 Found 138 hits with Last Name = 'lola' and Initial = 'd'
Found 138 hits with Last Name = 'lola' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-butyrobetaine dioxygenase
(Homo sapiens (Human)) | BDBM50007906
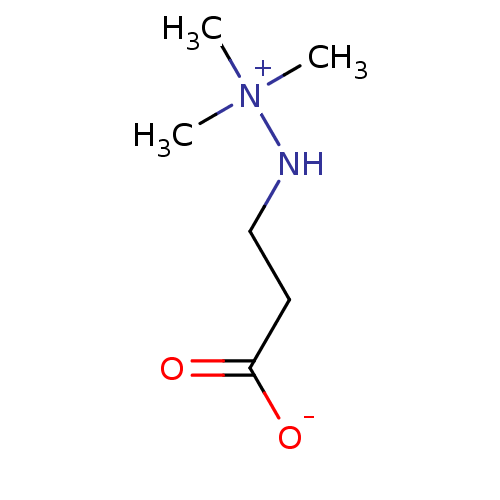
(Meldonium)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)7-5-4-6(9)10/h7H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Biomedical Research and Study centre
Curated by ChEMBL
| Assay Description
Inhibition of BBOX (unknown origin) |
J Med Chem 57: 2213-36 (2014)
Article DOI: 10.1021/jm401603e
BindingDB Entry DOI: 10.7270/Q22N53TP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268097
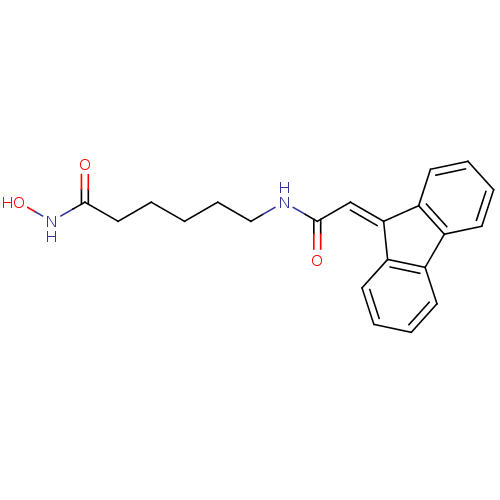
(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268063
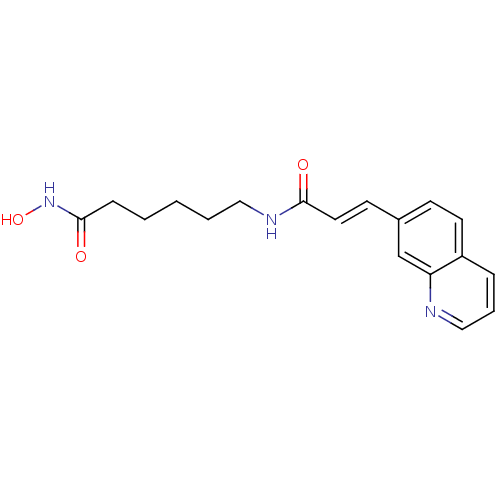
((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268121
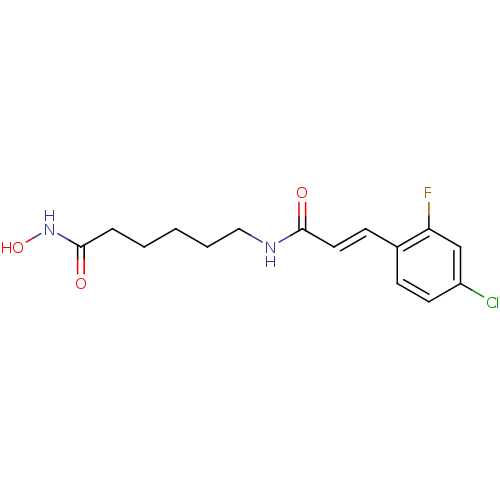
((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268042
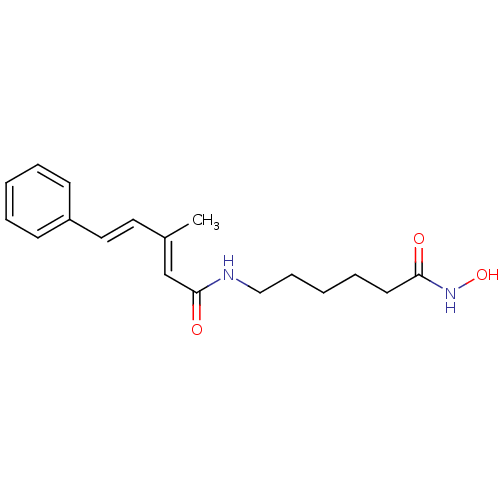
((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286774

(CHEMBL4163140)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccncc1 |r| Show InChI InChI=1S/C17H21N3O3S/c1-12(2)10-16(18)17(21)20-24(22,23)15-5-3-4-14(11-15)13-6-8-19-9-7-13/h3-9,11-12,16H,10,18H2,1-2H3,(H,20,21)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286767
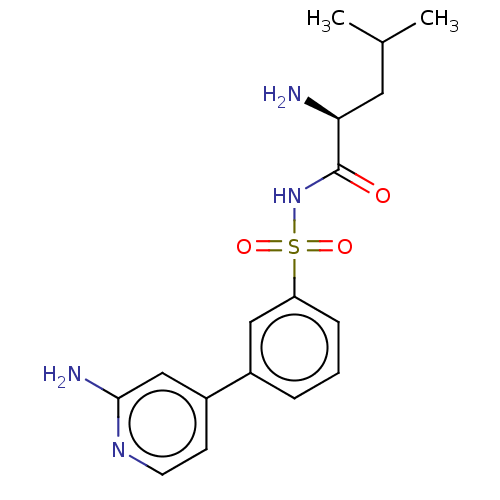
(CHEMBL4172643)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccnc(N)c1 |r| Show InChI InChI=1S/C17H22N4O3S/c1-11(2)8-15(18)17(22)21-25(23,24)14-5-3-4-12(9-14)13-6-7-20-16(19)10-13/h3-7,9-11,15H,8,18H2,1-2H3,(H2,19,20)(H,21,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286765
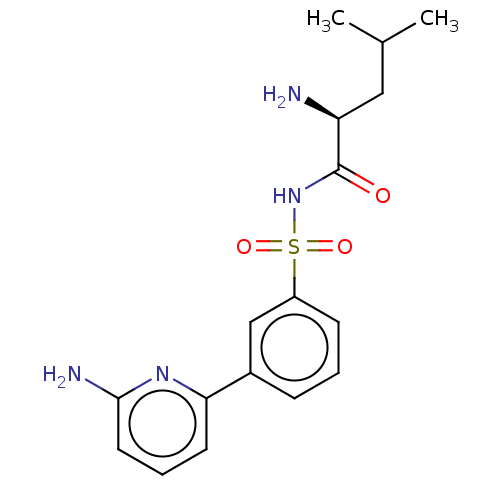
(CHEMBL4159778)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1cccc(N)n1 |r| Show InChI InChI=1S/C17H22N4O3S/c1-11(2)9-14(18)17(22)21-25(23,24)13-6-3-5-12(10-13)15-7-4-8-16(19)20-15/h3-8,10-11,14H,9,18H2,1-2H3,(H2,19,20)(H,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286770

(CHEMBL4171058)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccccc1 |r| Show InChI InChI=1S/C18H22N2O3S/c1-13(2)11-17(19)18(21)20-24(22,23)16-10-6-9-15(12-16)14-7-4-3-5-8-14/h3-10,12-13,17H,11,19H2,1-2H3,(H,20,21)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50286775
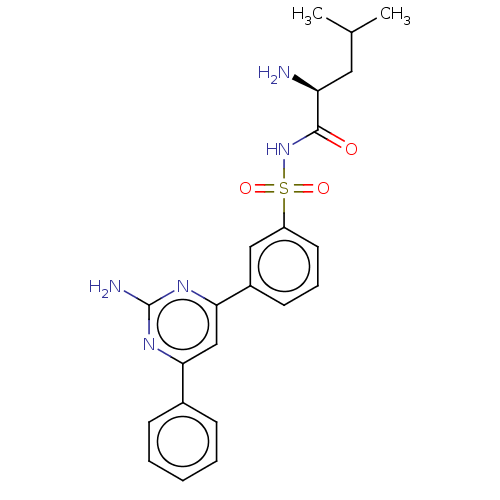
(CHEMBL4174152)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1cc(nc(N)n1)-c1ccccc1 |r| Show InChI InChI=1S/C22H25N5O3S/c1-14(2)11-18(23)21(28)27-31(29,30)17-10-6-9-16(12-17)20-13-19(25-22(24)26-20)15-7-4-3-5-8-15/h3-10,12-14,18H,11,23H2,1-2H3,(H,27,28)(H2,24,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human LeuRS assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286772

(CHEMBL4160841)Show InChI InChI=1S/C12H18N2O3S/c1-9(2)8-11(13)12(15)14-18(16,17)10-6-4-3-5-7-10/h3-7,9,11H,8,13H2,1-2H3,(H,14,15)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286766
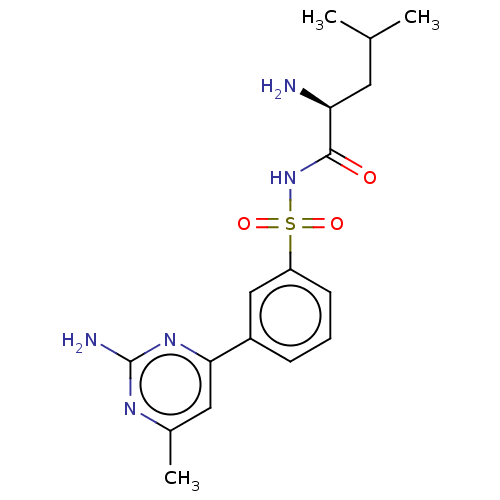
(CHEMBL4163450)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1cc(C)nc(N)n1 |r| Show InChI InChI=1S/C17H23N5O3S/c1-10(2)7-14(18)16(23)22-26(24,25)13-6-4-5-12(9-13)15-8-11(3)20-17(19)21-15/h4-6,8-10,14H,7,18H2,1-3H3,(H,22,23)(H2,19,20,21)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286771
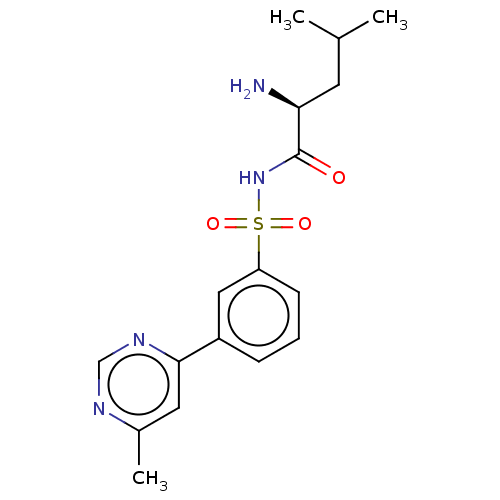
(CHEMBL4167692)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1cc(C)ncn1 |r| Show InChI InChI=1S/C17H22N4O3S/c1-11(2)7-15(18)17(22)21-25(23,24)14-6-4-5-13(9-14)16-8-12(3)19-10-20-16/h4-6,8-11,15H,7,18H2,1-3H3,(H,21,22)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286769

(CHEMBL4161986)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccnc(N)n1 |r| Show InChI InChI=1S/C16H21N5O3S/c1-10(2)8-13(17)15(22)21-25(23,24)12-5-3-4-11(9-12)14-6-7-19-16(18)20-14/h3-7,9-10,13H,8,17H2,1-2H3,(H,21,22)(H2,18,19,20)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50286768
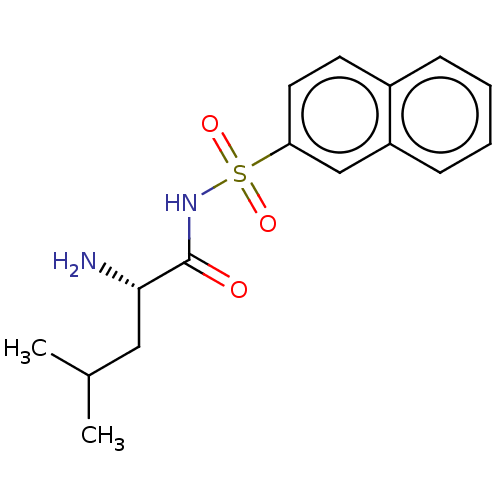
(CHEMBL4171458)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C16H20N2O3S/c1-11(2)9-15(17)16(19)18-22(20,21)14-8-7-12-5-3-4-6-13(12)10-14/h3-8,10-11,15H,9,17H2,1-2H3,(H,18,19)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LeuRS expressed in Escherichia coli M15 cells assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-butyrobetaine dioxygenase
(Homo sapiens (Human)) | BDBM50008027
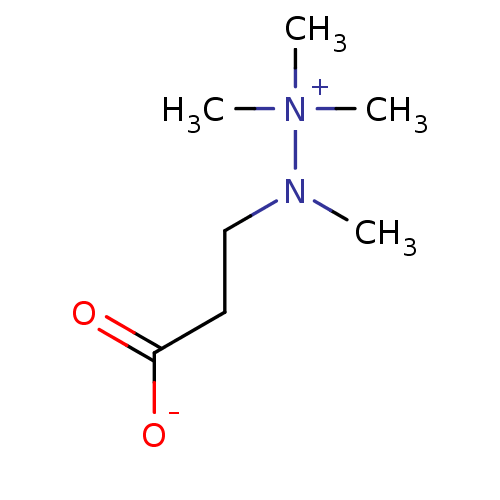
(CHEMBL3234384)Show InChI InChI=1S/C7H16N2O2/c1-8(9(2,3)4)6-5-7(10)11/h5-6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Biomedical Research and Study centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BBOX expressed in Saccharomyces cerevisiae AH22 assessed as formation of carnitine from gamma-butyrobetaine preincuba... |
J Med Chem 57: 2213-36 (2014)
Article DOI: 10.1021/jm401603e
BindingDB Entry DOI: 10.7270/Q22N53TP |
More data for this
Ligand-Target Pair | |
Gamma-butyrobetaine dioxygenase
(Homo sapiens (Human)) | BDBM50007999
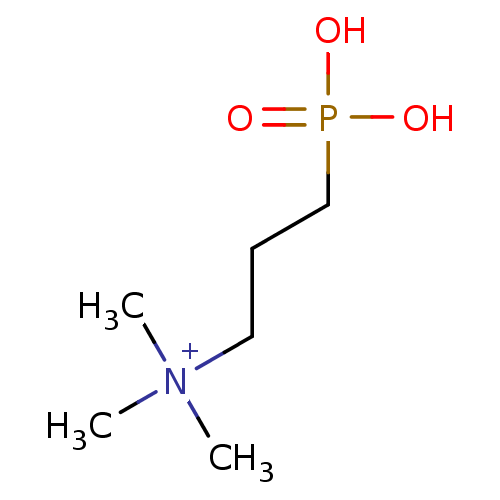
(CHEMBL3234371)Show InChI InChI=1S/C6H16NO3P.ClH/c1-7(2,3)5-4-6-11(8,9)10;/h4-6H2,1-3H3,(H-,8,9,10);1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Biomedical Research and Study centre
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BBOX expressed in Saccharomyces cerevisiae AH22 assessed as formation of carnitine from gamma-butyrobetaine preincuba... |
J Med Chem 57: 2213-36 (2014)
Article DOI: 10.1021/jm401603e
BindingDB Entry DOI: 10.7270/Q22N53TP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50286766

(CHEMBL4163450)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1cc(C)nc(N)n1 |r| Show InChI InChI=1S/C17H23N5O3S/c1-10(2)7-14(18)16(23)22-26(24,25)13-6-4-5-12(9-13)15-8-11(3)20-17(19)21-15/h4-6,8-10,14H,7,18H2,1-3H3,(H,22,23)(H2,19,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Drug Design Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human LeuRS assessed as reduction in ATP consumption |
ACS Med Chem Lett 9: 84-88 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00374
BindingDB Entry DOI: 10.7270/Q2BZ68KK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data