

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268509 (CHEMBL495623 | N-[3-(3-(3,4-Dichlorophenoxy)phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268510 (CHEMBL495624 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268511 (CHEMBL497634 | N-[3-(3-(4-Chlorophenoxy)phenyl)pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268512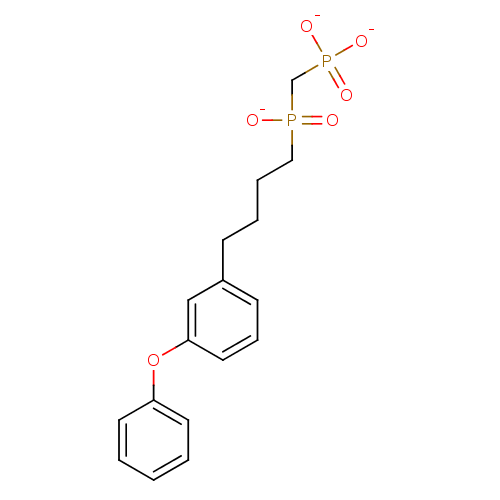 (3-(3-Phenoxyphenyl)propylphosphinylmethylphosphoni...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50388903 (CHEMBL2063253) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus His6-tagged CrtM expressed in Escherichia coli BL21(DE3) using Farnesyl pyrophosphate as substrate incubated for ... | ACS Med Chem Lett 3: 402-406 (2012) Article DOI: 10.1021/ml300038t BindingDB Entry DOI: 10.7270/Q2XW4KVQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268627 (CHEMBL496801 | N-Hydroxy-2-phosphono-5-(3-phenoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268563 (CHEMBL497617 | N-Hydroxy-N-[3-(3-phenoxyphenyl)pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268562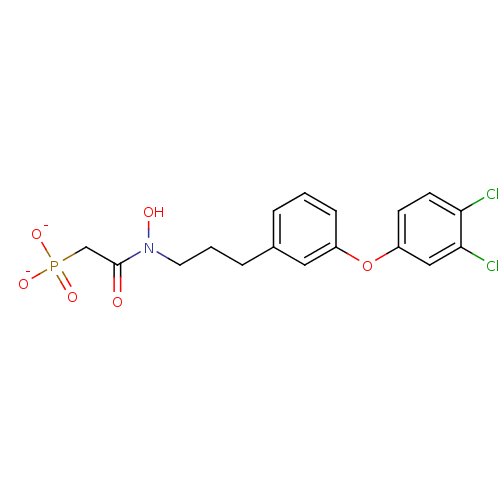 (CHEMBL497616 | N-Hydroxy-N-[3-(3-(3,4-dichlorophen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50388398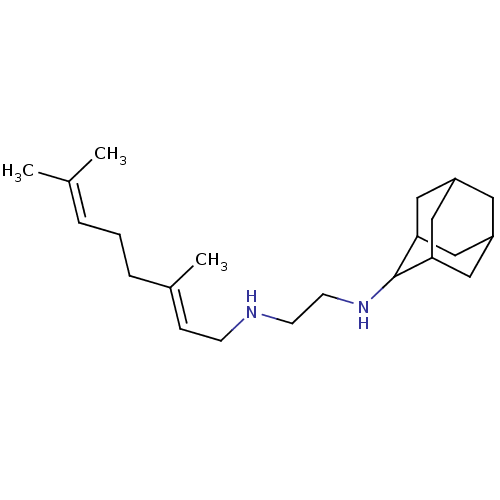 (CHEMBL561057 | SQ-109) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 27659 dehydrosqualene synthase expressed in Escherichia coli BL21(DE3) after 30 mins by spectrophotometric a... | J Med Chem 55: 4367-72 (2012) Article DOI: 10.1021/jm300208p BindingDB Entry DOI: 10.7270/Q2Z320QH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50388906 (CHEMBL2063256) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus His6-tagged CrtM expressed in Escherichia coli BL21(DE3) using Farnesyl pyrophosphate as substrate incubated for ... | ACS Med Chem Lett 3: 402-406 (2012) Article DOI: 10.1021/ml300038t BindingDB Entry DOI: 10.7270/Q2XW4KVQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268625 (CHEMBL447414 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268510 (CHEMBL495624 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50388398 (CHEMBL561057 | SQ-109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human squalene synthase | J Med Chem 55: 4367-72 (2012) Article DOI: 10.1021/jm300208p BindingDB Entry DOI: 10.7270/Q2Z320QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268509 (CHEMBL495623 | N-[3-(3-(3,4-Dichlorophenoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268564 (CHEMBL497815 | N-[3-(4-Biphenyl)propyl]phosphonoac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268565 (CHEMBL497410 | N-[3-(3-Phenoxyphenyl)propyl]sulfoa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268566 (CHEMBL497618 | N-Methyl-N-[3-(3-phenoxyphenyl)prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268625 (CHEMBL447414 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268566 (CHEMBL497618 | N-Methyl-N-[3-(3-phenoxyphenyl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268511 (CHEMBL497634 | N-[3-(3-(4-Chlorophenoxy)phenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50388397 (CHEMBL39581) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 27659 dehydrosqualene synthase expressed in Escherichia coli BL21(DE3) after 30 mins by spectrophotometric a... | J Med Chem 55: 4367-72 (2012) Article DOI: 10.1021/jm300208p BindingDB Entry DOI: 10.7270/Q2Z320QH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268626 (CHEMBL524084 | N-[2-(3-Phenoxyphenyl)ethyl]phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268562 (CHEMBL497616 | N-Hydroxy-N-[3-(3-(3,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268627 (CHEMBL496801 | N-Hydroxy-2-phosphono-5-(3-phenoxyp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268628 (CHEMBL496802 | N-[4-(3-Phenoxyphenyl)butyl]phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268629 (3-(3-Phenoxyphenyl)propyl Phosphonoacetate Dipotas...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268626 (CHEMBL524084 | N-[2-(3-Phenoxyphenyl)ethyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268677 (CHEMBL498628 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268678 (CHEMBL525377 | N-Hydroxy-N-[3-(4-methylbiphenyl)pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268679 (CHEMBL523897 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268676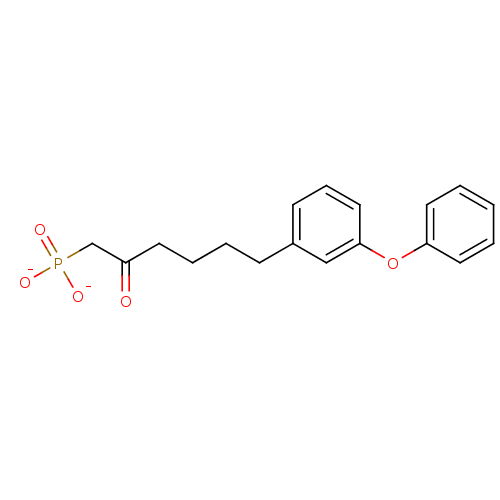 (2-Oxo-6-(4-phenoxyphenyl)hexylphosphonic Acid Dipo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268676 (2-Oxo-6-(4-phenoxyphenyl)hexylphosphonic Acid Dipo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268677 (CHEMBL498628 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268565 (CHEMBL497410 | N-[3-(3-Phenoxyphenyl)propyl]sulfoa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268628 (CHEMBL496802 | N-[4-(3-Phenoxyphenyl)butyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268629 (3-(3-Phenoxyphenyl)propyl Phosphonoacetate Dipotas...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268512 (3-(3-Phenoxyphenyl)propylphosphinylmethylphosphoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268563 (CHEMBL497617 | N-Hydroxy-N-[3-(3-phenoxyphenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268564 (CHEMBL497815 | N-[3-(4-Biphenyl)propyl]phosphonoac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268678 (CHEMBL525377 | N-Hydroxy-N-[3-(4-methylbiphenyl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268679 (CHEMBL523897 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human FPPS using pre-incubation of compound with enzyme | ACS Med Chem Lett 6: 349-54 (2015) Article DOI: 10.1021/ml500528x BindingDB Entry DOI: 10.7270/Q2251KW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50593269 (CHEMBL5177247) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00339 BindingDB Entry DOI: 10.7270/Q27P93DQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/ N-terminal GST-tagged recombinant human EGFR L858R/T790M/C797S mutant (668 to 1210 residues) expressed in a Bacu... | J Nat Prod 82: 3065-3073 (2019) Article DOI: 10.1021/acs.jnatprod.9b00659 BindingDB Entry DOI: 10.7270/Q2FX7DZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/ N-terminal GST-tagged recombinant human EGFR L858R/T790M double mutant (668 to 1210 residues) expressed in a Bac... | J Nat Prod 82: 3065-3073 (2019) Article DOI: 10.1021/acs.jnatprod.9b00659 BindingDB Entry DOI: 10.7270/Q2FX7DZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/ N-terminal GST-tagged recombinant human EGFR L858R/T790M double mutant (668 to 1210 residues) expressed in a Bac... | J Nat Prod 82: 3065-3073 (2019) Article DOI: 10.1021/acs.jnatprod.9b00659 BindingDB Entry DOI: 10.7270/Q2FX7DZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50185140 (AP-26113 | Brigatinib | US11248003, Example Brigat...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged/ N-terminal GST-tagged recombinant human EGFR L858R/T790M/C797S mutant (668 to 1210 residues) expressed in a Bacu... | J Nat Prod 82: 3065-3073 (2019) Article DOI: 10.1021/acs.jnatprod.9b00659 BindingDB Entry DOI: 10.7270/Q2FX7DZ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50593269 (CHEMBL5177247) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00339 BindingDB Entry DOI: 10.7270/Q27P93DQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50593269 (CHEMBL5177247) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00339 BindingDB Entry DOI: 10.7270/Q27P93DQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50593267 (CHEMBL5180468) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00339 BindingDB Entry DOI: 10.7270/Q27P93DQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 296 total ) | Next | Last >> |