 Found 1316 hits with Last Name = 'long' and Initial = 'c'
Found 1316 hits with Last Name = 'long' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572486
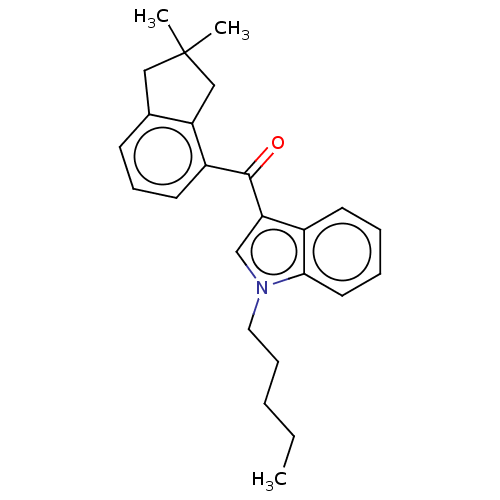
(CHEMBL4860950)Show SMILES CCCCCn1cc(C(=O)c2cccc3CC(C)(C)Cc23)c2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572486

(CHEMBL4860950)Show SMILES CCCCCn1cc(C(=O)c2cccc3CC(C)(C)Cc23)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572487
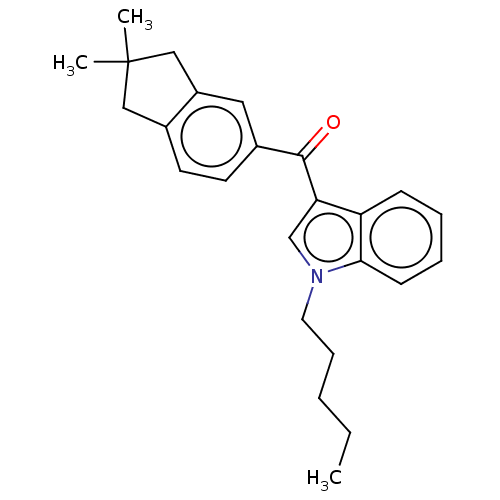
(CHEMBL4856192)Show SMILES CCCCCn1cc(C(=O)c2ccc3CC(C)(C)Cc3c2)c2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572485
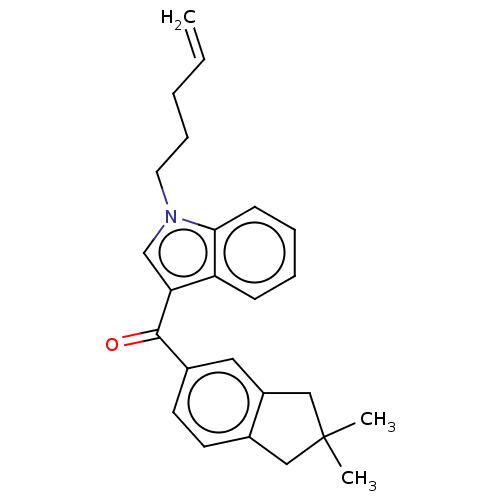
(CHEMBL4855206)Show SMILES CC1(C)Cc2ccc(cc2C1)C(=O)c1cn(CCCC=C)c2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572489
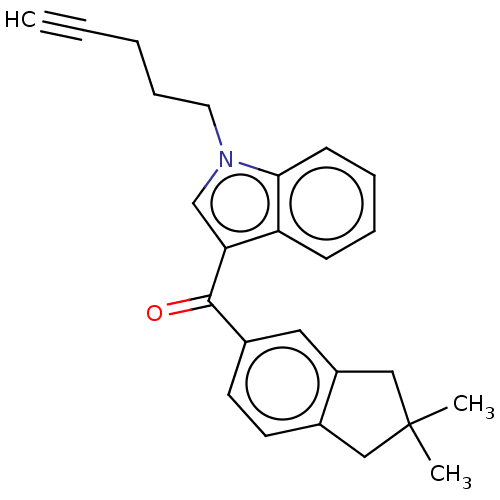
(CHEMBL4877815)Show SMILES CC1(C)Cc2ccc(cc2C1)C(=O)c1cn(CCCC#C)c2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50553588

(CHEMBL4747163)Show SMILES Fc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(43.82,-36.75,;45.16,-35.99,;45.17,-34.45,;46.51,-33.7,;47.83,-34.49,;49.17,-33.73,;49.18,-32.2,;50.09,-30.95,;49.18,-29.69,;49.17,-28.15,;50.49,-27.37,;51.84,-28.13,;53.16,-27.35,;53.15,-25.81,;51.82,-25.05,;52.57,-26.38,;51.08,-26.77,;50.48,-25.83,;49.15,-25.07,;47.71,-30.18,;46.37,-29.4,;45.03,-30.18,;45.03,-31.73,;46.37,-32.5,;47.71,-31.72,;47.82,-36.02,;46.49,-36.78,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572487

(CHEMBL4856192)Show SMILES CCCCCn1cc(C(=O)c2ccc3CC(C)(C)Cc3c2)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572488
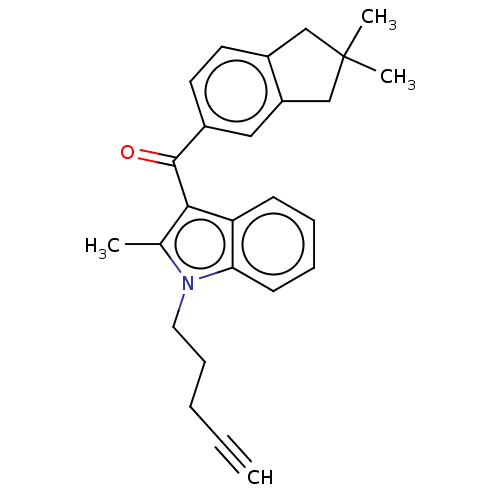
(CHEMBL4866238)Show SMILES Cc1c(C(=O)c2ccc3CC(C)(C)Cc3c2)c2ccccc2n1CCCC#C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50553585
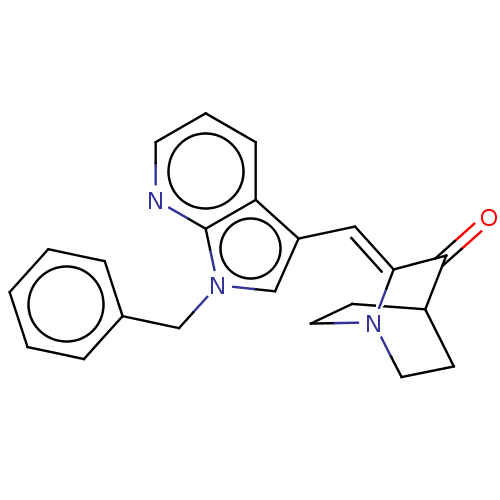
(CHEMBL4741229)Show SMILES O=C1C2CCN(CC2)\C1=C/c1cn(Cc2ccccc2)c2ncccc12 |(34.95,-5.65,;36.29,-6.41,;37.62,-5.64,;38.96,-6.4,;38.96,-7.94,;37.64,-8.71,;36.88,-7.36,;38.38,-6.96,;36.3,-7.95,;34.97,-8.73,;34.99,-10.28,;35.89,-11.53,;34.99,-12.78,;34.97,-14.32,;33.63,-15.07,;32.31,-14.28,;30.98,-15.04,;30.96,-16.58,;32.29,-17.36,;33.63,-16.6,;33.52,-12.31,;32.18,-13.09,;30.84,-12.32,;30.84,-10.77,;32.17,-9.99,;33.51,-10.76,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50553586
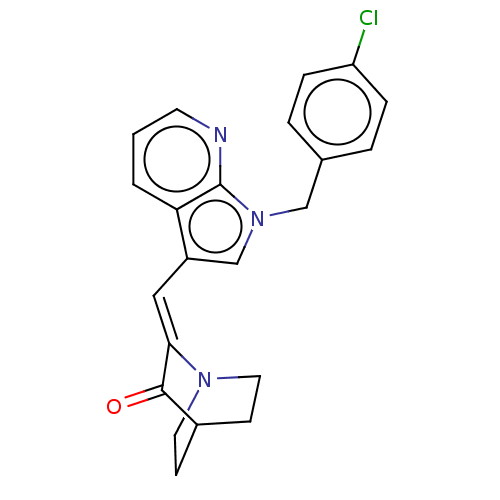
(CHEMBL4794483)Show SMILES Clc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(42.77,-16.76,;44.11,-16,;44.13,-14.46,;45.47,-13.71,;46.79,-14.5,;48.13,-13.74,;48.14,-12.21,;49.05,-10.96,;48.14,-9.7,;48.13,-8.16,;49.45,-7.38,;50.8,-8.14,;52.12,-7.36,;52.11,-5.82,;50.77,-5.06,;51.53,-6.39,;50.04,-6.78,;49.44,-5.84,;48.1,-5.08,;46.67,-10.19,;45.32,-9.41,;43.99,-10.19,;43.99,-11.74,;45.33,-12.51,;46.67,-11.73,;46.78,-16.03,;45.45,-16.78,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328879
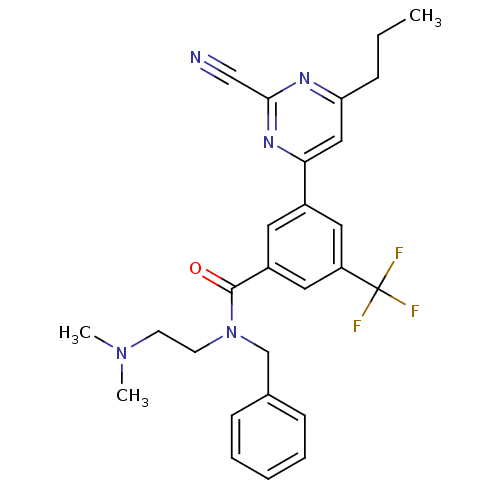
(CHEMBL1234898 | N-benzyl-3-(2-cyano-6-propylpyrimi...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(CCN(C)C)Cc1ccccc1 Show InChI InChI=1S/C27H28F3N5O/c1-4-8-23-16-24(33-25(17-31)32-23)20-13-21(15-22(14-20)27(28,29)30)26(36)35(12-11-34(2)3)18-19-9-6-5-7-10-19/h5-7,9-10,13-16H,4,8,11-12,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50572490
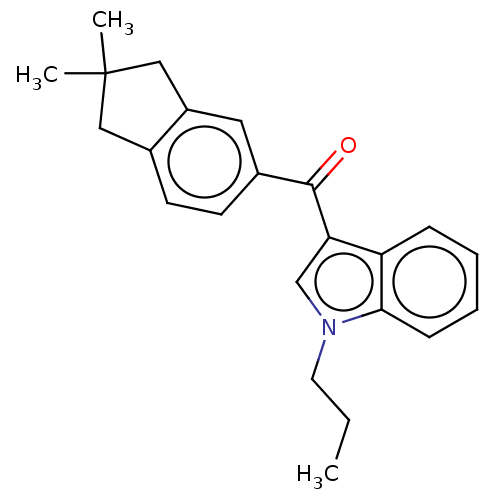
(CHEMBL4872576) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572485

(CHEMBL4855206)Show SMILES CC1(C)Cc2ccc(cc2C1)C(=O)c1cn(CCCC=C)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313476

(4-(3-(piperidin-1-yl)propyl)-6-(3-(trifluoromethyl...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCN2CCCCC2)nc(n1)C#N Show InChI InChI=1S/C20H21F3N4/c21-20(22,23)16-7-4-6-15(12-16)18-13-17(25-19(14-24)26-18)8-5-11-27-9-2-1-3-10-27/h4,6-7,12-13H,1-3,5,8-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Orotidine 5'-phosphate decarboxylase
(Methanobacterium thermoautotrophicum) | BDBM21337
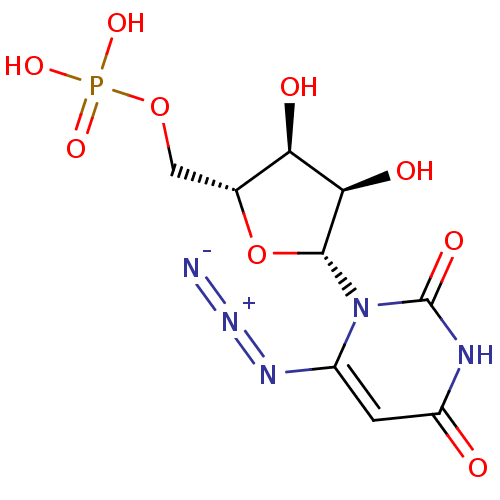
(6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)n1c(cc(=O)[nH]c1=O)N=[N+]=[N-] |r| Show InChI InChI=1S/C9H12N5O9P/c10-13-12-4-1-5(15)11-9(18)14(4)8-7(17)6(16)3(23-8)2-22-24(19,20)21/h1,3,6-8,16-17H,2H2,(H,11,15,18)(H2,19,20,21)/t3-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 200 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute
| Assay Description
An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... |
J Med Chem 52: 1648-58 (2009)
Article DOI: 10.1021/jm801224t
BindingDB Entry DOI: 10.7270/Q2RR1WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50553588

(CHEMBL4747163)Show SMILES Fc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(43.82,-36.75,;45.16,-35.99,;45.17,-34.45,;46.51,-33.7,;47.83,-34.49,;49.17,-33.73,;49.18,-32.2,;50.09,-30.95,;49.18,-29.69,;49.17,-28.15,;50.49,-27.37,;51.84,-28.13,;53.16,-27.35,;53.15,-25.81,;51.82,-25.05,;52.57,-26.38,;51.08,-26.77,;50.48,-25.83,;49.15,-25.07,;47.71,-30.18,;46.37,-29.4,;45.03,-30.18,;45.03,-31.73,;46.37,-32.5,;47.71,-31.72,;47.82,-36.02,;46.49,-36.78,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328887
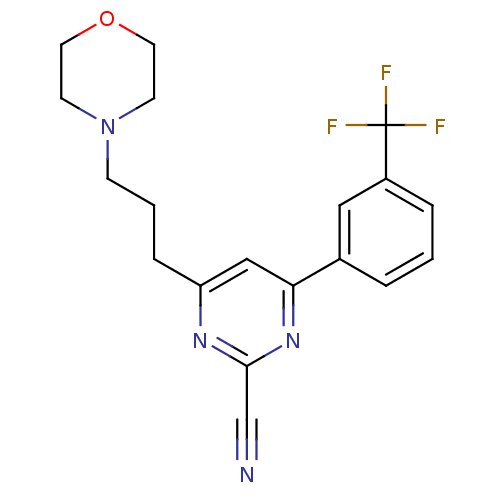
(4-(3-morpholinopropyl)-6-(3-(trifluoromethyl)pheny...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCN2CCOCC2)nc(n1)C#N Show InChI InChI=1S/C19H19F3N4O/c20-19(21,22)15-4-1-3-14(11-15)17-12-16(24-18(13-23)25-17)5-2-6-26-7-9-27-10-8-26/h1,3-4,11-12H,2,5-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313477

(4-(3-(pentan-3-ylamino)propyl)-6-(3-(trifluorometh...)Show SMILES CCC(CC)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H23F3N4/c1-3-16(4-2)25-10-6-9-17-12-18(27-19(13-24)26-17)14-7-5-8-15(11-14)20(21,22)23/h5,7-8,11-12,16,25H,3-4,6,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572489

(CHEMBL4877815)Show SMILES CC1(C)Cc2ccc(cc2C1)C(=O)c1cn(CCCC#C)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Uridine 5'-monophosphate synthase
(Homo sapiens (Human)) | BDBM27946
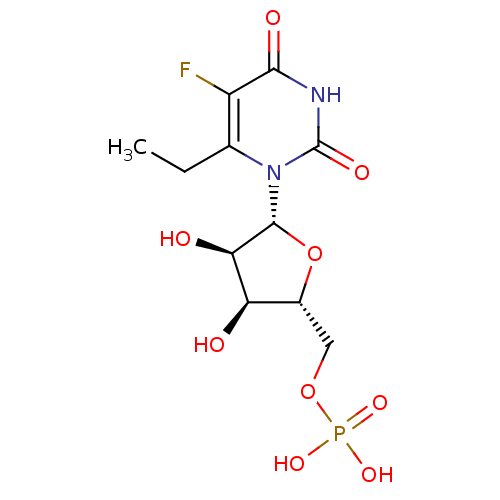
(uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...)Show SMILES CCc1c(F)c(=O)[nH]c(=O)n1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16FN2O9P/c1-2-4-6(12)9(17)13-11(18)14(4)10-8(16)7(15)5(23-10)3-22-24(19,20)21/h5,7-8,10,15-16H,2-3H2,1H3,(H,13,17,18)(H2,19,20,21)/t5-,7-,8-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute
| Assay Description
An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... |
J Med Chem 52: 1648-58 (2009)
Article DOI: 10.1021/jm801224t
BindingDB Entry DOI: 10.7270/Q2RR1WJB |
More data for this
Ligand-Target Pair | |
Orotidine 5'-phosphate decarboxylase
(Methanobacterium thermoautotrophicum) | BDBM27944
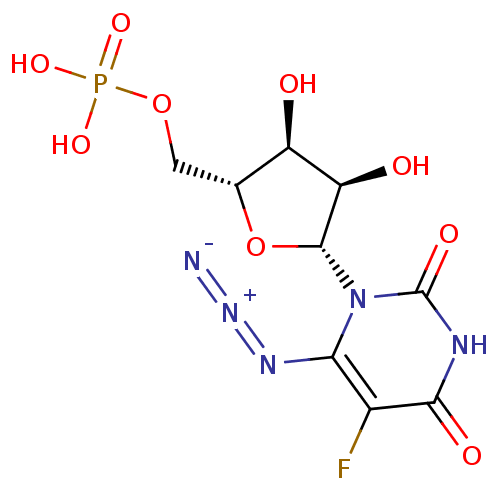
(uridine derivative, 41 | {[(2R,3S,4R,5R)-5-(6-azid...)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(O)=O)n1c(N=[N+]=[N-])c(F)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H11FN5O9P/c10-3-6(13-14-11)15(9(19)12-7(3)18)8-5(17)4(16)2(24-8)1-23-25(20,21)22/h2,4-5,8,16-17H,1H2,(H,12,18,19)(H2,20,21,22)/t2-,4-,5-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute
| Assay Description
An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... |
J Med Chem 52: 1648-58 (2009)
Article DOI: 10.1021/jm801224t
BindingDB Entry DOI: 10.7270/Q2RR1WJB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328880
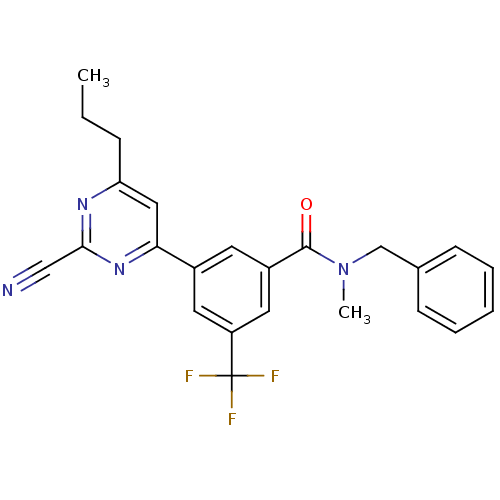
(CHEMBL1271174 | N-benzyl-3-(2-cyano-6-propylpyrimi...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(C)Cc1ccccc1 Show InChI InChI=1S/C24H21F3N4O/c1-3-7-20-13-21(30-22(14-28)29-20)17-10-18(12-19(11-17)24(25,26)27)23(32)31(2)15-16-8-5-4-6-9-16/h4-6,8-13H,3,7,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328890
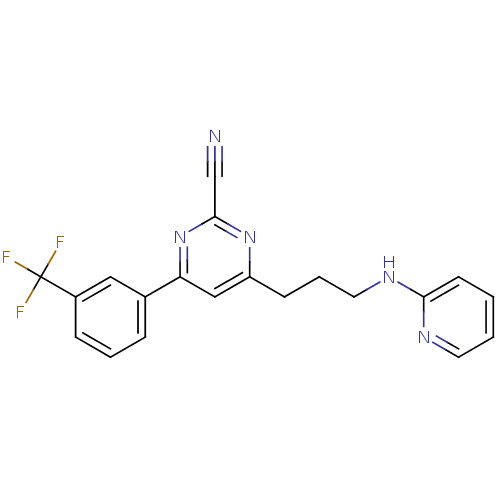
(4-(3-(pyridin-2-ylamino)propyl)-6-(3-(trifluoromet...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCNc2ccccn2)nc(n1)C#N Show InChI InChI=1S/C20H16F3N5/c21-20(22,23)15-6-3-5-14(11-15)17-12-16(27-19(13-24)28-17)7-4-10-26-18-8-1-2-9-25-18/h1-3,5-6,8-9,11-12H,4,7,10H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328885

(4-(3-(2,2,2-trifluoroethylamino)propyl)-6-(3-(trif...)Show SMILES FC(F)(F)CNCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H14F6N4/c18-16(19,20)10-25-6-2-5-13-8-14(27-15(9-24)26-13)11-3-1-4-12(7-11)17(21,22)23/h1,3-4,7-8,25H,2,5-6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328888
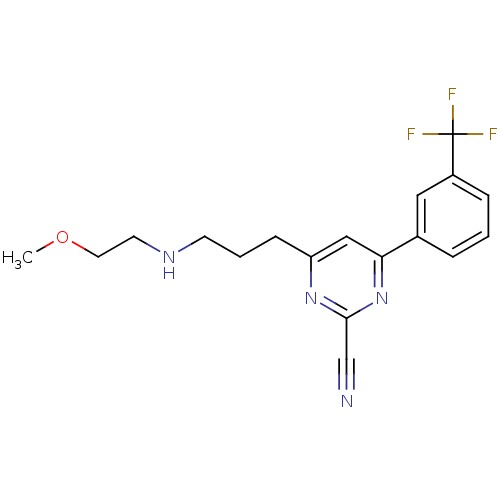
(4-(3-(2-methoxyethylamino)propyl)-6-(3-(trifluorom...)Show InChI InChI=1S/C18H19F3N4O/c1-26-9-8-23-7-3-6-15-11-16(25-17(12-22)24-15)13-4-2-5-14(10-13)18(19,20)21/h2,4-5,10-11,23H,3,6-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50553587
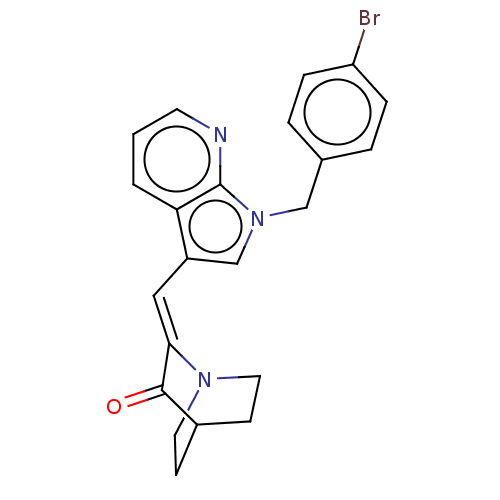
(CHEMBL4780917)Show SMILES Brc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(29.73,-37.45,;31.07,-36.69,;31.09,-35.15,;32.43,-34.4,;33.75,-35.19,;35.08,-34.43,;35.1,-32.9,;36.01,-31.64,;35.1,-30.39,;35.08,-28.85,;36.41,-28.07,;37.75,-28.83,;39.08,-28.05,;39.07,-26.51,;37.73,-25.75,;38.49,-27.07,;37,-27.47,;36.4,-26.53,;35.06,-25.77,;33.63,-30.87,;32.28,-30.1,;30.95,-30.88,;30.95,-32.43,;32.29,-33.2,;33.63,-32.42,;33.74,-36.72,;32.41,-37.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328884
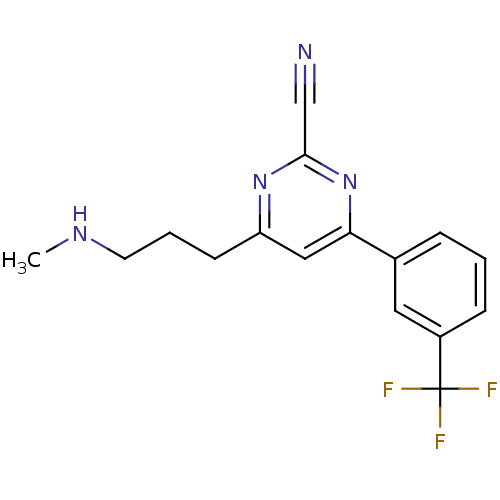
(4-(3-(methylamino)propyl)-6-(3-(trifluoromethyl)ph...)Show InChI InChI=1S/C16H15F3N4/c1-21-7-3-6-13-9-14(23-15(10-20)22-13)11-4-2-5-12(8-11)16(17,18)19/h2,4-5,8-9,21H,3,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50313479

(4-cycloheptyl-6-(3-(piperidin-1-yl)propyl)pyrimidi...)Show InChI InChI=1S/C20H30N4/c21-16-20-22-18(11-8-14-24-12-6-3-7-13-24)15-19(23-20)17-9-4-1-2-5-10-17/h15,17H,1-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50553586

(CHEMBL4794483)Show SMILES Clc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(42.77,-16.76,;44.11,-16,;44.13,-14.46,;45.47,-13.71,;46.79,-14.5,;48.13,-13.74,;48.14,-12.21,;49.05,-10.96,;48.14,-9.7,;48.13,-8.16,;49.45,-7.38,;50.8,-8.14,;52.12,-7.36,;52.11,-5.82,;50.77,-5.06,;51.53,-6.39,;50.04,-6.78,;49.44,-5.84,;48.1,-5.08,;46.67,-10.19,;45.32,-9.41,;43.99,-10.19,;43.99,-11.74,;45.33,-12.51,;46.67,-11.73,;46.78,-16.03,;45.45,-16.78,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 704 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328878
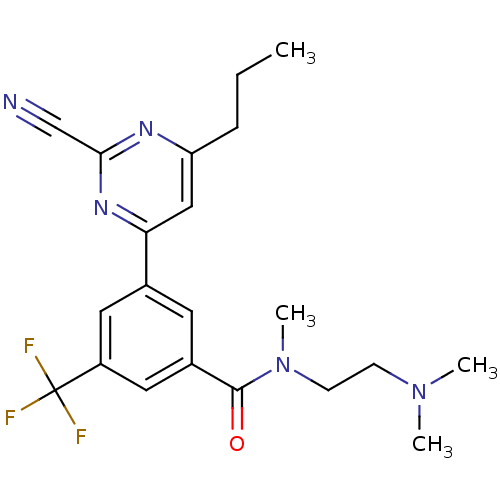
(3-(2-cyano-6-propylpyrimidin-4-yl)-N-(2-(dimethyla...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(C)CCN(C)C Show InChI InChI=1S/C21H24F3N5O/c1-5-6-17-12-18(27-19(13-25)26-17)14-9-15(11-16(10-14)21(22,23)24)20(30)29(4)8-7-28(2)3/h9-12H,5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Orotidine 5'-phosphate decarboxylase
(Methanobacterium thermoautotrophicum) | BDBM21338
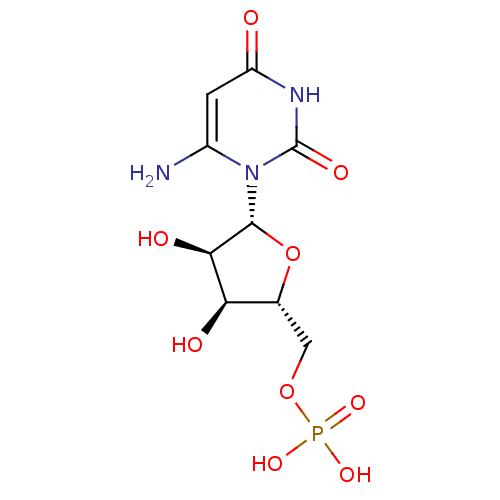
(6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...)Show SMILES Nc1cc(=O)[nH]c(=O)n1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H14N3O9P/c10-4-1-5(13)11-9(16)12(4)8-7(15)6(14)3(21-8)2-20-22(17,18)19/h1,3,6-8,14-15H,2,10H2,(H,11,13,16)(H2,17,18,19)/t3-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute
| Assay Description
An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... |
J Med Chem 52: 1648-58 (2009)
Article DOI: 10.1021/jm801224t
BindingDB Entry DOI: 10.7270/Q2RR1WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328886
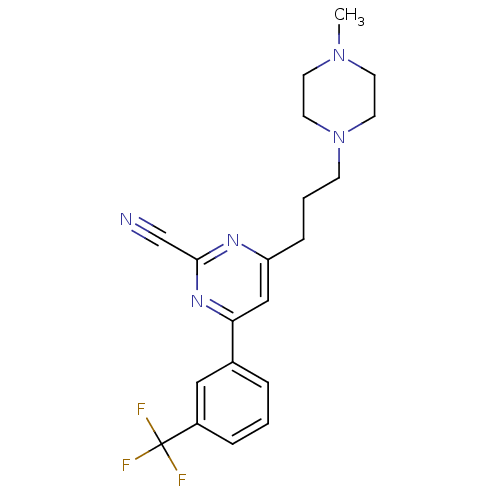
(4-(3-(4-methylpiperazin-1-yl)propyl)-6-(3-(trifluo...)Show SMILES CN1CCN(CCCc2cc(nc(n2)C#N)-c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C20H22F3N5/c1-27-8-10-28(11-9-27)7-3-6-17-13-18(26-19(14-24)25-17)15-4-2-5-16(12-15)20(21,22)23/h2,4-5,12-13H,3,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50553587

(CHEMBL4780917)Show SMILES Brc1ccc(Cn2cc(\C=C3/N4CCC(CC4)C3=O)c3cccnc23)cc1 |(29.73,-37.45,;31.07,-36.69,;31.09,-35.15,;32.43,-34.4,;33.75,-35.19,;35.08,-34.43,;35.1,-32.9,;36.01,-31.64,;35.1,-30.39,;35.08,-28.85,;36.41,-28.07,;37.75,-28.83,;39.08,-28.05,;39.07,-26.51,;37.73,-25.75,;38.49,-27.07,;37,-27.47,;36.4,-26.53,;35.06,-25.77,;33.63,-30.87,;32.28,-30.1,;30.95,-30.88,;30.95,-32.43,;32.29,-33.2,;33.63,-32.42,;33.74,-36.72,;32.41,-37.47,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328889
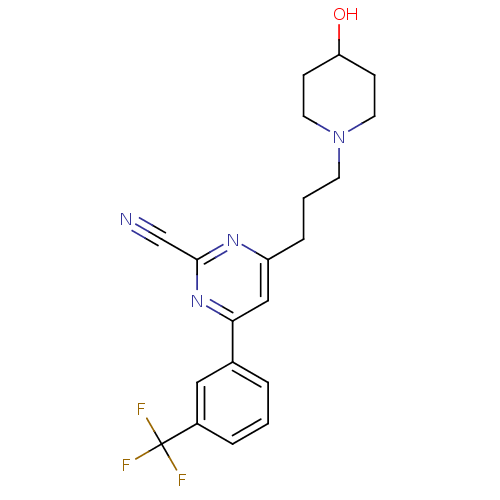
(4-(3-(4-hydroxypiperidin-1-yl)propyl)-6-(3-(triflu...)Show SMILES OC1CCN(CCCc2cc(nc(n2)C#N)-c2cccc(c2)C(F)(F)F)CC1 Show InChI InChI=1S/C20H21F3N4O/c21-20(22,23)15-4-1-3-14(11-15)18-12-16(25-19(13-24)26-18)5-2-8-27-9-6-17(28)7-10-27/h1,3-4,11-12,17,28H,2,5-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328895

(1-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)C1(CC1)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C19H18F3N5O/c20-19(21,22)13-4-1-3-12(9-13)15-10-14(26-16(11-23)27-15)5-2-8-25-18(6-7-18)17(24)28/h1,3-4,9-10,25H,2,5-8H2,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572488

(CHEMBL4866238)Show SMILES Cc1c(C(=O)c2ccc3CC(C)(C)Cc3c2)c2ccccc2n1CCCC#C | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328896
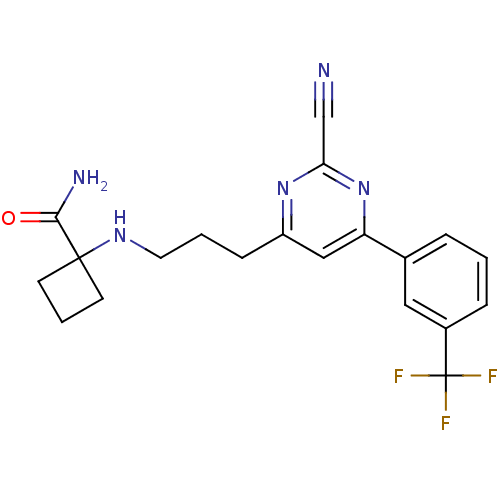
(1-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)C1(CCC1)NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H20F3N5O/c21-20(22,23)14-5-1-4-13(10-14)16-11-15(27-17(12-24)28-16)6-2-9-26-19(18(25)29)7-3-8-19/h1,4-5,10-11,26H,2-3,6-9H2,(H2,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50572490

(CHEMBL4872576) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00442
BindingDB Entry DOI: 10.7270/Q2B56PHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50553585

(CHEMBL4741229)Show SMILES O=C1C2CCN(CC2)\C1=C/c1cn(Cc2ccccc2)c2ncccc12 |(34.95,-5.65,;36.29,-6.41,;37.62,-5.64,;38.96,-6.4,;38.96,-7.94,;37.64,-8.71,;36.88,-7.36,;38.38,-6.96,;36.3,-7.95,;34.97,-8.73,;34.99,-10.28,;35.89,-11.53,;34.99,-12.78,;34.97,-14.32,;33.63,-15.07,;32.31,-14.28,;30.98,-15.04,;30.96,-16.58,;32.29,-17.36,;33.63,-16.6,;33.52,-12.31,;32.18,-13.09,;30.84,-12.32,;30.84,-10.77,;32.17,-9.99,;33.51,-10.76,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor (unknown origin) by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127501
BindingDB Entry DOI: 10.7270/Q2NV9NW2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328894
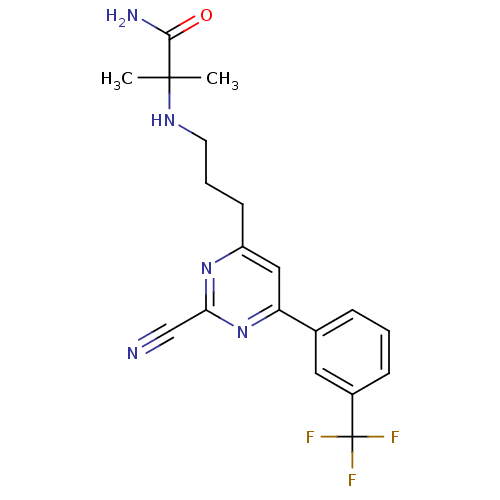
(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES CC(C)(NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F)C(N)=O Show InChI InChI=1S/C19H20F3N5O/c1-18(2,17(24)28)25-8-4-7-14-10-15(27-16(11-23)26-14)12-5-3-6-13(9-12)19(20,21)22/h3,5-6,9-10,25H,4,7-8H2,1-2H3,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by patch clamp assay |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328883

(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimidin-...)Show SMILES CCC(CC)NC(=O)CCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C20H21F3N4O/c1-3-15(4-2)26-19(28)9-8-16-11-17(27-18(12-24)25-16)13-6-5-7-14(10-13)20(21,22)23/h5-7,10-11,15H,3-4,8-9H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328894

(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES CC(C)(NCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F)C(N)=O Show InChI InChI=1S/C19H20F3N5O/c1-18(2,17(24)28)25-8-4-7-14-10-15(27-16(11-23)26-14)12-5-3-6-13(9-12)19(20,21)22/h3,5-6,9-10,25H,4,7-8H2,1-2H3,(H2,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328893
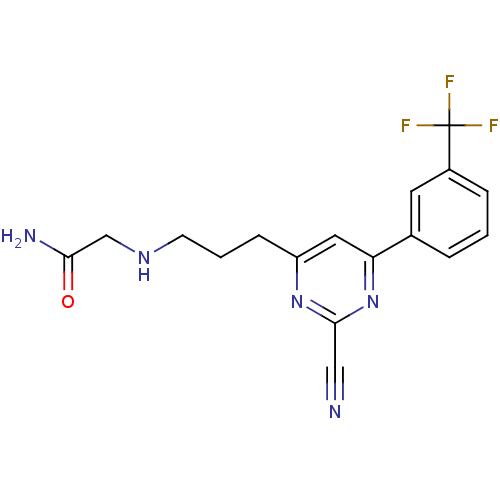
(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES NC(=O)CNCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H16F3N5O/c18-17(19,20)12-4-1-3-11(7-12)14-8-13(24-16(9-21)25-14)5-2-6-23-10-15(22)26/h1,3-4,7-8,23H,2,5-6,10H2,(H2,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328898

((S)-4-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyr...)Show SMILES NC(=O)[C@@H]1COCCN1CCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2/c21-20(22,23)14-4-1-3-13(9-14)16-10-15(26-18(11-24)27-16)5-2-6-28-7-8-30-12-17(28)19(25)29/h1,3-4,9-10,17H,2,5-8,12H2,(H2,25,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328892
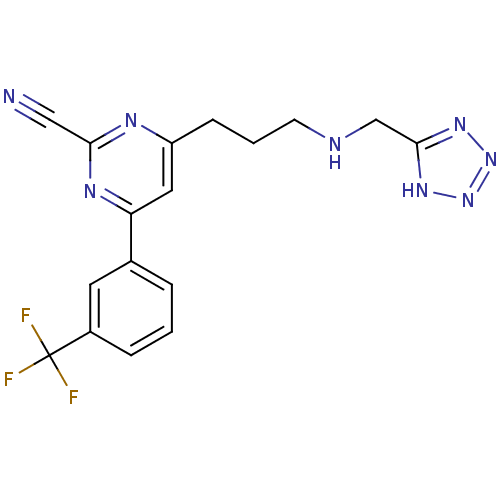
(4-(3-((1H-tetrazol-5-yl)methylamino)propyl)-6-(3-(...)Show SMILES FC(F)(F)c1cccc(c1)-c1cc(CCCNCc2nnn[nH]2)nc(n1)C#N Show InChI InChI=1S/C17H15F3N8/c18-17(19,20)12-4-1-3-11(7-12)14-8-13(23-15(9-21)24-14)5-2-6-22-10-16-25-27-28-26-16/h1,3-4,7-8,22H,2,5-6,10H2,(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328891
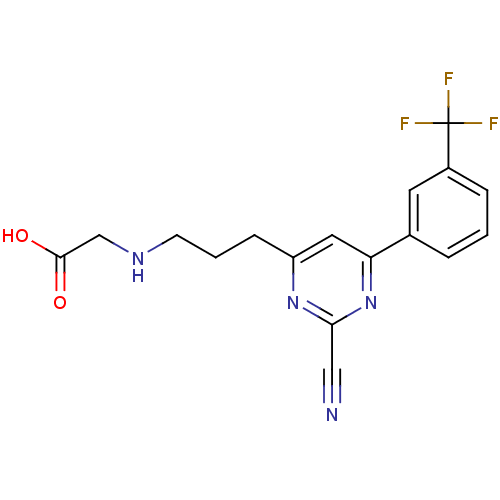
(2-(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimid...)Show SMILES OC(=O)CNCCCc1cc(nc(n1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2/c18-17(19,20)12-4-1-3-11(7-12)14-8-13(23-15(9-21)24-14)5-2-6-22-10-16(25)26/h1,3-4,7-8,22H,2,5-6,10H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328877
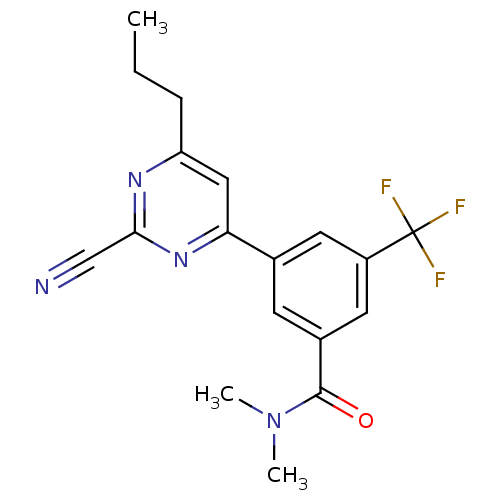
(3-(2-cyano-6-propylpyrimidin-4-yl)-N,N-dimethyl-5-...)Show SMILES CCCc1cc(nc(n1)C#N)-c1cc(cc(c1)C(F)(F)F)C(=O)N(C)C Show InChI InChI=1S/C18H17F3N4O/c1-4-5-14-9-15(24-16(10-22)23-14)11-6-12(17(26)25(2)3)8-13(7-11)18(19,20)21/h6-9H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50328882

(3-(2-cyano-6-(3-(trifluoromethyl)phenyl)pyrimidin-...)Show InChI InChI=1S/C15H10F3N3O2/c16-15(17,18)10-3-1-2-9(6-10)12-7-11(4-5-14(22)23)20-13(8-19)21-12/h1-3,6-7H,4-5H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H] dofetilide from human ERG channel expressed in HEK293 cells |
Bioorg Med Chem Lett 20: 6237-41 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.101
BindingDB Entry DOI: 10.7270/Q2RR1ZFT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data