

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant human H3 receptor expressed in CHO-K1 cell membranes incubated for 60 mins by [35S]GTPgammaS binding assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112667 BindingDB Entry DOI: 10.7270/Q2HD80BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546246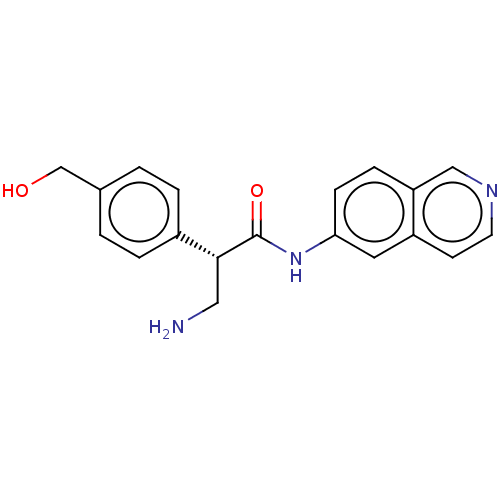 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546246 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528182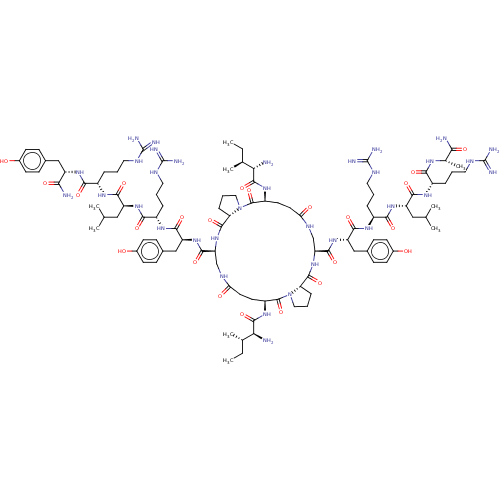 (CHEMBL4454036) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50073587 (CHEMBL3408947 | US10358436, Example A185 | US20230...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Competitive inhibition of PLK-4 (unknown origin) | Eur J Med Chem 95: 35-40 (2015) Article DOI: 10.1016/j.ejmech.2015.03.020 BindingDB Entry DOI: 10.7270/Q2NG4SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528179 (CHEMBL4476074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631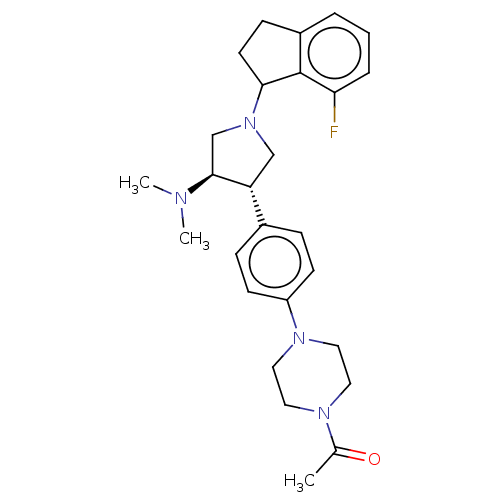 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235630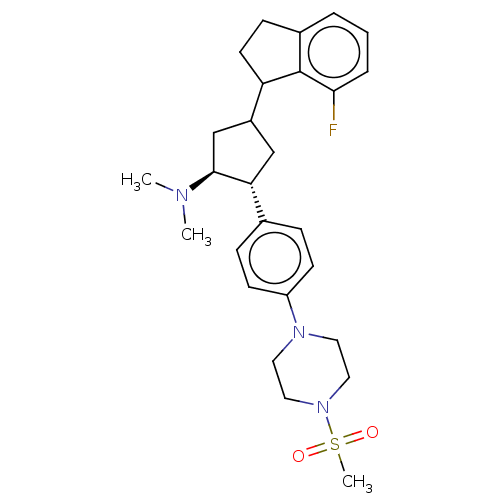 (CHEMBL4093096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50409919 (CHEMBL12282) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50080322 (Acetic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding of Indolactam-V(ILV) to wild-type Protein kinase C delta C1b domain | J Med Chem 42: 3436-46 (1999) Article DOI: 10.1021/jm990129n BindingDB Entry DOI: 10.7270/Q25T3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528186 (CHEMBL4445317) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987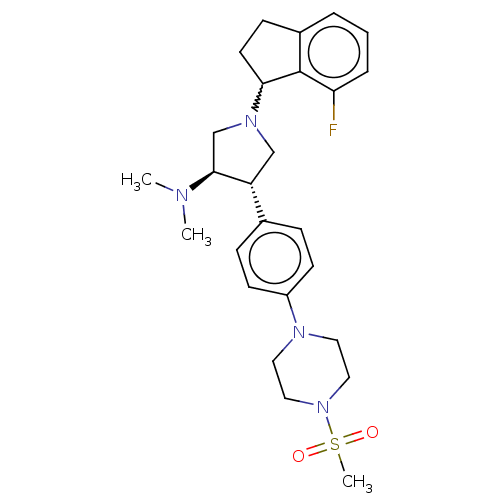 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235643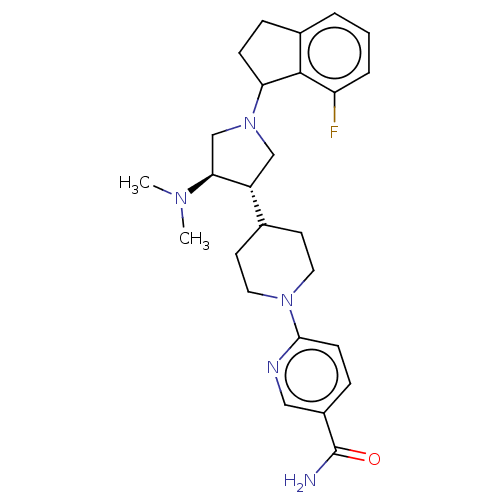 (CHEMBL4076017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096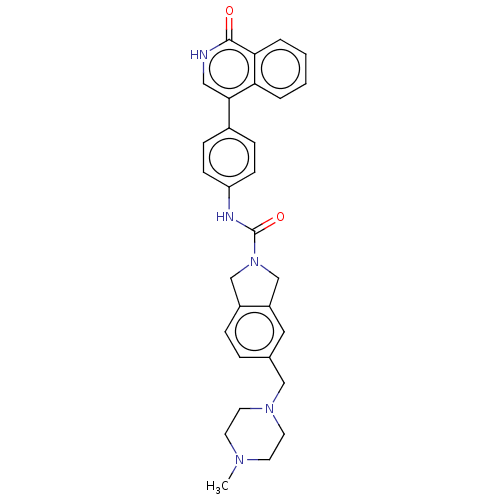 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50528181 (CHEMBL4519035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235658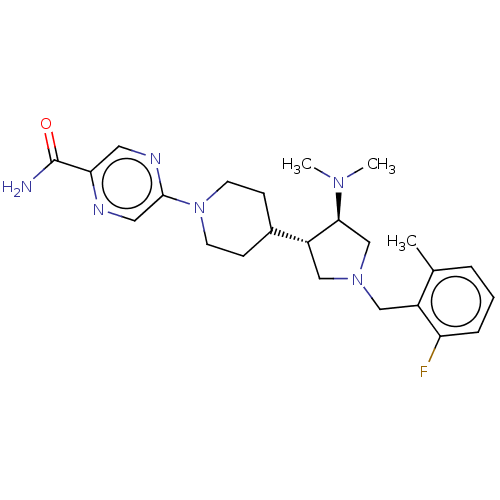 (CHEMBL4073166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235644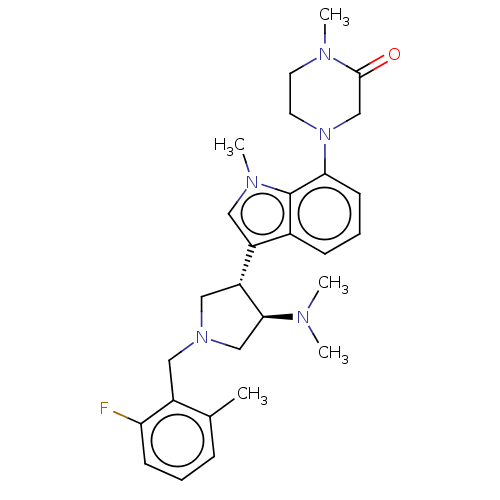 (CHEMBL4065766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Mus musculus) | BDBM50080322 (Acetic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9-acet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Binding of Indolactam-V(ILV) to mutant Protein kinase C delta C1b domain (Phe13 to Gly) | J Med Chem 42: 3436-46 (1999) Article DOI: 10.1021/jm990129n BindingDB Entry DOI: 10.7270/Q25T3JP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50224090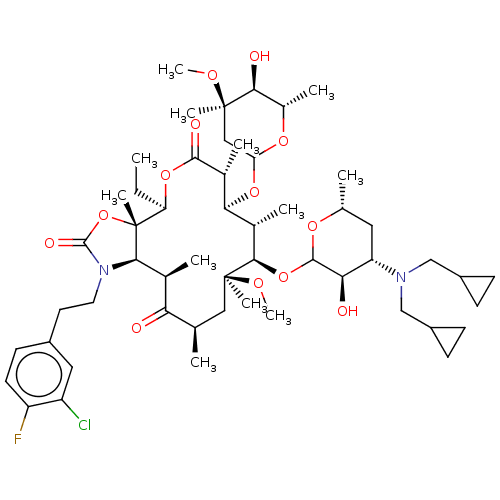 (CHEMBL410940) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human luteinizing releasing hormone receptor cloned in CHO cells | Bioorg Med Chem Lett 14: 1599-602 (2004) BindingDB Entry DOI: 10.7270/Q27083NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196890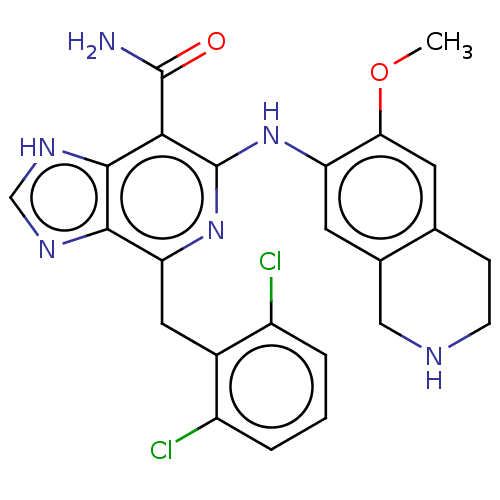 (US9212192, 53) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196891 (US9212192, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112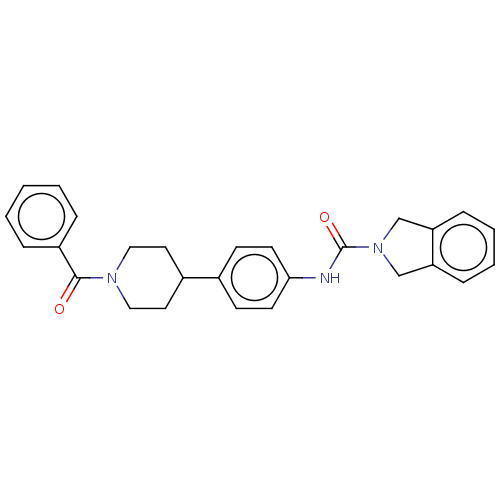 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50409214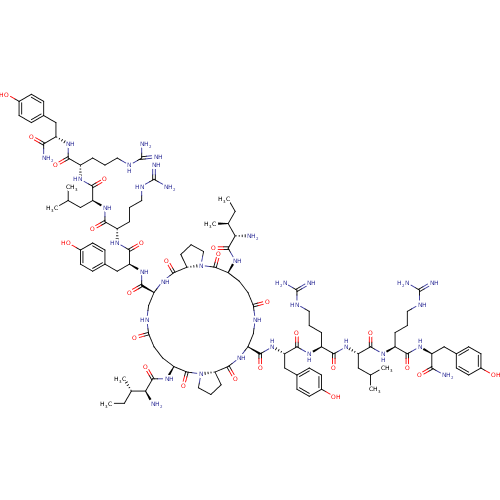 (CHEMBL2110365 | GR-231118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Displacement of (sCy5)-[Lys2 Arg4]-BVD15 from GFP-tagged Y1R in human HEK293T cells assessed as inhibitory constant incubated for 5 mins followed by ... | J Med Chem 63: 5274-5286 (2020) Article DOI: 10.1021/acs.jmedchem.0c00027 BindingDB Entry DOI: 10.7270/Q2RB782R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235632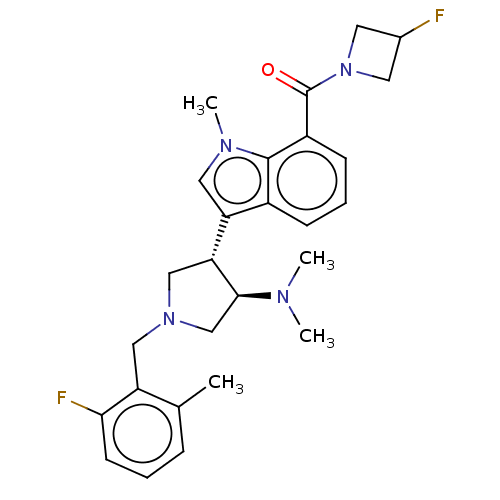 (CHEMBL4077363) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196903 (US9212192, 66) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196897 (US9212192, 60) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196888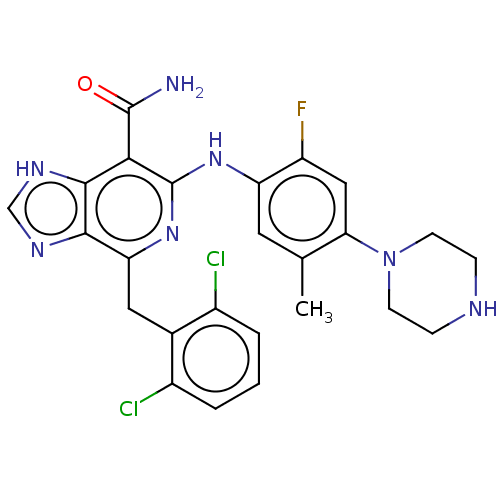 (US9212192, 51) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196887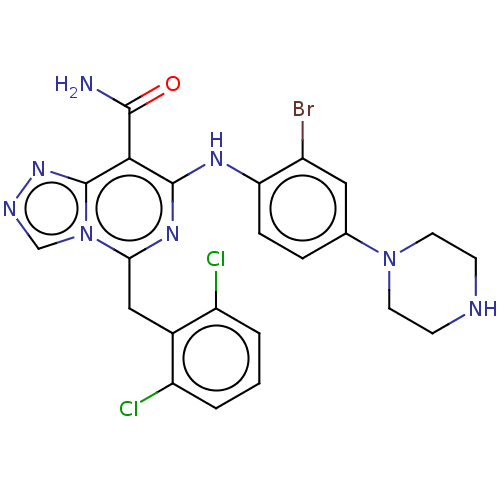 (US9212192, 50) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196886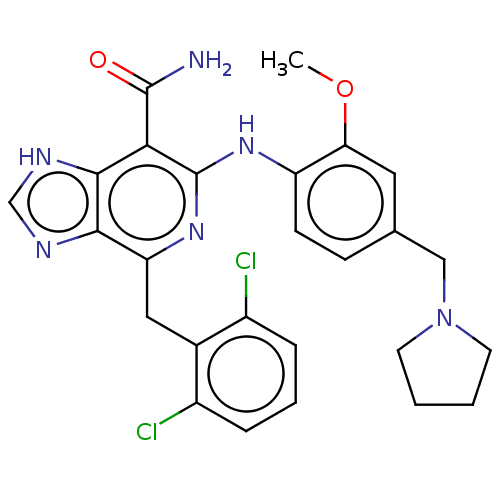 (US9212192, 49) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196885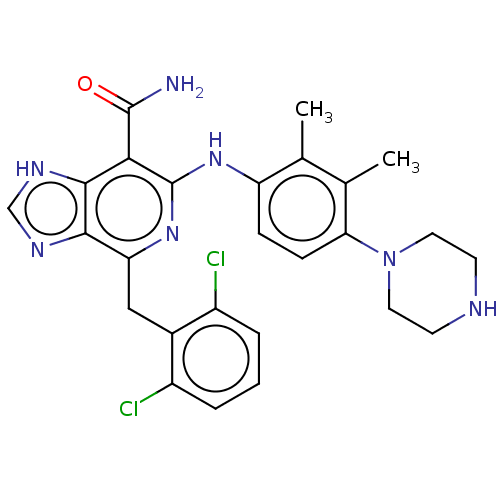 (US9212192, 48) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223986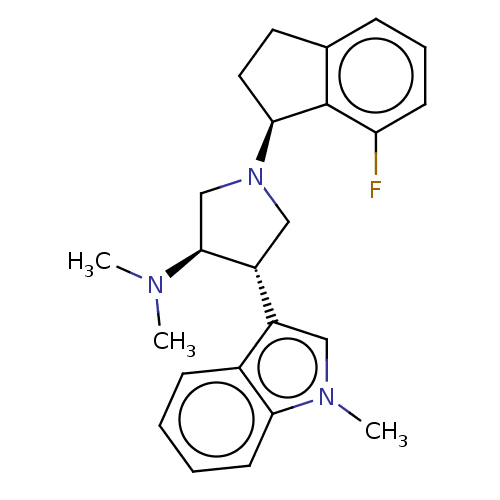 ((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15956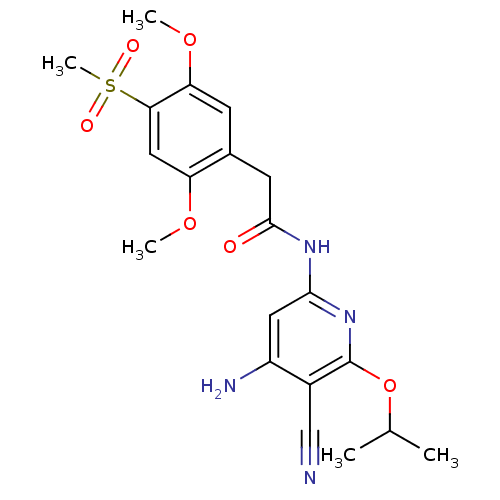 (Aminopyridine-Based Inhibitor 18b | N-(4-Amino-5-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Ser/Thr-kinase selectivity assays were performed using a radioactive FlashPlate-based assay platform. Substrate incorporated radioactivity was counte... | J Med Chem 49: 3563-80 (2006) Article DOI: 10.1021/jm060199b BindingDB Entry DOI: 10.7270/Q2P26WDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196849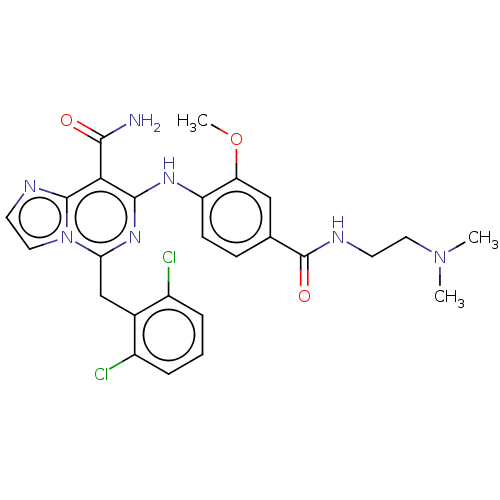 (US9212192, 12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196852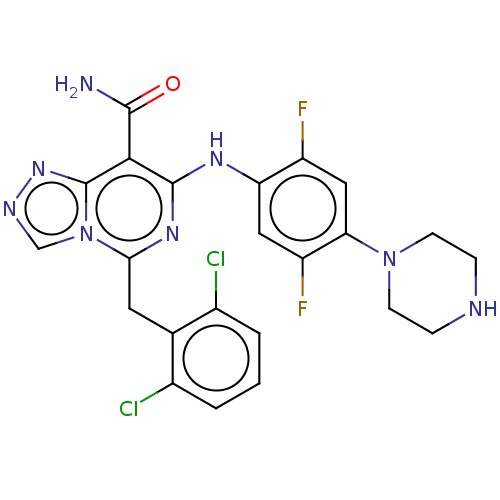 (US9212192, 15) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196854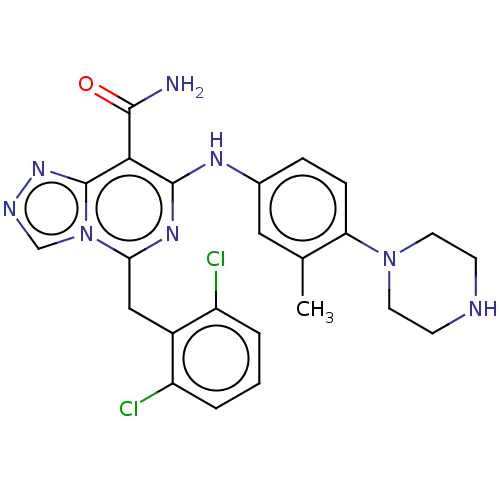 (US9212192, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196855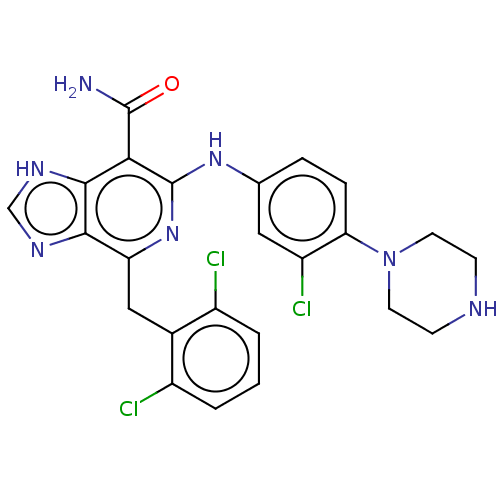 (US9212192, 18) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196857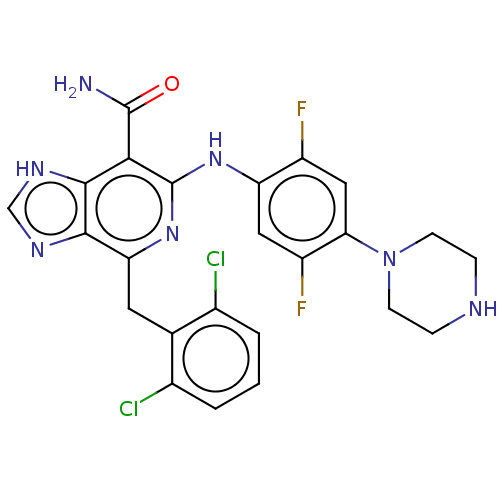 (US9212192, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196862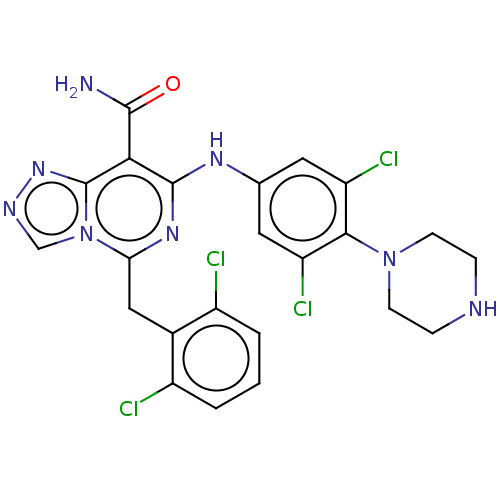 (US9212192, 25) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196863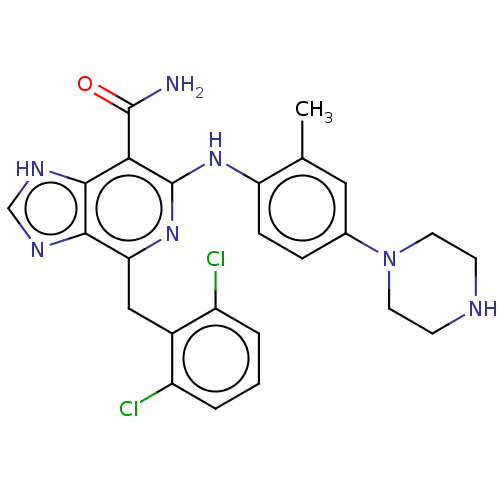 (US9212192, 26) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196864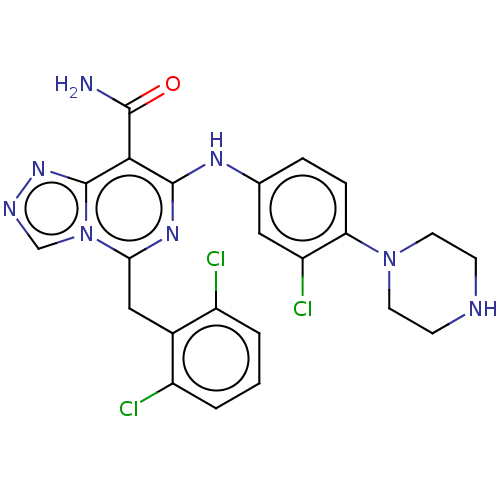 (US9212192, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196865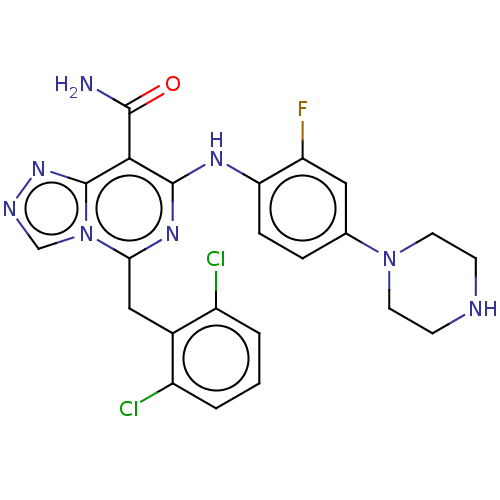 (US9212192, 28) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196866 (US9212192, 29) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196868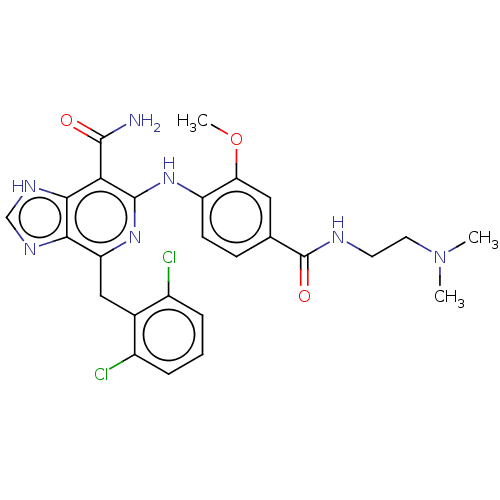 (US9212192, 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196869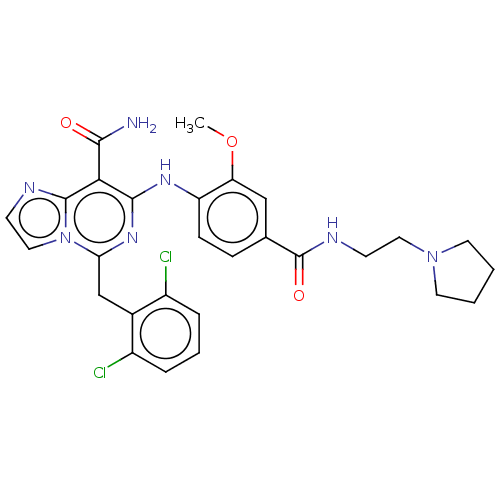 (US9212192, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196870 (US9212192, 33) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196873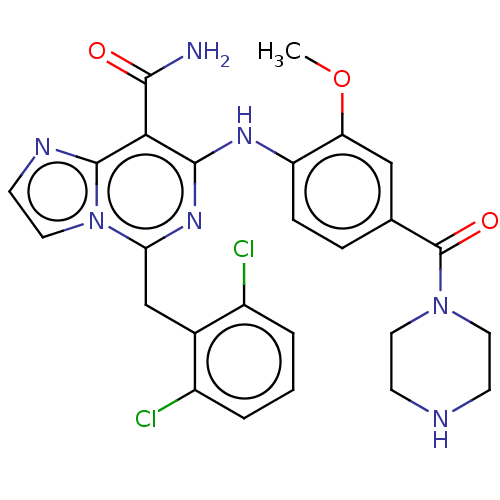 (US9212192, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM196875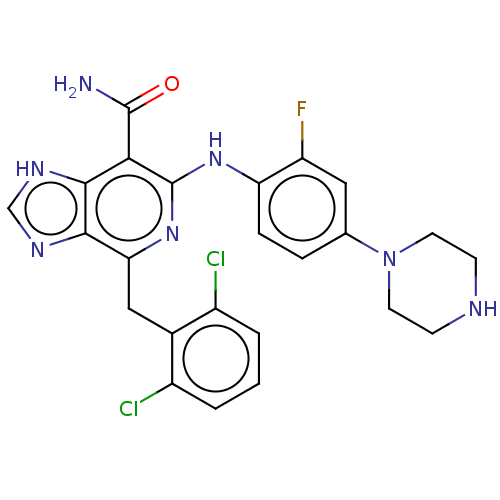 (US9212192, 38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Inc. US Patent | Assay Description ALK kinase assays were conducted with the indicated final concentrations unless otherwise specified. In 384 well black plates (Axygen), 8 ul of compo... | US Patent US9212192 (2015) BindingDB Entry DOI: 10.7270/Q2D79971 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10063 total ) | Next | Last >> |