392897)Show SMILES Cc1nc(nc(NC[C@H](NCCCCN2CCNCC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C26H34ClN7/c1-20-24(27)26(33-25(32-20)22-11-5-6-12-29-22)31-19-23(21-9-3-2-4-10-21)30-13-7-8-16-34-17-14-28-15-18-34/h2-6,9-12,23,28,30H,7-8,13-19H2,1H3,(H,31,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112358
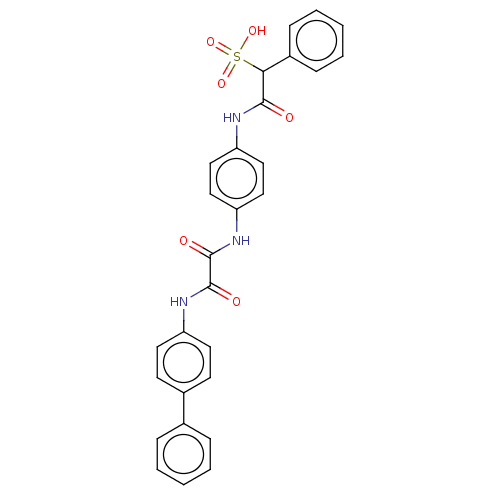
(CHEMBL3609374)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(cc2)-c2ccccc2)cc1)c1ccccc1 Show InChI InChI=1S/C28H23N3O6S/c32-26(25(38(35,36)37)21-9-5-2-6-10-21)29-23-15-17-24(18-16-23)31-28(34)27(33)30-22-13-11-20(12-14-22)19-7-3-1-4-8-19/h1-18,25H,(H,29,32)(H,30,33)(H,31,34)(H,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435222

(CHEMBL2392895)Show SMILES Cc1nc(nc(NC[C@H](NCCCN2CCNCC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C25H32ClN7/c1-19-23(26)25(32-24(31-19)21-10-5-6-11-28-21)30-18-22(20-8-3-2-4-9-20)29-12-7-15-33-16-13-27-14-17-33/h2-6,8-11,22,27,29H,7,12-18H2,1H3,(H,30,31,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM17847
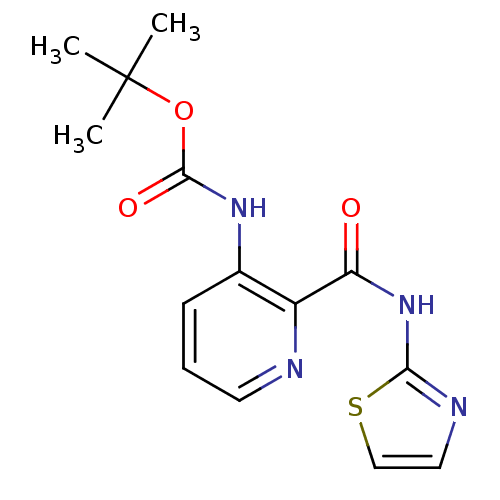
(CHEMBL327579 | pyridine-2-carboxylic acid inhibito...)Show InChI InChI=1S/C14H16N4O3S/c1-14(2,3)21-13(20)17-9-5-4-6-15-10(9)11(19)18-12-16-7-8-22-12/h4-8H,1-3H3,(H,17,20)(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University
| Assay Description
The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... |
Proc Natl Acad Sci U S A 103: 18148-53 (2006)
Article DOI: 10.1073/pnas.0608389103
BindingDB Entry DOI: 10.7270/Q2RB72VK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435231
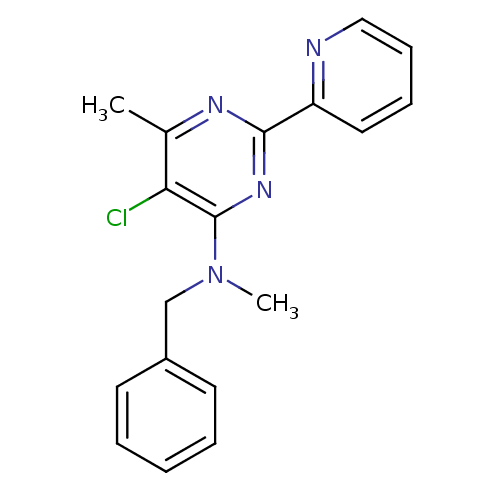
(CHEMBL2392769)Show InChI InChI=1S/C18H17ClN4/c1-13-16(19)18(23(2)12-14-8-4-3-5-9-14)22-17(21-13)15-10-6-7-11-20-15/h3-11H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112356

(CHEMBL3609373)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(I)cc2)cc1)c1ccccc1 Show InChI InChI=1S/C22H18IN3O6S/c23-15-6-8-16(9-7-15)25-21(28)22(29)26-18-12-10-17(11-13-18)24-20(27)19(33(30,31)32)14-4-2-1-3-5-14/h1-13,19H,(H,24,27)(H,25,28)(H,26,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50435221
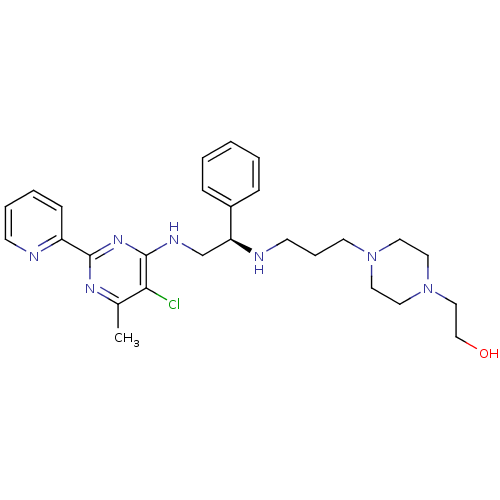
(CHEMBL2392896)Show SMILES Cc1nc(nc(NC[C@H](NCCCN2CCN(CCO)CC2)c2ccccc2)c1Cl)-c1ccccn1 |r| Show InChI InChI=1S/C27H36ClN7O/c1-21-25(28)27(33-26(32-21)23-10-5-6-11-29-23)31-20-24(22-8-3-2-4-9-22)30-12-7-13-34-14-16-35(17-15-34)18-19-36/h2-6,8-11,24,30,36H,7,12-20H2,1H3,(H,31,32,33)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM68281
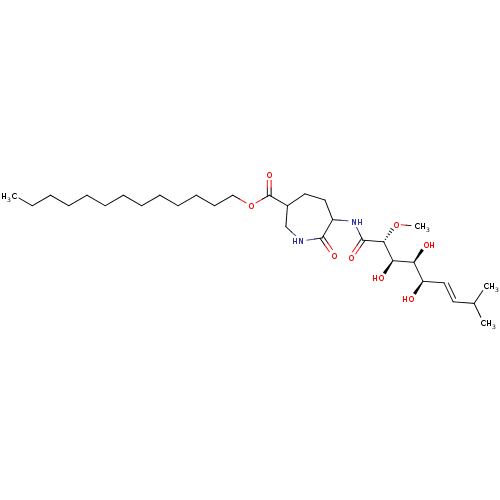
(Bengamide A)Show SMILES CCCCCCCCCCCCCOC(=O)C1CCC(NC(=O)[C@H](OC)[C@H](O)[C@@H](O)[C@H](O)\C=C\C(C)C)C(=O)NC1 |r| Show InChI InChI=1S/C31H56N2O8/c1-5-6-7-8-9-10-11-12-13-14-15-20-41-31(39)23-17-18-24(29(37)32-21-23)33-30(38)28(40-4)27(36)26(35)25(34)19-16-22(2)3/h16,19,22-28,34-36H,5-15,17-18,20-21H2,1-4H3,(H,32,37)(H,33,38)/b19-16+/t23?,24?,25-,26+,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
| Assay Description
The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. |
Chem Biol 14: 764-74 (2007)
Article DOI: 10.1016/j.chembiol.2007.05.010
BindingDB Entry DOI: 10.7270/Q2NC5ZN9 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM50433377
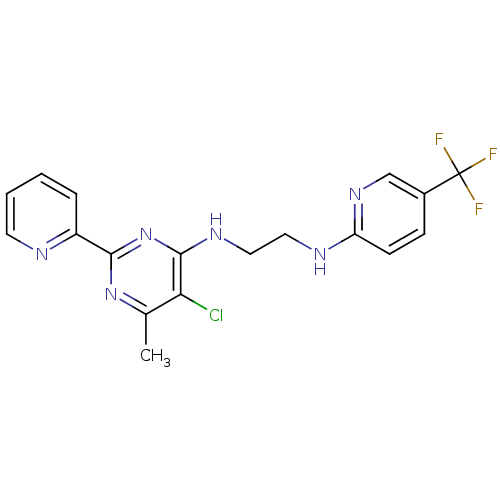
(CHEMBL2375628)Show SMILES Cc1nc(nc(NCCNc2ccc(cn2)C(F)(F)F)c1Cl)-c1ccccn1 Show InChI InChI=1S/C18H16ClF3N6/c1-11-15(19)17(28-16(27-11)13-4-2-3-7-23-13)25-9-8-24-14-6-5-12(10-26-14)18(20,21)22/h2-7,10H,8-9H2,1H3,(H,24,26)(H,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... |
Bioorg Med Chem 21: 2600-17 (2013)
Article DOI: 10.1016/j.bmc.2013.02.023
BindingDB Entry DOI: 10.7270/Q290256X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Plasmodium falciparum) | BDBM17446

((3R,4S,5S,6R)-5-methoxy-4-[(2R,3R)-2-methyl-3-(3-m...)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C19H28ClNO6/c1-11(2)5-6-13-18(3,27-13)16-15(24-4)12(7-8-19(16)10-25-19)26-17(23)21-14(22)9-20/h5,12-13,15-16H,6-10H2,1-4H3,(H,21,22,23)/t12-,13-,15-,16-,18+,19+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins School of Medicine
| Assay Description
Enzyme affinity assay using Fumarranol analogs with plasmodium falciparum methionine aminopeptidases (PfMetAPs). |
Chem Biol 16: 193-202 (2009)
Article DOI: 10.1016/j.chembiol.2009.01.006
BindingDB Entry DOI: 10.7270/Q2WS8RQ5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50112357

(CHEMBL3609375)Show SMILES CC(C)c1ccc(NC(=O)C(=O)Nc2ccc(NC(=O)C(c3ccccc3)S(O)(=O)=O)cc2)cc1 Show InChI InChI=1S/C25H25N3O6S/c1-16(2)17-8-10-19(11-9-17)27-24(30)25(31)28-21-14-12-20(13-15-21)26-23(29)22(35(32,33)34)18-6-4-3-5-7-18/h3-16,22H,1-2H3,(H,26,29)(H,27,30)(H,28,31)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of SHP2 (unknown origin) using pNPP as a substrate after 10 mins by spectrophotometer analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50541823

(CHEMBL4644787)Show SMILES CCC(C)n1ncn(-c2ccc(cc2)N2CCN(CC2)c2ccc(OC[C@@H]3CO[C@](Cn4ccnc4)(O3)c3ccc(Cl)cc3Cl)cn2)c1=O |r| Show InChI InChI=1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)28-7-5-27(6-8-28)42-14-16-43(17-15-42)33-11-9-29(19-39-33)47-20-30-21-48-35(49-30,22-41-13-12-38-23-41)31-10-4-26(36)18-32(31)37/h4-13,18-19,23-25,30H,3,14-17,20-22H2,1-2H3/t25?,30-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 11: 1111-1117 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00438
BindingDB Entry DOI: 10.7270/Q2QR51P0 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM68286
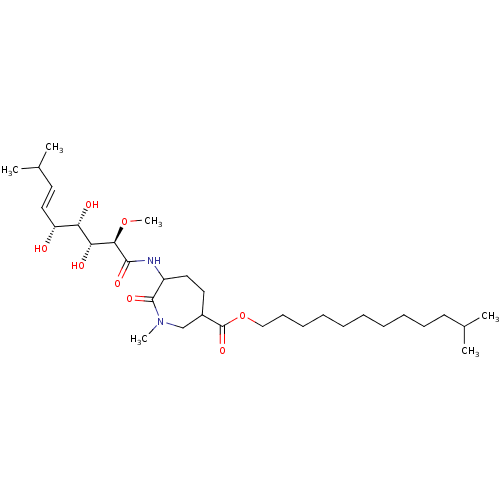
(Bengamide N | Bengamide O)Show SMILES CO[C@H]([C@H](O)[C@@H](O)[C@H](O)\C=C\C(C)C)C(=O)NC1CCC(CN(C)C1=O)C(=O)OCCCCCCCCCCC(C)C |r| Show InChI InChI=1S/C32H58N2O8/c1-22(2)15-13-11-9-7-8-10-12-14-20-42-32(40)24-17-18-25(31(39)34(5)21-24)33-30(38)29(41-6)28(37)27(36)26(35)19-16-23(3)4/h16,19,22-29,35-37H,7-15,17-18,20-21H2,1-6H3,(H,33,38)/b19-16+/t24?,25?,26-,27+,28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
| Assay Description
The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. |
Chem Biol 14: 764-74 (2007)
Article DOI: 10.1016/j.chembiol.2007.05.010
BindingDB Entry DOI: 10.7270/Q2NC5ZN9 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 1
(Homo sapiens (Human)) | BDBM17849
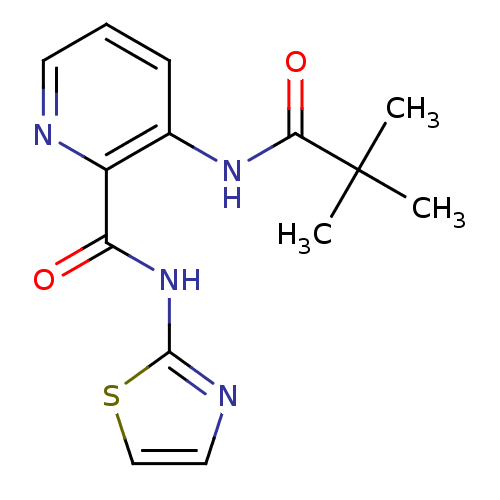
(3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...)Show InChI InChI=1S/C14H16N4O2S/c1-14(2,3)12(20)17-9-5-4-6-15-10(9)11(19)18-13-16-7-8-21-13/h4-8H,1-3H3,(H,17,20)(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University
| Assay Description
The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... |
Proc Natl Acad Sci U S A 103: 18148-53 (2006)
Article DOI: 10.1073/pnas.0608389103
BindingDB Entry DOI: 10.7270/Q2RB72VK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase subunit gamma-1
(Homo sapiens (Human)) | BDBM50448482

(CHEMBL3126408)Show SMILES C[C@H]1C\C(C)=C\C=C/C(=O)O[C@@H](Cc2nc(CCCCC(=O)O1)cs2)\C=C(/C)\C=C\C(\C)=C\CN(C)C |r,c:6,t:4| Show InChI InChI=1S/C30H42N2O4S/c1-22(16-17-32(5)6)14-15-24(3)19-27-20-28-31-26(21-37-28)11-7-8-12-29(33)35-25(4)18-23(2)10-9-13-30(34)36-27/h9-10,13-16,19,21,25,27H,7-8,11-12,17-18,20H2,1-6H3/b13-9-,15-14+,22-16+,23-10+,24-19+/t25-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase-gamma (unknown origin) assessed as incorporation of Br(d)UTP by colorimetric analysis |
Bioorg Med Chem 22: 116-25 (2013)
Article DOI: 10.1016/j.bmc.2013.11.046
BindingDB Entry DOI: 10.7270/Q23T9JQ8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50112358

(CHEMBL3609374)Show SMILES OS(=O)(=O)C(C(=O)Nc1ccc(NC(=O)C(=O)Nc2ccc(cc2)-c2ccccc2)cc1)c1ccccc1 Show InChI InChI=1S/C28H23N3O6S/c32-26(25(38(35,36)37)21-9-5-2-6-10-21)29-23-15-17-24(18-16-23)31-28(34)27(33)30-22-13-11-20(12-14-22)19-7-3-1-4-8-19/h1-18,25H,(H,29,32)(H,30,33)(H,31,34)(H,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of phosphatase activity of human PTP1B using pNPP as a substrate after 10 mins by spectrophotometer analysis |
ACS Med Chem Lett 6: 782-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00118
BindingDB Entry DOI: 10.7270/Q2251M0S |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM36593
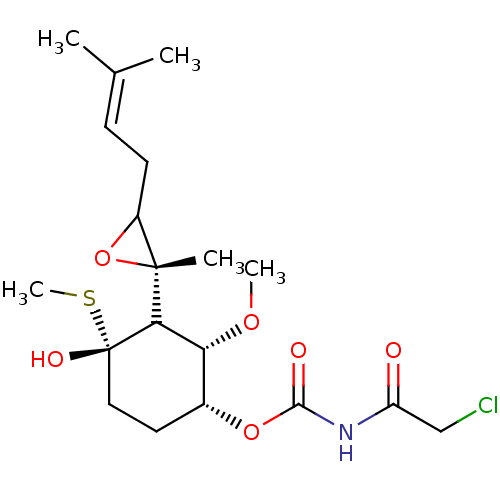
(FOS-64)Show SMILES [#6]-[#8]-[#6@@H]-1-[#6@@H](-[#6]-[#6][C@]([#8])([#16]-[#6])[#6]-1[C@]1([#6])[#8]-[#6]1-[#6]\[#6]=[#6](\[#6])-[#6])-[#8]-[#6](=O)-[#7]-[#6](=O)-[#6]Cl |r| Show InChI InChI=1S/C19H30ClNO6S/c1-11(2)6-7-13-18(3,27-13)16-15(25-4)12(8-9-19(16,24)28-5)26-17(23)21-14(22)10-20/h6,12-13,15-16,24H,7-10H2,1-5H3,(H,21,22,23)/t12-,13?,15-,16?,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.35 | 37 |
Massachusetts Institute of Technology
| Assay Description
Enzymatic assay using recombinant human type 2 methionine aminopeptidase (MetAP2). |
Chem Biol 4: 461-71 (1997)
Article DOI: 10.1016/s1074-5521(97)90198-8
BindingDB Entry DOI: 10.7270/Q2NG4P04 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data