

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326399 ((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326399 ((+/-)-trans-4-Butyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326400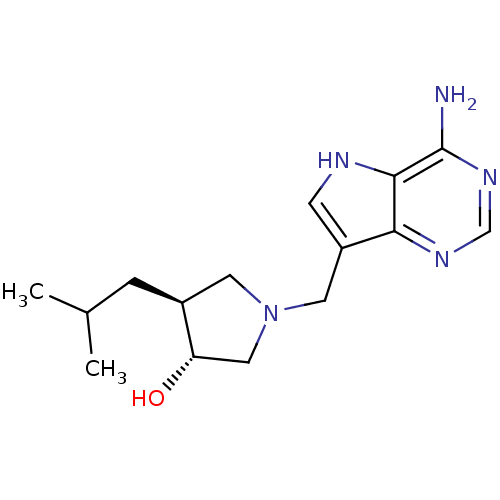 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326402 ((+/-)-trans-4-Cyclopropyl-1-[(9-deazaadenin-9-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326403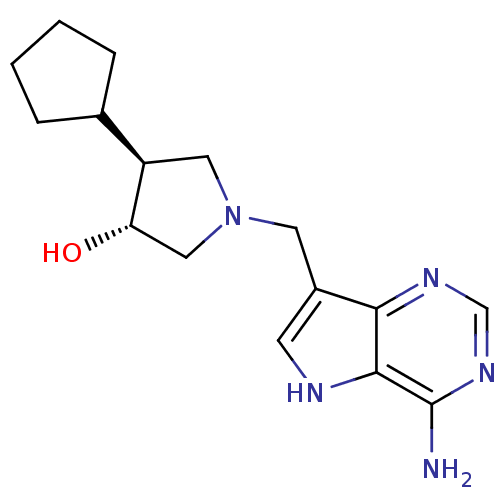 ((+/-)-trans-4-Cyclopentyl-1-[(9-deazaadenin-9-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326407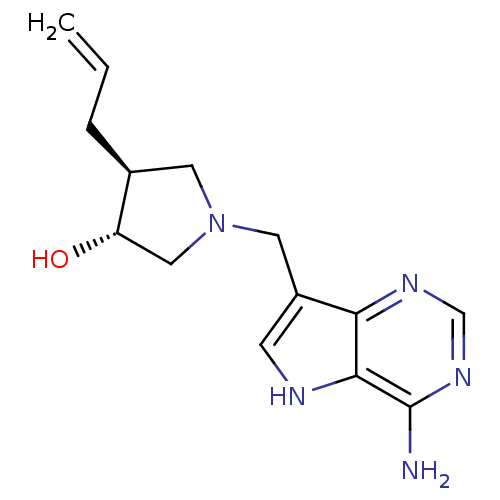 ((+/-)-trans-4-Allyl-1-[(9-deazaadenin-9-yl)methyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326406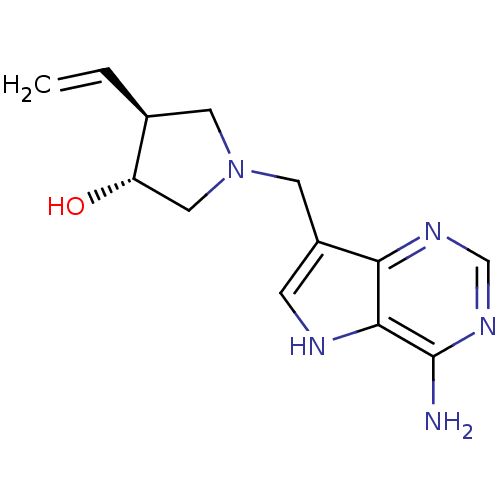 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326398 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326398 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326405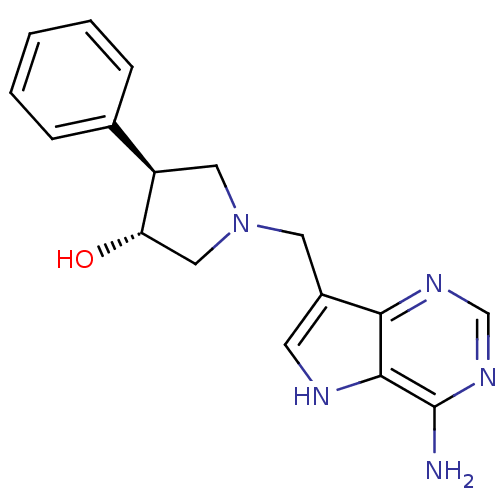 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326404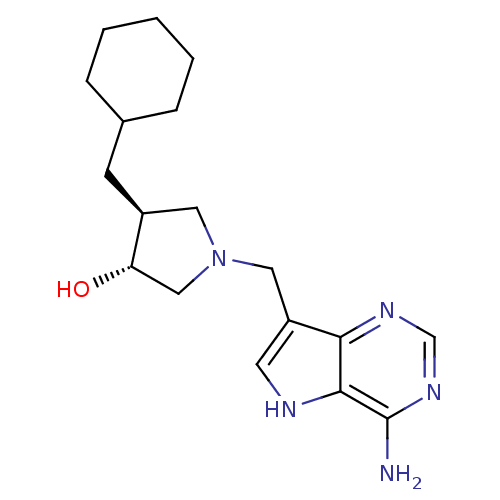 ((+/-)-trans-4-(Cyclohexylmethyl)-1-[(9-deaza-adeni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326401 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326408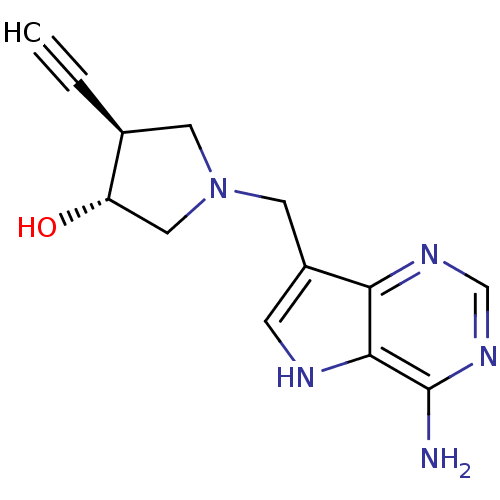 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-4-ethyn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326411 ((+/-)-Benzyl cis-3-(Benzoyloxy)-4-ethylpyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326409 ((+/-)-trans-1-[(9-Deazaadenin-9-yl)methyl]-3-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50326410 ((+/-)-trans-4-[3-(Benzylthio)propyl]-1-[(9-deazaad...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTAP | J Med Chem 53: 6730-46 (2010) Article DOI: 10.1021/jm100898v BindingDB Entry DOI: 10.7270/Q22R3RWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM205305 (US9249125, 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determining the l... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205298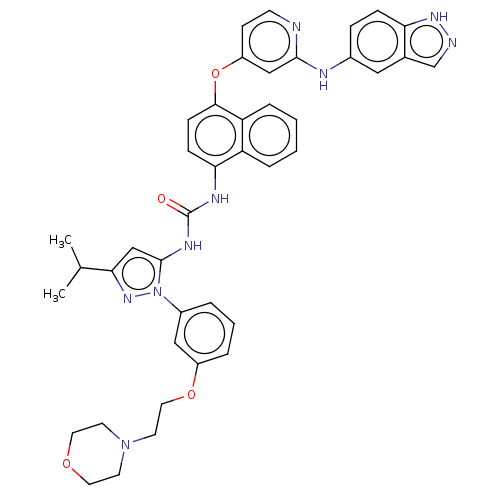 (US9249125, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205302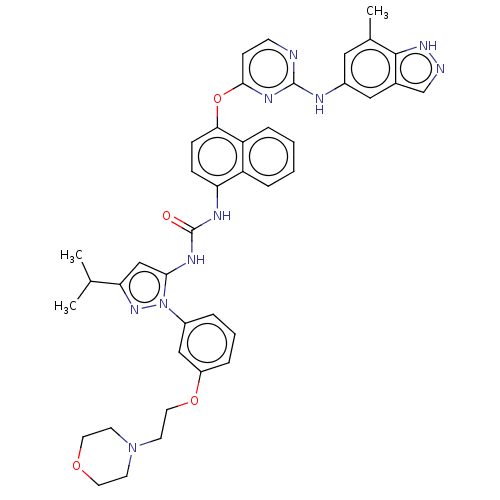 (US9249125, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205314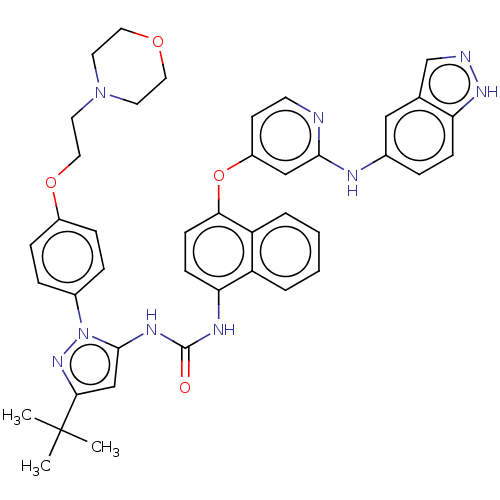 (US9249125, 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205315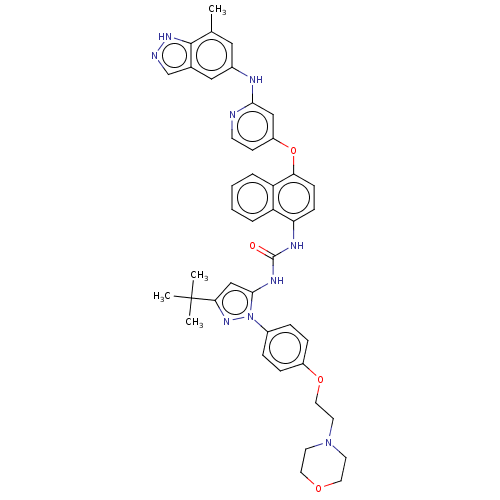 (US9249125, 68) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205321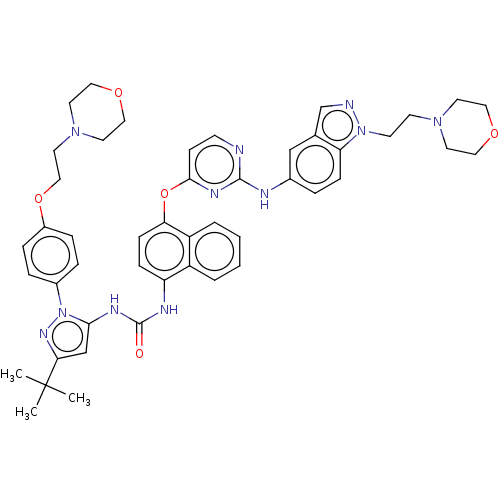 (US9249125, 74) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205322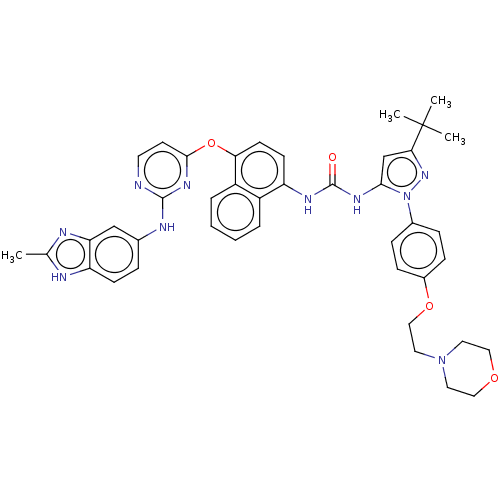 (US9249125, 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205337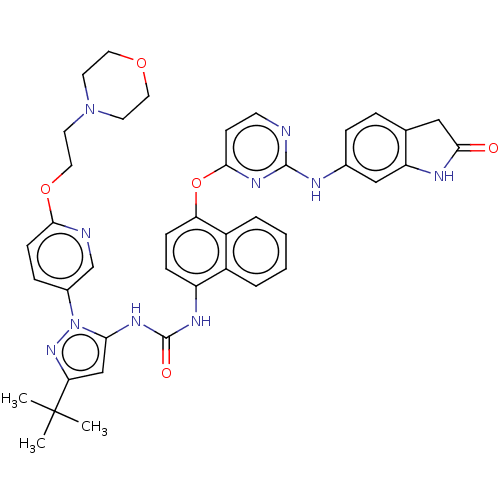 (US9249125, 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205338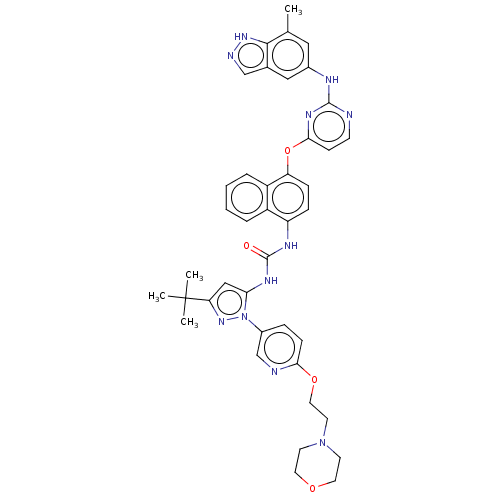 (US9249125, 91) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205339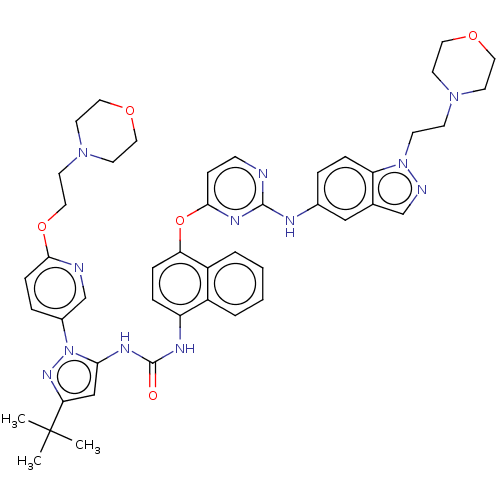 (US9249125, 92) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205321 (US9249125, 74) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205322 (US9249125, 75) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205333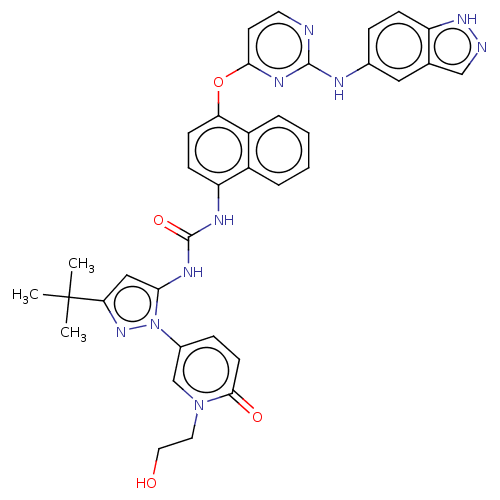 (US9249125, 86) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205336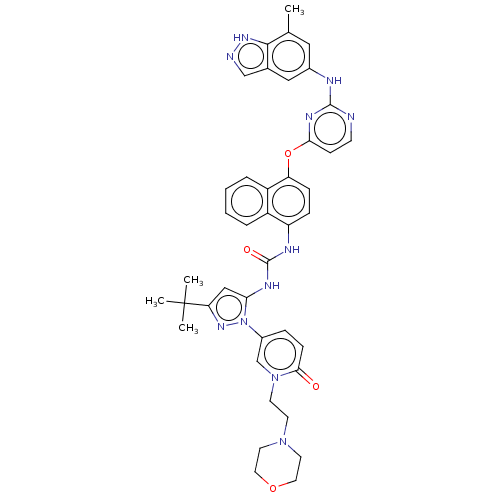 (US9249125, 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205337 (US9249125, 90) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205338 (US9249125, 91) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205339 (US9249125, 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205257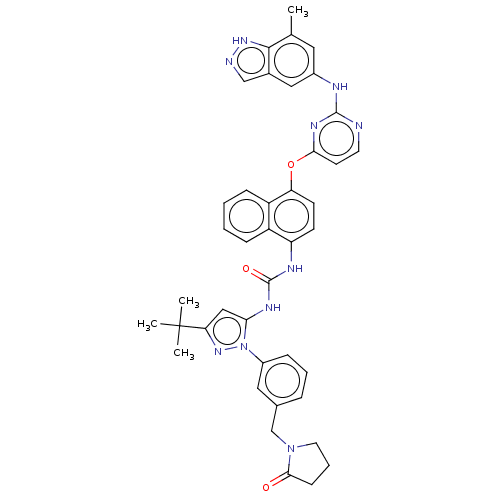 (US9249125, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205262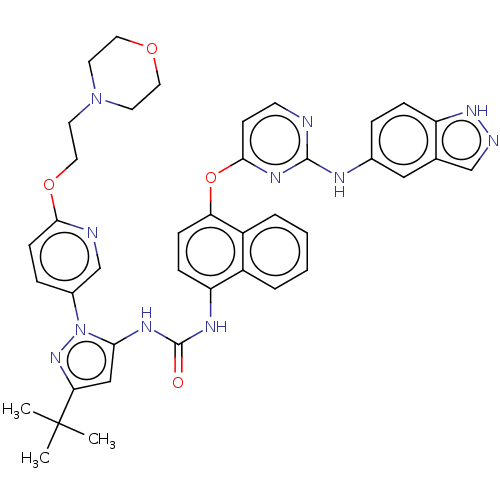 (US9249125, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205293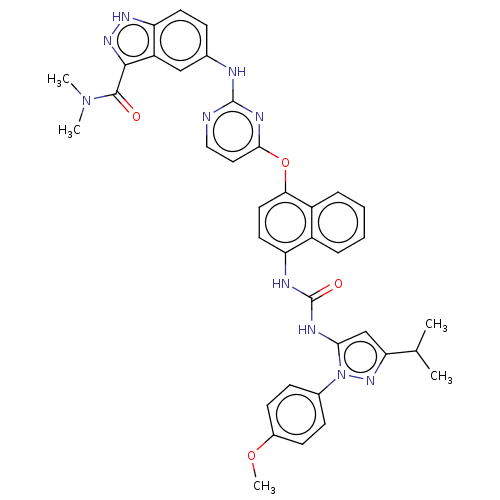 (US9249125, 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205294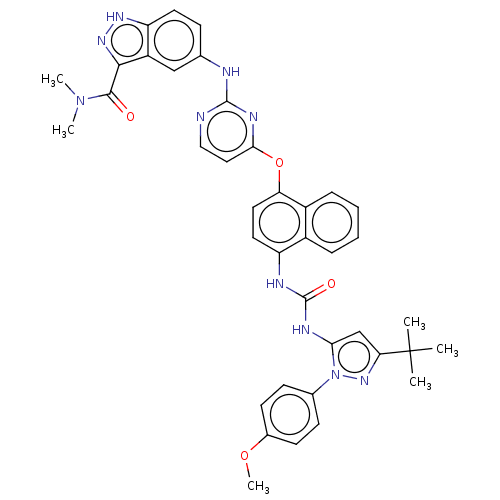 (US9249125, 47) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205297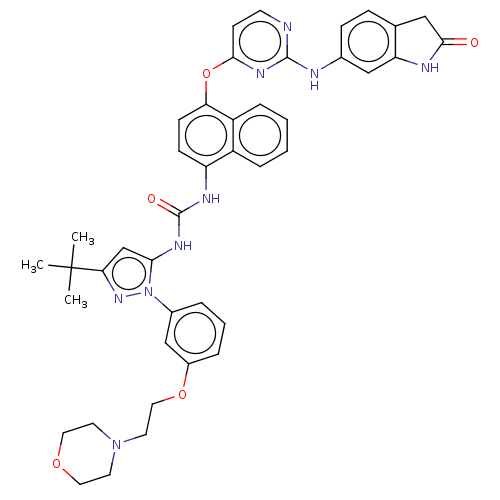 (US9249125, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205298 (US9249125, 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205302 (US9249125, 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205305 (US9249125, 58) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205308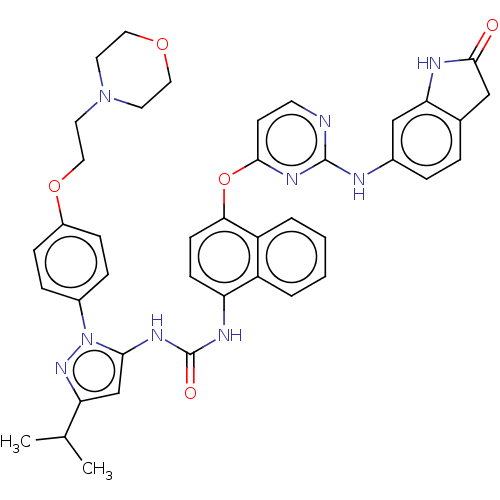 (US9249125, 61) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205310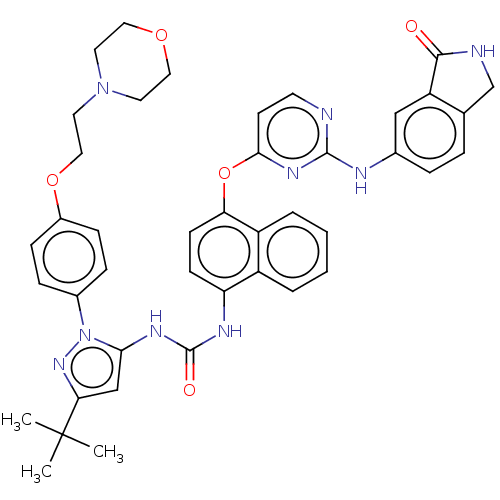 (US9249125, 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205314 (US9249125, 67) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM205331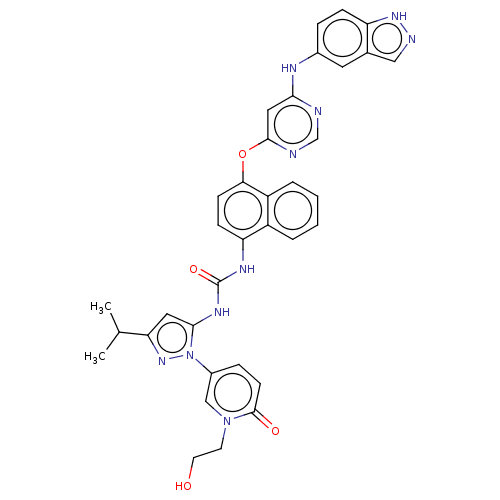 (US9249125, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determining the l... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205255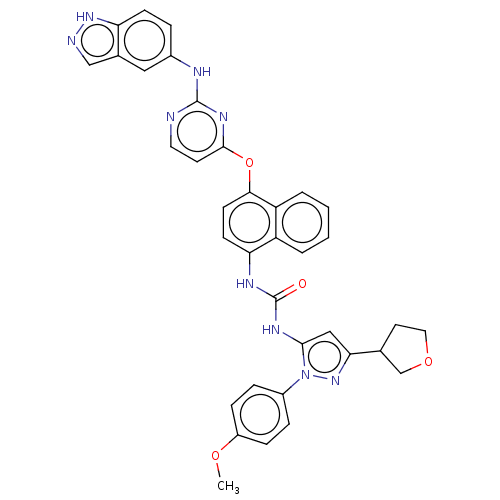 (US9249125, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM205298 (US9249125, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of test compounds against the p38 MAPKα isoform (MAPK14: Invitrogen), are evaluated indirectly by determining the l... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205293 (US9249125, 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM205297 (US9249125, 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM205319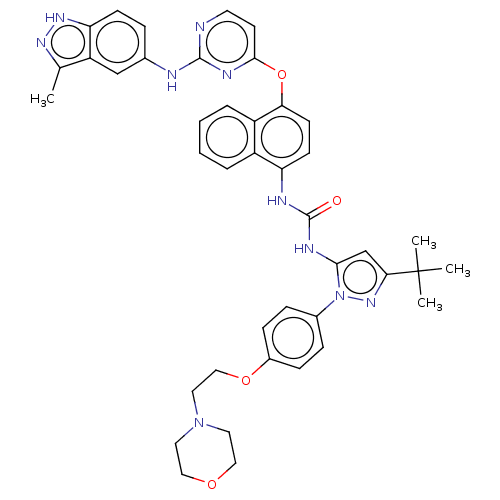 (US9249125, 72) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
RESPIVERT LIMITED; TOPIVERT PHARMA LIMITED US Patent | Assay Description The inhibitory activities of compounds of the invention against c-Src and Syk enzymes (Invitrogen), are evaluated in a similar fashion to that descri... | US Patent US9249125 (2016) BindingDB Entry DOI: 10.7270/Q2CN72QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 863 total ) | Next | Last >> |