 Found 19163 hits with Last Name = 'liu' and Initial = 'q'
Found 19163 hits with Last Name = 'liu' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344099
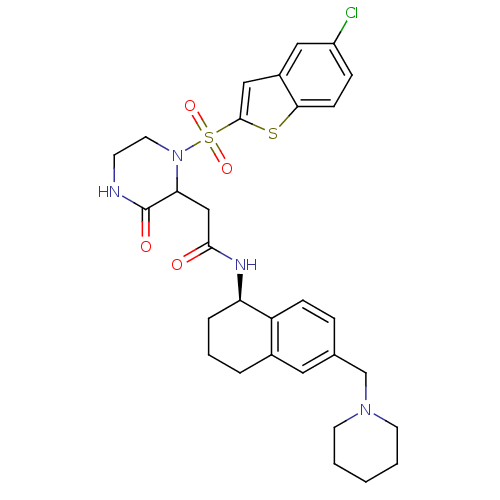
(2-(1-(5-chlorobenzo[b]thiophen-2-ylsulfonyl)-3-oxo...)Show SMILES Clc1ccc2sc(cc2c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C30H35ClN4O4S2/c31-23-8-10-27-22(16-23)17-29(40-27)41(38,39)35-14-11-32-30(37)26(35)18-28(36)33-25-6-4-5-21-15-20(7-9-24(21)25)19-34-12-2-1-3-13-34/h7-10,15-17,25-26H,1-6,11-14,18-19H2,(H,32,37)(H,33,36)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146490
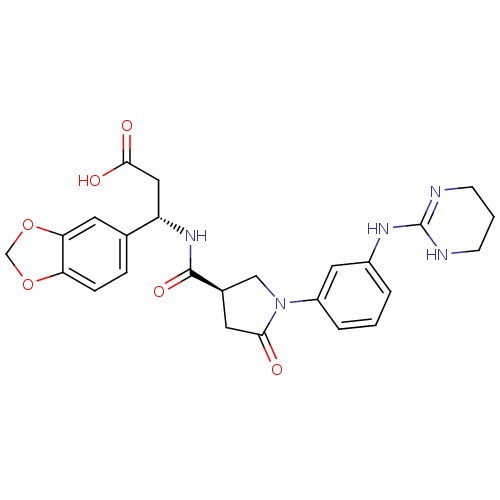
(3-(S)-Benzo[1,3]dioxol-5-yl-3-({(R)-5-oxo-1-[3-(1,...)Show SMILES OC(=O)C[C@H](NC(=O)[C@H]1CN(C(=O)C1)c1cccc(NC2=NCCCN2)c1)c1ccc2OCOc2c1 |t:21| Show InChI InChI=1S/C25H27N5O6/c31-22-10-16(13-30(22)18-4-1-3-17(11-18)28-25-26-7-2-8-27-25)24(34)29-19(12-23(32)33)15-5-6-20-21(9-15)36-14-35-20/h1,3-6,9,11,16,19H,2,7-8,10,12-14H2,(H,29,34)(H,32,33)(H2,26,27,28)/t16-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344098
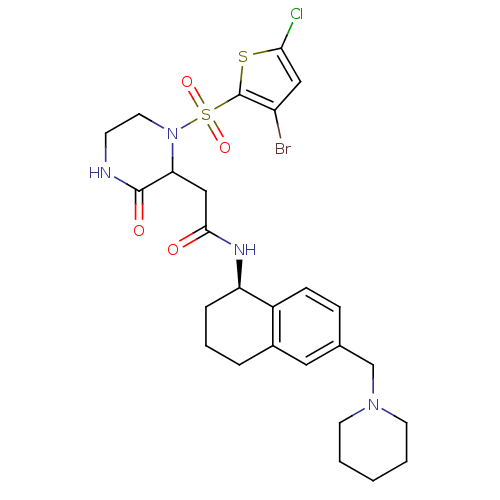
(2-(1-(3-bromo-5-chlorothiophen-2-ylsulfonyl)-3-oxo...)Show SMILES Clc1cc(Br)c(s1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C26H32BrClN4O4S2/c27-20-14-23(28)37-26(20)38(35,36)32-12-9-29-25(34)22(32)15-24(33)30-21-6-4-5-18-13-17(7-8-19(18)21)16-31-10-2-1-3-11-31/h7-8,13-14,21-22H,1-6,9-12,15-16H2,(H,29,34)(H,30,33)/t21-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50272453
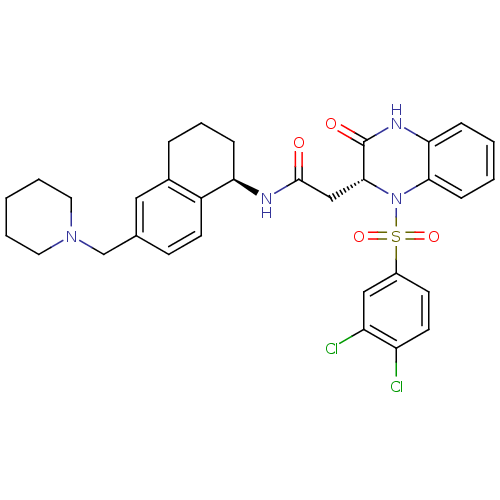
(2-((R)-1-(3,4-dichlorophenylsulfonyl)-3-oxo-1,2,3,...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C32H34Cl2N4O4S/c33-25-14-12-23(18-26(25)34)43(41,42)38-29-10-3-2-8-28(29)36-32(40)30(38)19-31(39)35-27-9-6-7-22-17-21(11-13-24(22)27)20-37-15-4-1-5-16-37/h2-3,8,10-14,17-18,27,30H,1,4-7,9,15-16,19-20H2,(H,35,39)(H,36,40)/t27-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344111
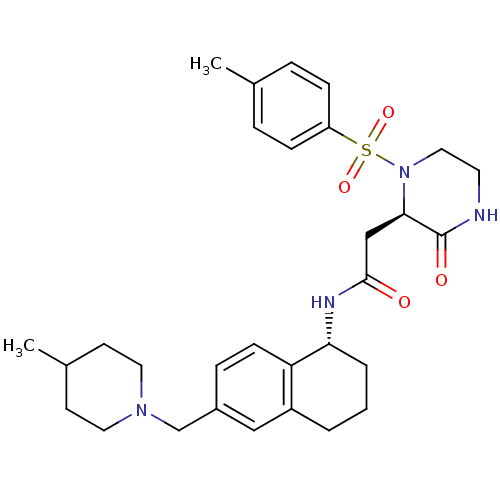
(CHEMBL1777969 | N-((R)-6-((4-methylpiperidin-1-yl)...)Show SMILES CC1CCN(Cc2ccc3[C@@H](CCCc3c2)NC(=O)C[C@H]2N(CCNC2=O)S(=O)(=O)c2ccc(C)cc2)CC1 |r| Show InChI InChI=1S/C30H40N4O4S/c1-21-6-9-25(10-7-21)39(37,38)34-17-14-31-30(36)28(34)19-29(35)32-27-5-3-4-24-18-23(8-11-26(24)27)20-33-15-12-22(2)13-16-33/h6-11,18,22,27-28H,3-5,12-17,19-20H2,1-2H3,(H,31,36)(H,32,35)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344097
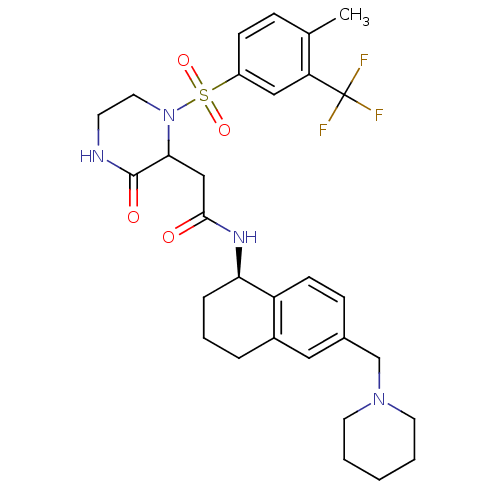
(2-(1-(4-methyl-3-(trifluoromethyl)phenylsulfonyl)-...)Show SMILES Cc1ccc(cc1C(F)(F)F)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C30H37F3N4O4S/c1-20-8-10-23(17-25(20)30(31,32)33)42(40,41)37-15-12-34-29(39)27(37)18-28(38)35-26-7-5-6-22-16-21(9-11-24(22)26)19-36-13-3-2-4-14-36/h8-11,16-17,26-27H,2-7,12-15,18-19H2,1H3,(H,34,39)(H,35,38)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50227856
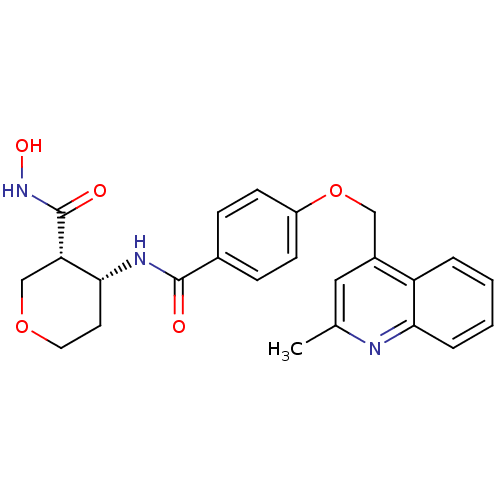
((3R,4R)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C24H25N3O5/c1-15-12-17(19-4-2-3-5-21(19)25-15)13-32-18-8-6-16(7-9-18)23(28)26-22-10-11-31-14-20(22)24(29)27-30/h2-9,12,20,22,30H,10-11,13-14H2,1H3,(H,26,28)(H,27,29)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of pig TACE |
Bioorg Med Chem Lett 18: 241-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.093
BindingDB Entry DOI: 10.7270/Q2JM2BGZ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344096
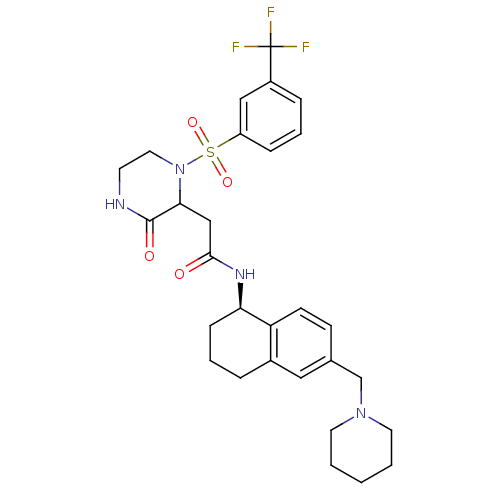
(2-(3-oxo-1-(3-(trifluoromethyl)phenylsulfonyl)pipe...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H35F3N4O4S/c30-29(31,32)22-7-5-8-23(17-22)41(39,40)36-15-12-33-28(38)26(36)18-27(37)34-25-9-4-6-21-16-20(10-11-24(21)25)19-35-13-2-1-3-14-35/h5,7-8,10-11,16-17,25-26H,1-4,6,9,12-15,18-19H2,(H,33,38)(H,34,37)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344100
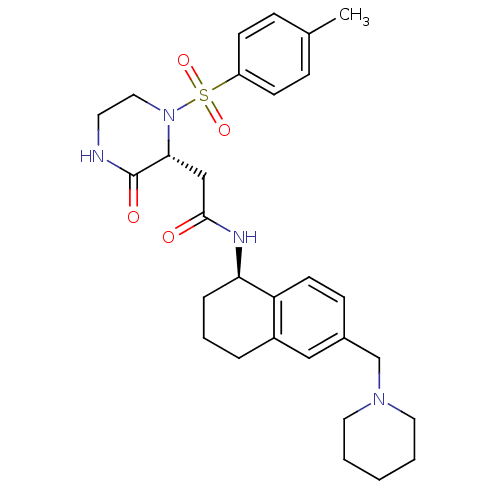
(2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344087
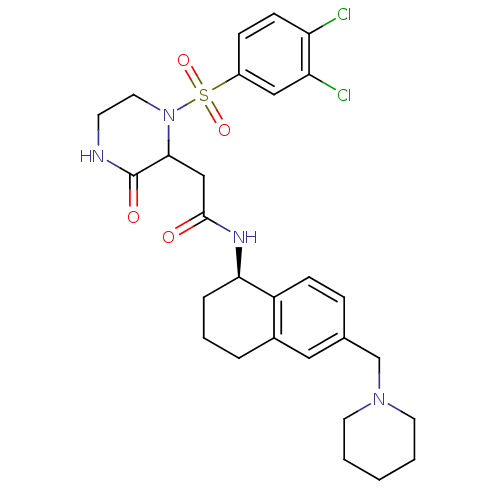
(2-(1-(3,4-dichlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H34Cl2N4O4S/c29-23-10-8-21(16-24(23)30)39(37,38)34-14-11-31-28(36)26(34)17-27(35)32-25-6-4-5-20-15-19(7-9-22(20)25)18-33-12-2-1-3-13-33/h7-10,15-16,25-26H,1-6,11-14,17-18H2,(H,31,36)(H,32,35)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344110

(2-((R)-3-oxo-1-tosylpiperazin-2-yl)-N-((R)-7-(pipe...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O5S/c1-20-5-8-22(9-6-20)38(35,36)32-15-12-29-28(34)25(32)18-27(33)30-24-11-16-37-26-17-21(7-10-23(24)26)19-31-13-3-2-4-14-31/h5-10,17,24-25H,2-4,11-16,18-19H2,1H3,(H,29,34)(H,30,33)/t24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II |
Bioorg Med Chem Lett 13: 2097-100 (2003)
BindingDB Entry DOI: 10.7270/Q2Z60NF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrate |
J Med Chem 46: 1989-96 (2003)
Article DOI: 10.1021/jm020515w
BindingDB Entry DOI: 10.7270/Q2SQ8ZRG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
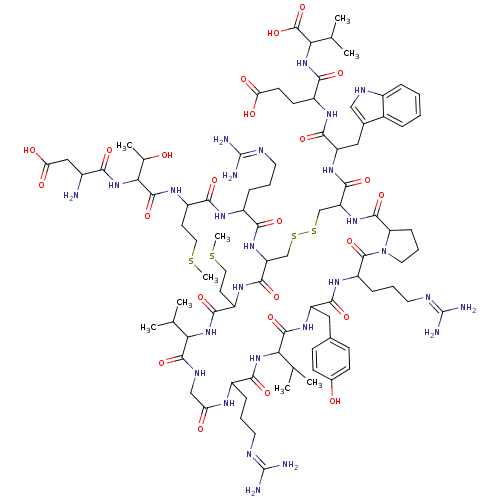
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344092
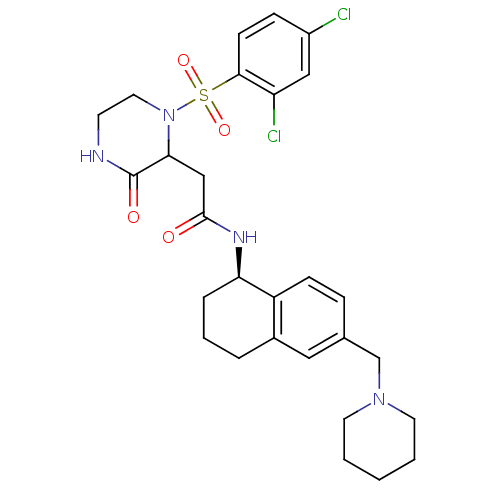
(2-(1-(2,4-dichlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H34Cl2N4O4S/c29-21-8-10-26(23(30)16-21)39(37,38)34-14-11-31-28(36)25(34)17-27(35)32-24-6-4-5-20-15-19(7-9-22(20)24)18-33-12-2-1-3-13-33/h7-10,15-16,24-25H,1-6,11-14,17-18H2,(H,31,36)(H,32,35)/t24-,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344119
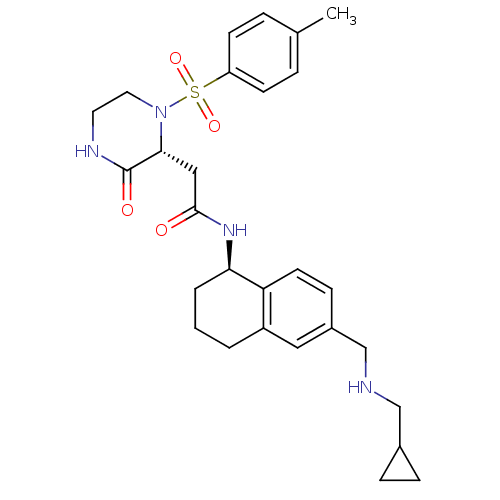
(CHEMBL1777977 | N-((R)-6-((cyclopropylmethylamino)...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CNCC3CC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O4S/c1-19-5-10-23(11-6-19)37(35,36)32-14-13-30-28(34)26(32)16-27(33)31-25-4-2-3-22-15-21(9-12-24(22)25)18-29-17-20-7-8-20/h5-6,9-12,15,20,25-26,29H,2-4,7-8,13-14,16-18H2,1H3,(H,30,34)(H,31,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344120
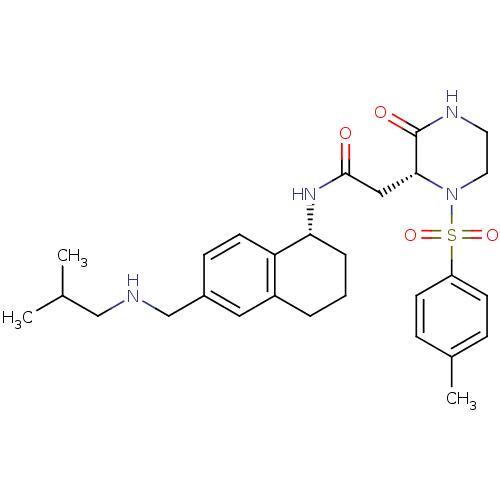
(CHEMBL1777978 | N-((R)-6-((isobutylamino)methyl)-1...)Show SMILES CC(C)CNCc1ccc2[C@@H](CCCc2c1)NC(=O)C[C@H]1N(CCNC1=O)S(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C28H38N4O4S/c1-19(2)17-29-18-21-9-12-24-22(15-21)5-4-6-25(24)31-27(33)16-26-28(34)30-13-14-32(26)37(35,36)23-10-7-20(3)8-11-23/h7-12,15,19,25-26,29H,4-6,13-14,16-18H2,1-3H3,(H,30,34)(H,31,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344093
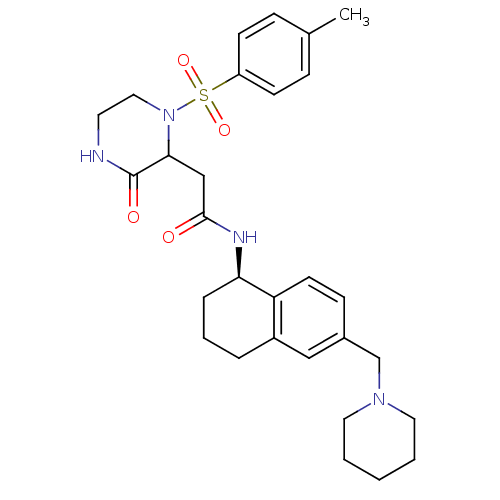
(2-(3-oxo-1-tosylpiperazin-2-yl)-N-((R)-6-(piperidi...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
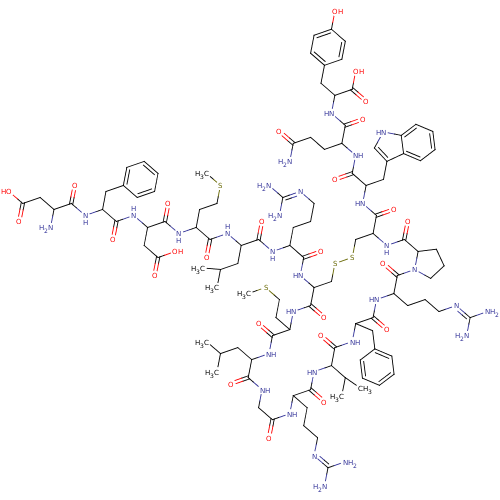
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344109
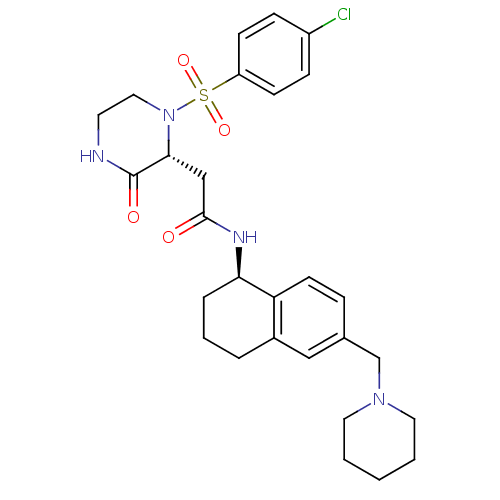
(2-((R)-1-(4-chlorophenylsulfonyl)-3-oxopiperazin-2...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-22-8-10-23(11-9-22)38(36,37)33-16-13-30-28(35)26(33)18-27(34)31-25-6-4-5-21-17-20(7-12-24(21)25)19-32-14-2-1-3-15-32/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,30,35)(H,31,34)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50183715
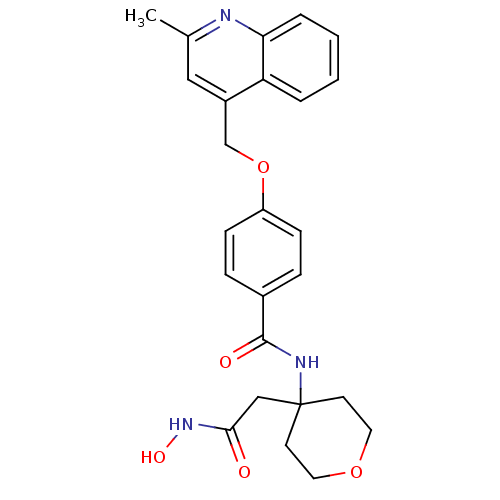
(CHEMBL207305 | N-(4-(2-(hydroxyamino)-2-oxoethyl)-...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)NC2(CC(=O)NO)CCOCC2)c2ccccc2n1 Show InChI InChI=1S/C25H27N3O5/c1-17-14-19(21-4-2-3-5-22(21)26-17)16-33-20-8-6-18(7-9-20)24(30)27-25(15-23(29)28-31)10-12-32-13-11-25/h2-9,14,31H,10-13,15-16H2,1H3,(H,27,30)(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of porcine TACE |
Bioorg Med Chem Lett 16: 2699-704 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.015
BindingDB Entry DOI: 10.7270/Q2TB16H3 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50209744
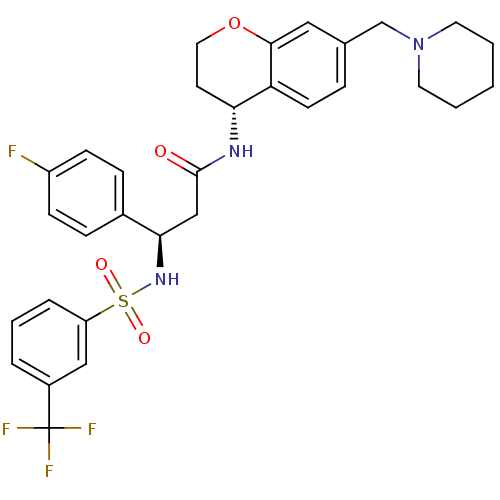
((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...)Show SMILES Fc1ccc(cc1)[C@@H](CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12)NS(=O)(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H33F4N3O4S/c32-24-10-8-22(9-11-24)28(37-43(40,41)25-6-4-5-23(18-25)31(33,34)35)19-30(39)36-27-13-16-42-29-17-21(7-12-26(27)29)20-38-14-2-1-3-15-38/h4-12,17-18,27-28,37H,1-3,13-16,19-20H2,(H,36,39)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11551
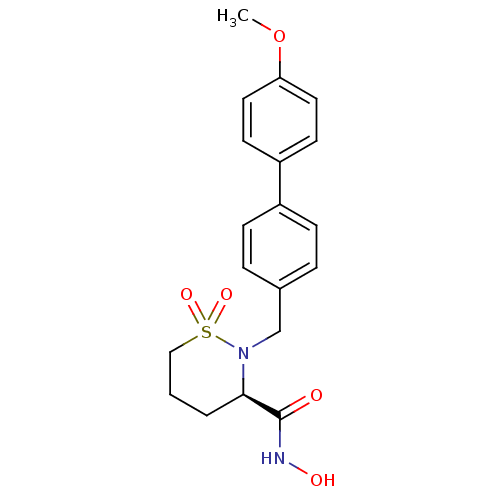
((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50209744

((R)-3-(4-fluorophenyl)-N-((R)-7-(piperidin-1-ylmet...)Show SMILES Fc1ccc(cc1)[C@@H](CC(=O)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12)NS(=O)(=O)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H33F4N3O4S/c32-24-10-8-22(9-11-24)28(37-43(40,41)25-6-4-5-23(18-25)31(33,34)35)19-30(39)36-27-13-16-42-29-17-21(7-12-26(27)29)20-38-14-2-1-3-15-38/h4-12,17-18,27-28,37H,1-3,13-16,19-20H2,(H,36,39)/t27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells |
J Med Chem 50: 2200-12 (2007)
Article DOI: 10.1021/jm070055c
BindingDB Entry DOI: 10.7270/Q2MS3SG2 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344090
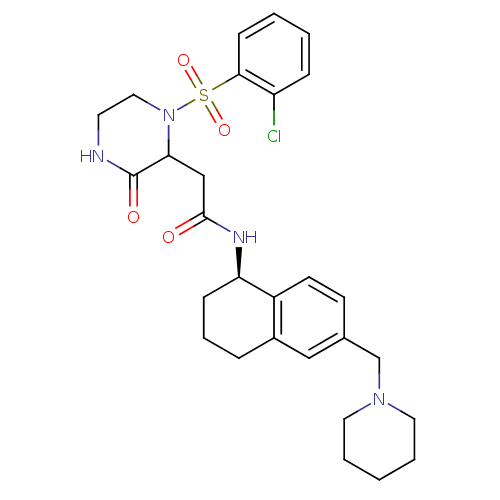
(2-(1-(2-chlorophenylsulfonyl)-3-oxopiperazin-2-yl)...)Show SMILES Clc1ccccc1S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-23-8-2-3-10-26(23)38(36,37)33-16-13-30-28(35)25(33)18-27(34)31-24-9-6-7-21-17-20(11-12-22(21)24)19-32-14-4-1-5-15-32/h2-3,8,10-12,17,24-25H,1,4-7,9,13-16,18-19H2,(H,30,35)(H,31,34)/t24-,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50469353

(CHEMBL4283353)Show SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)-c1ccc(CN2CCCN(C)CC2)cc1 |r,wU:13.12,wD:16.16,3.3,(17.07,-10.92,;18.4,-10.15,;19.74,-10.92,;21.07,-10.15,;21.07,-8.61,;22.4,-10.92,;23.74,-10.15,;23.74,-8.61,;25.07,-7.84,;26.4,-8.6,;27.88,-8.12,;28.79,-9.38,;27.88,-10.63,;28.63,-11.97,;30.16,-12,;30.91,-13.35,;30.11,-14.67,;30.85,-16.02,;28.57,-14.64,;27.83,-13.29,;26.4,-10.15,;25.07,-10.92,;28.59,-6.76,;30.13,-6.7,;30.84,-5.34,;30.02,-4.03,;30.73,-2.67,;32.27,-2.6,;33,-3.97,;34.51,-4.24,;35.67,-3.22,;35.61,-1.69,;36.96,-.95,;34.36,-.79,;32.87,-1.2,;28.47,-4.1,;27.77,-5.47,)| Show InChI InChI=1S/C30H44N6O/c1-4-6-22(2)32-30-31-19-27-28(21-36(29(27)33-30)25-11-13-26(37)14-12-25)24-9-7-23(8-10-24)20-35-16-5-15-34(3)17-18-35/h7-10,19,21-22,25-26,37H,4-6,11-18,20H2,1-3H3,(H,31,32,33)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay |
J Med Chem 61: 10242-10254 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01229
BindingDB Entry DOI: 10.7270/Q2K076ZK |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104967
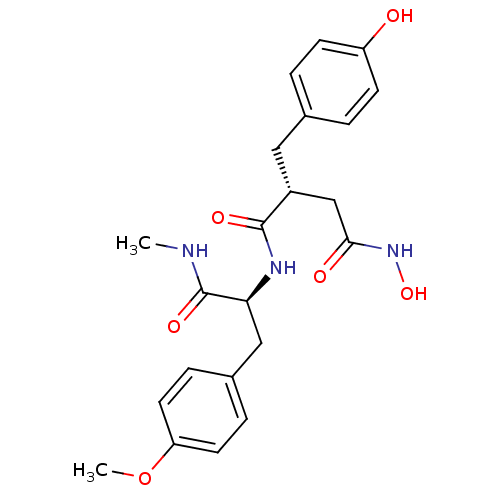
(CHEMBL419751 | N*4*-Hydroxy-2-(4-hydroxy-benzyl)-N...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@@H](CC(=O)NO)Cc1ccc(O)cc1 Show InChI InChI=1S/C22H27N3O6/c1-23-22(29)19(12-15-5-9-18(31-2)10-6-15)24-21(28)16(13-20(27)25-30)11-14-3-7-17(26)8-4-14/h3-10,16,19,26,30H,11-13H2,1-2H3,(H,23,29)(H,24,28)(H,25,27)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344095
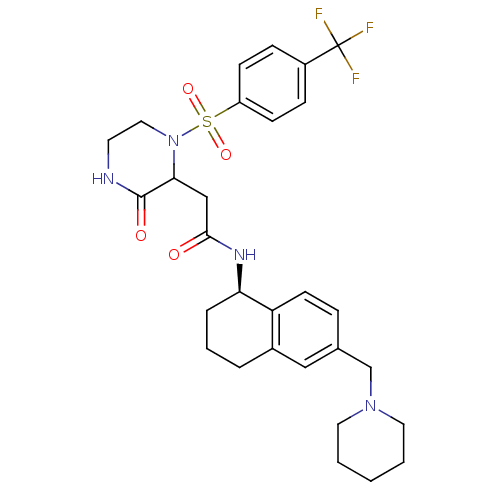
(2-(3-oxo-1-(4-(trifluoromethyl)phenylsulfonyl)pipe...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H35F3N4O4S/c30-29(31,32)22-8-10-23(11-9-22)41(39,40)36-16-13-33-28(38)26(36)18-27(37)34-25-6-4-5-21-17-20(7-12-24(21)25)19-35-14-2-1-3-15-35/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,33,38)(H,34,37)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344108
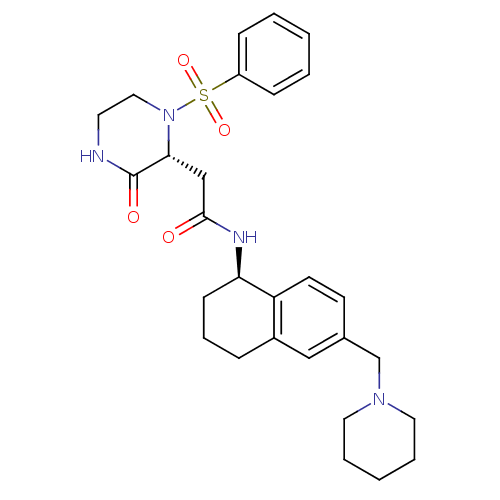
(2-((R)-3-oxo-1-(phenylsulfonyl)piperazin-2-yl)-N-(...)Show SMILES O=C(C[C@H]1N(CCNC1=O)S(=O)(=O)c1ccccc1)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H36N4O4S/c33-27(19-26-28(34)29-14-17-32(26)37(35,36)23-9-3-1-4-10-23)30-25-11-7-8-22-18-21(12-13-24(22)25)20-31-15-5-2-6-16-31/h1,3-4,9-10,12-13,18,25-26H,2,5-8,11,14-17,19-20H2,(H,29,34)(H,30,33)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146492
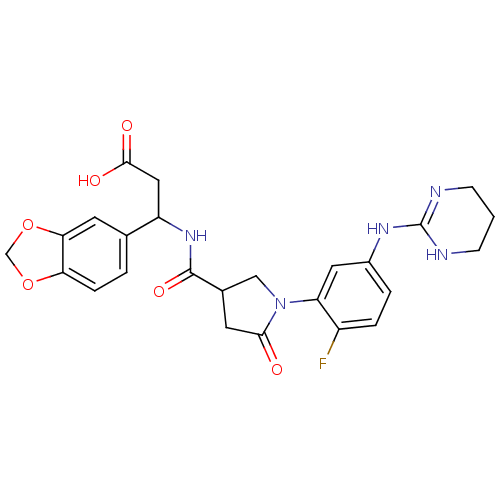
(3-Benzo[1,3]dioxol-5-yl-3-({1-[2-fluoro-5-(1,4,5,6...)Show SMILES OC(=O)CC(NC(=O)C1CN(C(=O)C1)c1cc(NC2=NCCCN2)ccc1F)c1ccc2OCOc2c1 |t:19| Show InChI InChI=1S/C25H26FN5O6/c26-17-4-3-16(29-25-27-6-1-7-28-25)10-19(17)31-12-15(9-22(31)32)24(35)30-18(11-23(33)34)14-2-5-20-21(8-14)37-13-36-20/h2-5,8,10,15,18H,1,6-7,9,11-13H2,(H,30,35)(H,33,34)(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards vitronectin receptor (AlphaV-beta3 integrin). |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
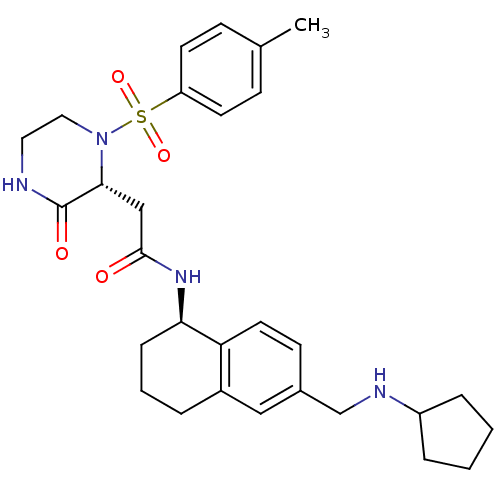
(Homo sapiens (Human)) | BDBM50344117
(CHEMBL1777975 | N-((R)-6-((cyclopentylamino)methyl...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CNC3CCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-20-9-12-24(13-10-20)38(36,37)33-16-15-30-29(35)27(33)18-28(34)32-26-8-4-5-22-17-21(11-14-25(22)26)19-31-23-6-2-3-7-23/h9-14,17,23,26-27,31H,2-8,15-16,18-19H2,1H3,(H,30,35)(H,32,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
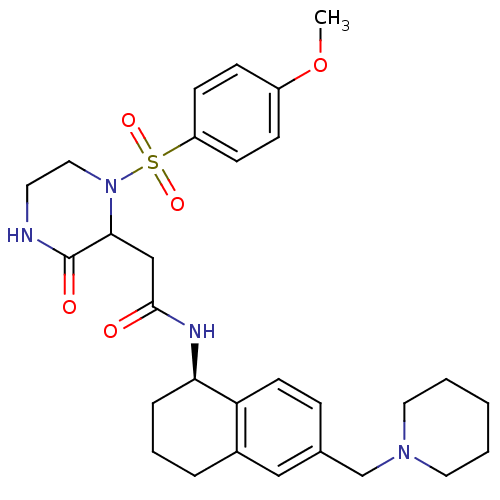
(Homo sapiens (Human)) | BDBM50344094
(2-(1-(4-methoxyphenylsulfonyl)-3-oxopiperazin-2-yl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O5S/c1-38-23-9-11-24(12-10-23)39(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-22-18-21(8-13-25(22)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
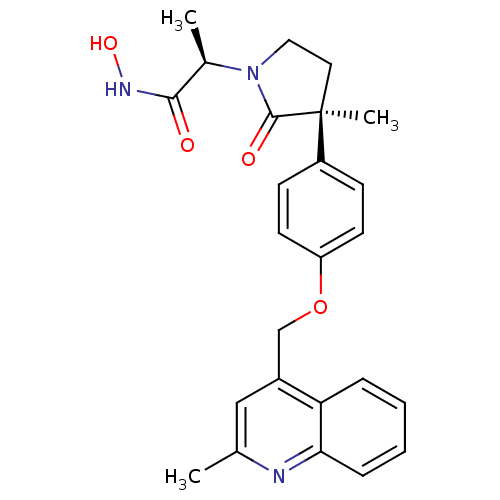
(Sus scrofa (pig)) | BDBM26526
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Affinity for Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 4954-7 (2002)
BindingDB Entry DOI: 10.7270/Q2XP7497 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314782

(CHEMBL1089866 | N-Cyclopropyl-3-(1-(2,4-difluoroph...)Show SMILES CCn1c2n(ncc2cc(-c2cc(ccc2C)C(=O)NC2CC2)c1=O)-c1ccc(F)cc1F Show InChI InChI=1S/C25H22F2N4O2/c1-3-30-24-16(13-28-31(24)22-9-6-17(26)12-21(22)27)11-20(25(30)33)19-10-15(5-4-14(19)2)23(32)29-18-7-8-18/h4-6,9-13,18H,3,7-8H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated AFT2 phosphorylation after 1 hr by HTRF assay |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged recombinant Aurora 1 (62 to 344) (unknown origin) expressed in baculovirus expression system by radiometric ass... |
Eur J Med Chem 78: 65-71 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.027
BindingDB Entry DOI: 10.7270/Q2Z60QKP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344091
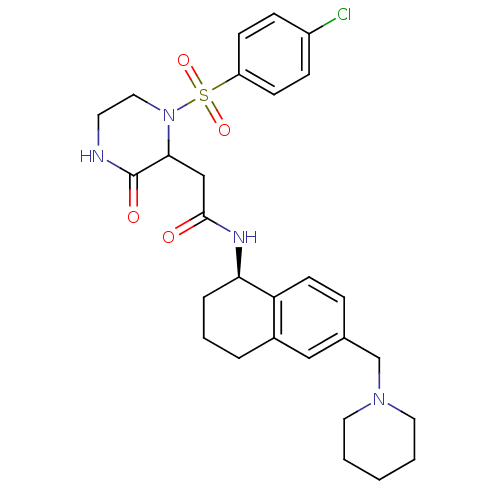
(2-(1-(4-chlorophenylsulfonyl)-3-oxopiperazin-2-yl)...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1CCNC(=O)C1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C28H35ClN4O4S/c29-22-8-10-23(11-9-22)38(36,37)33-16-13-30-28(35)26(33)18-27(34)31-25-6-4-5-21-17-20(7-12-24(21)25)19-32-14-2-1-3-15-32/h7-12,17,25-26H,1-6,13-16,18-19H2,(H,30,35)(H,31,34)/t25-,26?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 21: 3384-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.115
BindingDB Entry DOI: 10.7270/Q2FX79R6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104981
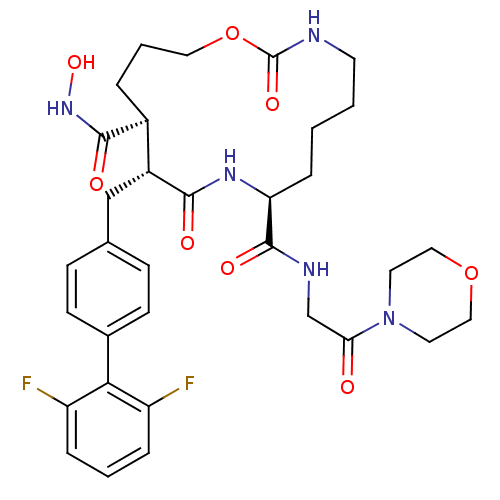
(11-(2',6'-Difluoro-biphenyl-4-ylmethyl)-2,10-dioxo...)Show SMILES ONC(=O)[C@H]1CCCOC(=O)NCCCC[C@H](NC(=O)[C@@H]1Cc1ccc(cc1)-c1c(F)cccc1F)C(=O)NCC(=O)N1CCOCC1 Show InChI InChI=1S/C33H41F2N5O8/c34-25-6-3-7-26(35)29(25)22-11-9-21(10-12-22)19-24-23(31(43)39-46)5-4-16-48-33(45)36-13-2-1-8-27(38-30(24)42)32(44)37-20-28(41)40-14-17-47-18-15-40/h3,6-7,9-12,23-24,27,46H,1-2,4-5,8,13-20H2,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 3351-4 (2001)
BindingDB Entry DOI: 10.7270/Q2VD6XRT |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50322858
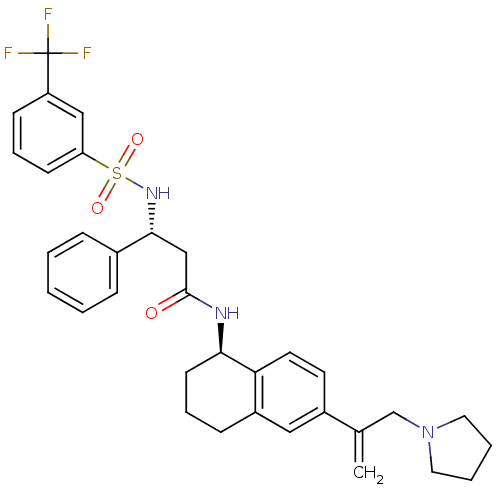
((R)-3-phenyl-N-((R)-6-(3-(pyrrolidin-1-yl)prop-1-e...)Show SMILES FC(F)(F)c1cccc(c1)S(=O)(=O)N[C@H](CC(=O)N[C@@H]1CCCc2cc(ccc12)C(=C)CN1CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C33H36F3N3O3S/c1-23(22-39-17-5-6-18-39)25-15-16-29-26(19-25)11-7-14-30(29)37-32(40)21-31(24-9-3-2-4-10-24)38-43(41,42)28-13-8-12-27(20-28)33(34,35)36/h2-4,8-10,12-13,15-16,19-20,30-31,38H,1,5-7,11,14,17-18,21-22H2,(H,37,40)/t30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]Lys-desArg9-BK from human bradykinin B1 receptor |
Bioorg Med Chem Lett 20: 4593-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.010
BindingDB Entry DOI: 10.7270/Q25Q4W88 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104969
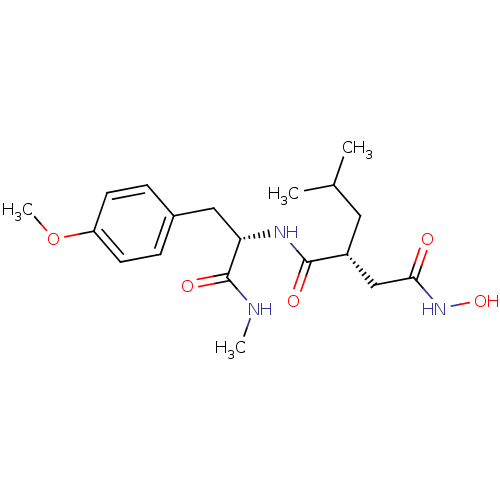
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50203200
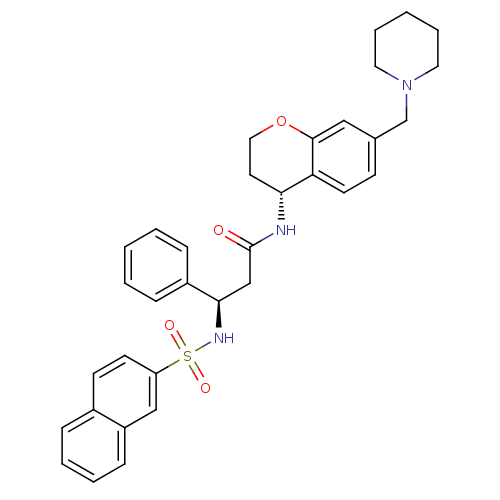
((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...)Show SMILES O=C(C[C@@H](NS(=O)(=O)c1ccc2ccccc2c1)c1ccccc1)N[C@@H]1CCOc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C34H37N3O4S/c38-34(35-31-17-20-41-33-21-25(13-16-30(31)33)24-37-18-7-2-8-19-37)23-32(27-10-3-1-4-11-27)36-42(39,40)29-15-14-26-9-5-6-12-28(26)22-29/h1,3-6,9-16,21-22,31-32,36H,2,7-8,17-20,23-24H2,(H,35,38)/t31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells |
J Med Chem 50: 2200-12 (2007)
Article DOI: 10.1021/jm070055c
BindingDB Entry DOI: 10.7270/Q2MS3SG2 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50146497
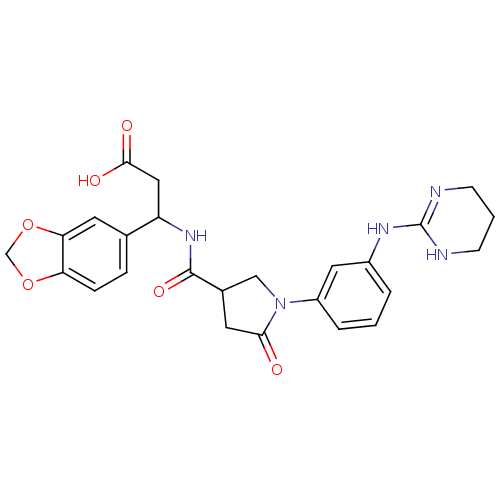
(3-Benzo[1,3]dioxol-5-yl-3-({5-oxo-1-[3-(1,4,5,6-te...)Show SMILES OC(=O)CC(NC(=O)C1CN(C(=O)C1)c1cccc(NC2=NCCCN2)c1)c1ccc2OCOc2c1 |t:21| Show InChI InChI=1S/C25H27N5O6/c31-22-10-16(13-30(22)18-4-1-3-17(11-18)28-25-26-7-2-8-27-25)24(34)29-19(12-23(32)33)15-5-6-20-21(9-15)36-14-35-20/h1,3-6,9,11,16,19H,2,7-8,10,12-14H2,(H,29,34)(H,32,33)(H2,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Affinity for alphaIIb-beta3 integrin |
Bioorg Med Chem Lett 14: 2905-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.033
BindingDB Entry DOI: 10.7270/Q2416WJD |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314785

(CHEMBL1091929 | N-Cyclopropyl-3-(1-(2,6-difluoroph...)Show SMILES Cc1c(F)cc(cc1-c1cc2cnn(-c3c(F)cccc3F)c2n(C)c1=O)C(=O)NC1CC1 |(1.2,-3.81,;-.13,-3.04,;-1.47,-3.81,;-1.46,-5.35,;-2.8,-3.04,;-2.8,-1.5,;-1.47,-.73,;-.14,-1.5,;1.2,-.73,;2.53,-1.49,;3.86,-.72,;5.33,-1.2,;6.23,.05,;5.3,1.31,;6.07,2.64,;7.56,2.72,;8.35,1.37,;8.33,4.06,;7.49,5.35,;5.95,5.35,;5.29,3.99,;3.7,4.17,;3.86,.82,;2.53,1.59,;2.53,3.13,;1.19,.81,;-.14,1.58,;-4.14,-.73,;-4.14,.81,;-5.47,-1.51,;-6.81,-.74,;-8.35,-.74,;-7.58,.6,)| Show InChI InChI=1S/C24H19F3N4O2/c1-12-16(8-13(10-20(12)27)22(32)29-15-6-7-15)17-9-14-11-28-31(23(14)30(2)24(17)33)21-18(25)4-3-5-19(21)26/h3-5,8-11,15H,6-7H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated AFT2 phosphorylation after 1 hr by HTRF assay |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314778
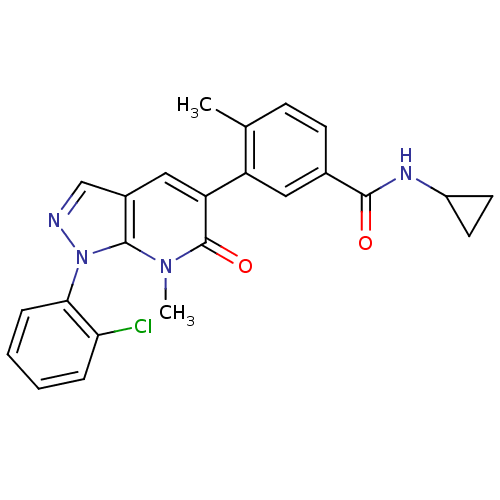
(3-(1-(2-Chlorophenyl)-7-methyl-6-oxo-6,7-dihydro-1...)Show SMILES Cc1ccc(cc1-c1cc2cnn(-c3ccccc3Cl)c2n(C)c1=O)C(=O)NC1CC1 Show InChI InChI=1S/C24H21ClN4O2/c1-14-7-8-15(22(30)27-17-9-10-17)11-18(14)19-12-16-13-26-29(23(16)28(2)24(19)31)21-6-4-3-5-20(21)25/h3-8,11-13,17H,9-10H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated AFT2 phosphorylation after 1 hr by HTRF assay |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314788

(3-(1-(2,6-Difluorophenyl)-7-methyl-6-oxo-6,7-dihyd...)Show SMILES Cc1ccc(cc1-c1cc2cnn(-c3c(F)cccc3F)c2n(C)c1=O)C(=O)Nc1ccon1 |(1.64,-4.57,;.31,-3.8,;-1.02,-4.57,;-2.36,-3.8,;-2.36,-2.26,;-1.02,-1.49,;.31,-2.26,;1.64,-1.49,;2.98,-2.26,;4.31,-1.49,;5.78,-1.97,;6.68,-.72,;5.75,.53,;6.52,1.87,;8.01,1.95,;8.8,.59,;8.78,3.28,;7.94,4.57,;6.41,4.57,;5.74,3.22,;4.16,3.4,;4.31,.05,;2.98,.82,;2.98,2.36,;1.64,.05,;.31,.82,;-3.69,-1.49,;-3.69,.05,;-5.02,-2.26,;-6.36,-1.49,;-7.77,-2.12,;-8.8,-.98,;-8.03,.36,;-6.52,.04,)| Show InChI InChI=1S/C24H17F2N5O3/c1-13-6-7-14(22(32)28-20-8-9-34-29-20)10-16(13)17-11-15-12-27-31(23(15)30(2)24(17)33)21-18(25)4-3-5-19(21)26/h3-12H,1-2H3,(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha-mediated AFT2 phosphorylation after 1 hr by HTRF assay |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data