

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191945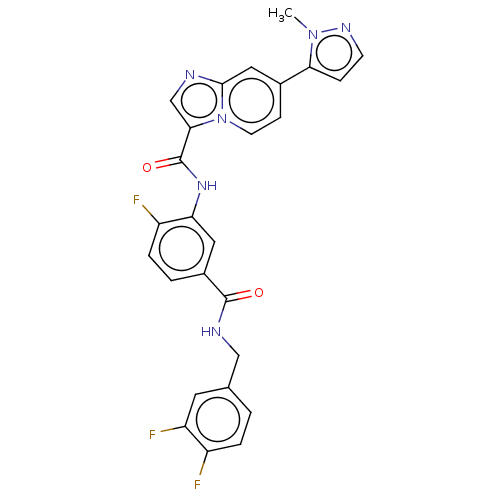 (CHEMBL3904768) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191977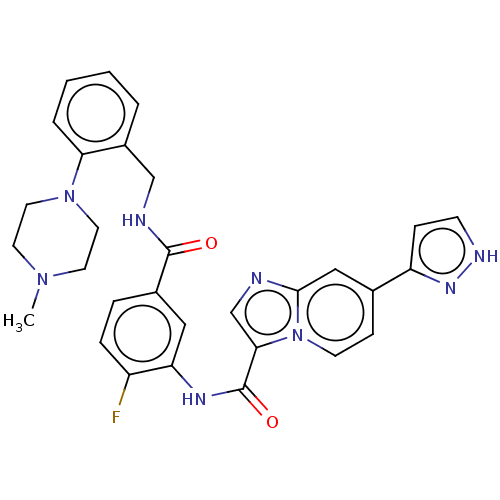 (CHEMBL3983564) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191981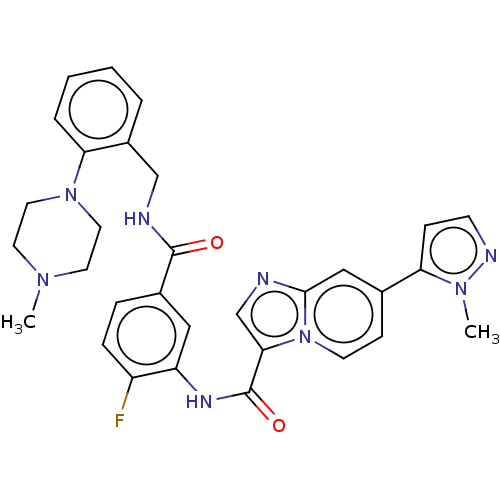 (CHEMBL3979322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191973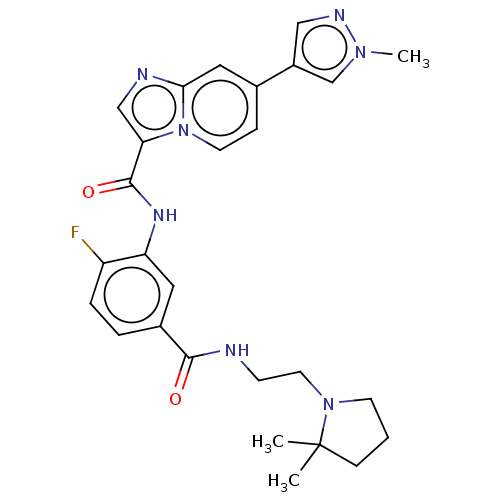 (CHEMBL3940697) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191978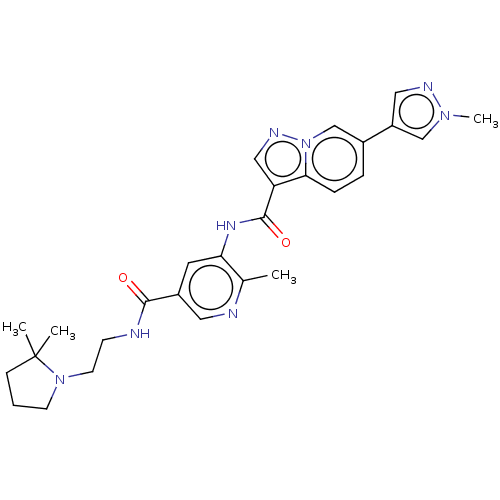 (CHEMBL3915941) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191974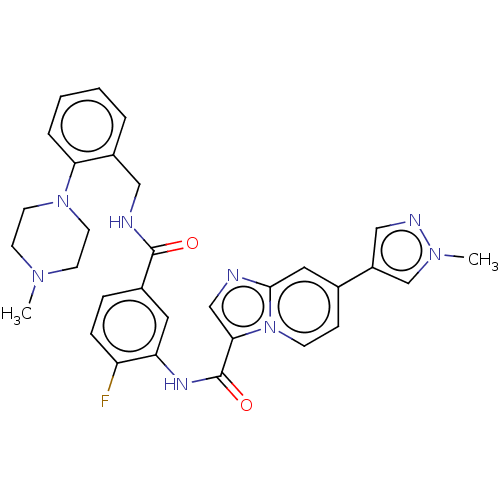 (CHEMBL3913766) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191975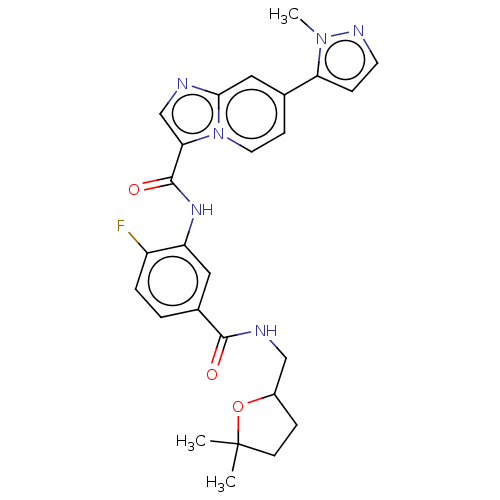 (CHEMBL3975580) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191946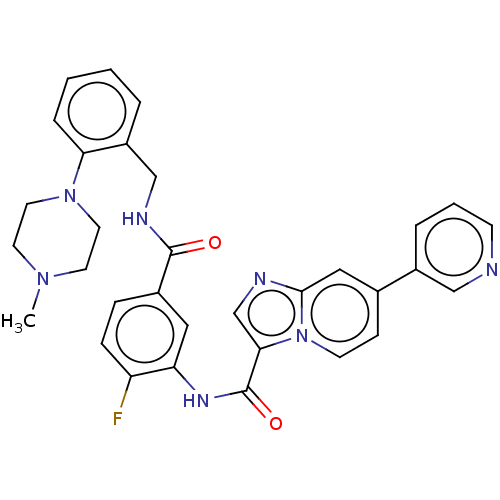 (CHEMBL3950278) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191980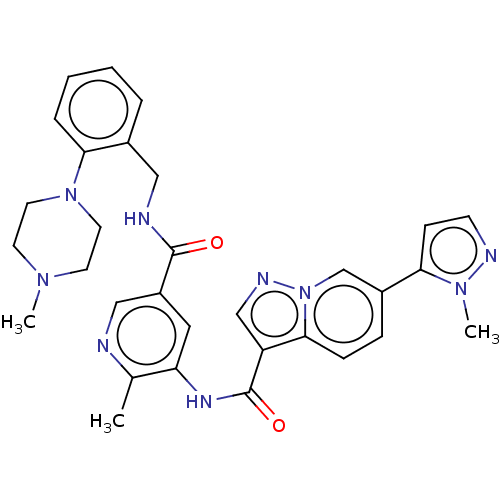 (CHEMBL3985689) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192005 (CHEMBL3947262) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192006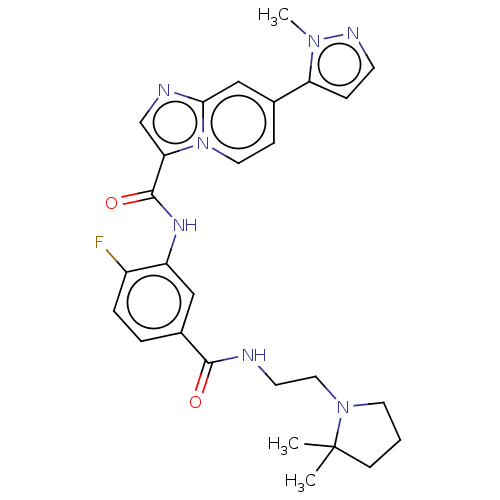 (CHEMBL3955987) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191972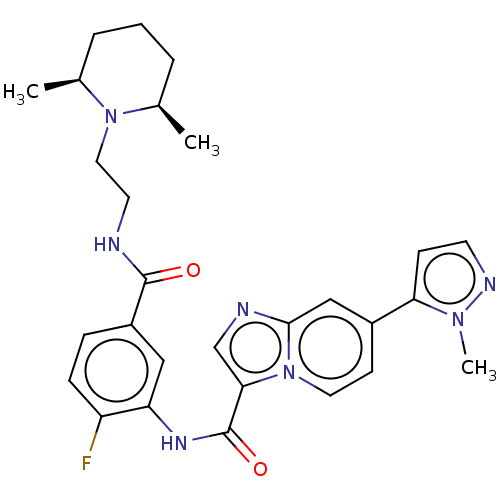 (CHEMBL3895824) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192007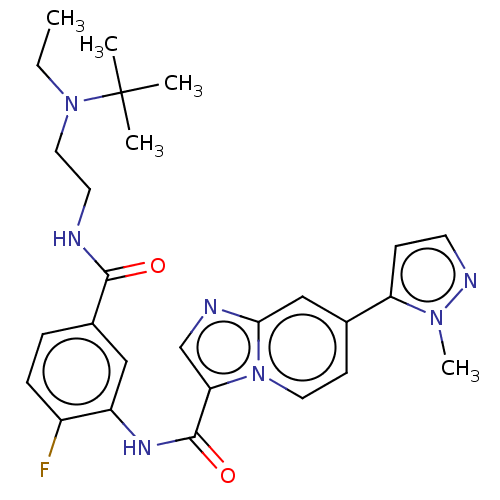 (CHEMBL3902237) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191973 (CHEMBL3940697) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191975 (CHEMBL3975580) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191945 (CHEMBL3904768) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191976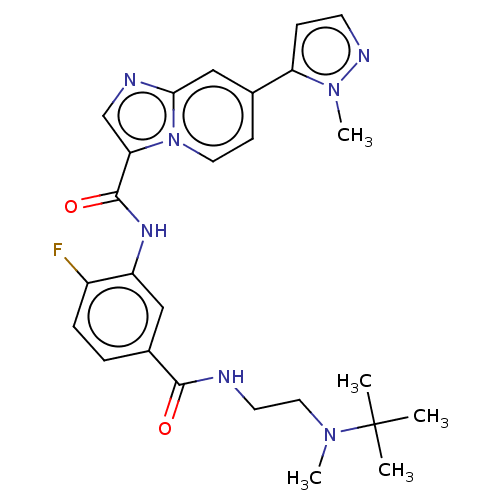 (CHEMBL3967097) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191974 (CHEMBL3913766) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191946 (CHEMBL3950278) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191972 (CHEMBL3895824) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191981 (CHEMBL3979322) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50192006 (CHEMBL3955987) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50192005 (CHEMBL3947262) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50300042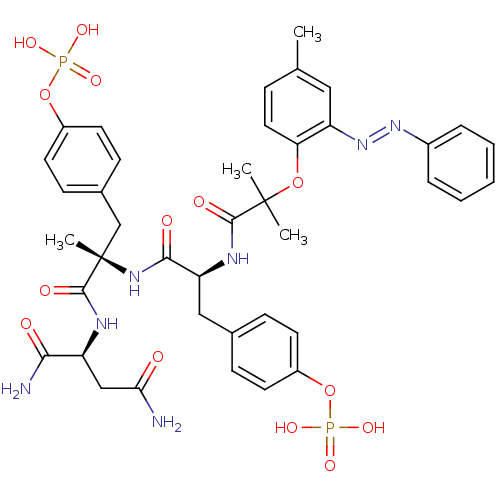 (4-[(2S)-2-{[(1S)-1-{[(1S)-1,2-dicarbamoylethyl]car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 176 Curated by ChEMBL | Assay Description Inhibition of Grb2 by ELISA | Eur J Med Chem 45: 244-55 (2010) Article DOI: 10.1016/j.ejmech.2009.10.003 BindingDB Entry DOI: 10.7270/Q21C1WZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191979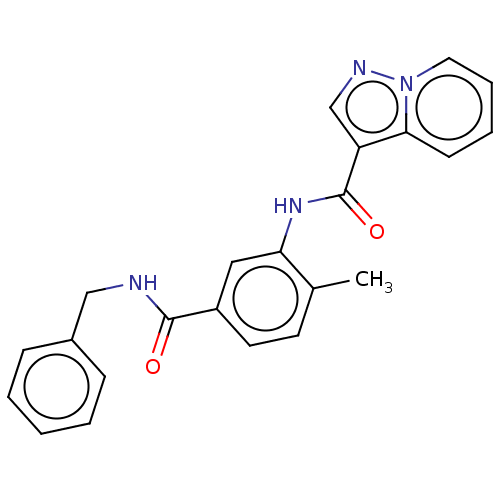 (CHEMBL3906976) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191978 (CHEMBL3915941) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192008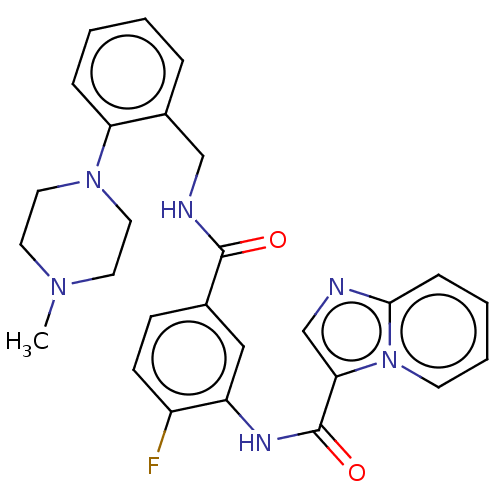 (CHEMBL3971140) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191977 (CHEMBL3983564) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50192007 (CHEMBL3902237) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191976 (CHEMBL3967097) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191980 (CHEMBL3985689) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50192008 (CHEMBL3971140) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50191981 (CHEMBL3979322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50191978 (CHEMBL3915941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of H3 receptor (unknown origin) | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50191978 (CHEMBL3915941) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of M1 receptor (unknown origin) | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50191978 (CHEMBL3915941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth factor receptor-bound protein 2 (Homo sapiens (Human)) | BDBM50300043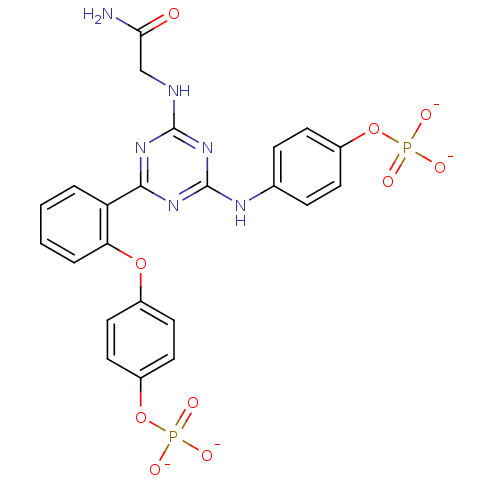 (CHEMBL573405 | Sodium 2-(4-(2-(4-phosphonatooxyphe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 176 Curated by ChEMBL | Assay Description Displacement of biotin-Ahx-PSpYVNVQN peptide from GST fused Grb2 SH2 domain by ELISA | Eur J Med Chem 45: 244-55 (2010) Article DOI: 10.1016/j.ejmech.2009.10.003 BindingDB Entry DOI: 10.7270/Q21C1WZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057805 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057810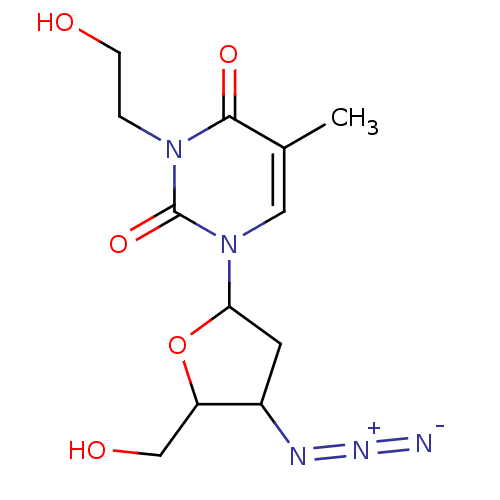 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057818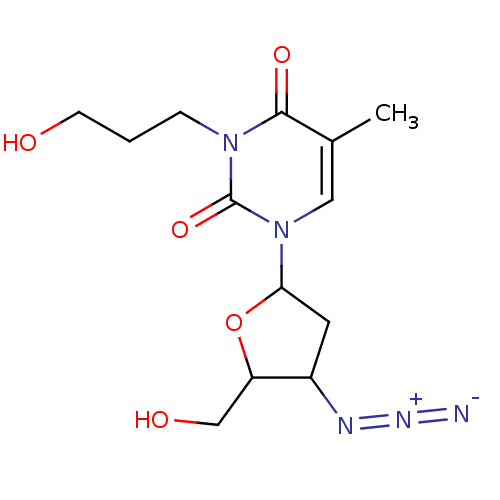 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057814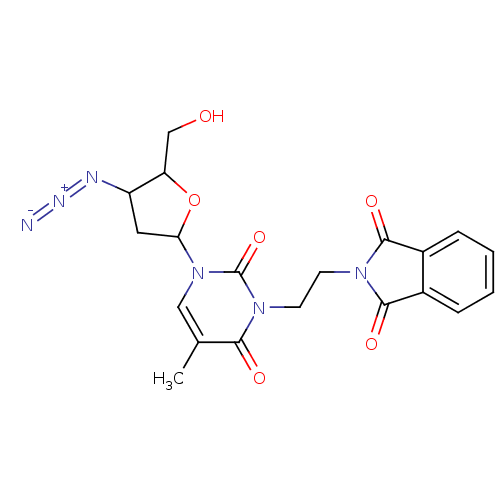 (2-{2-[3-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057804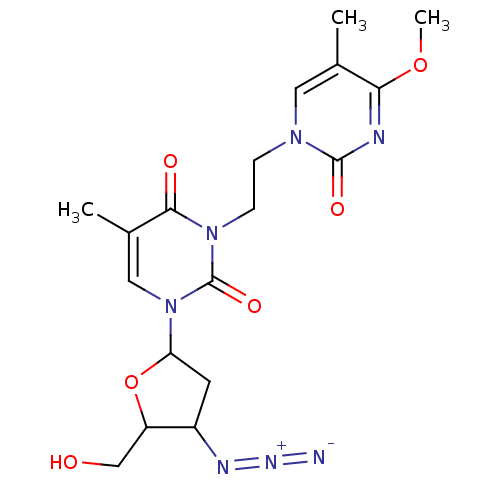 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.90E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057821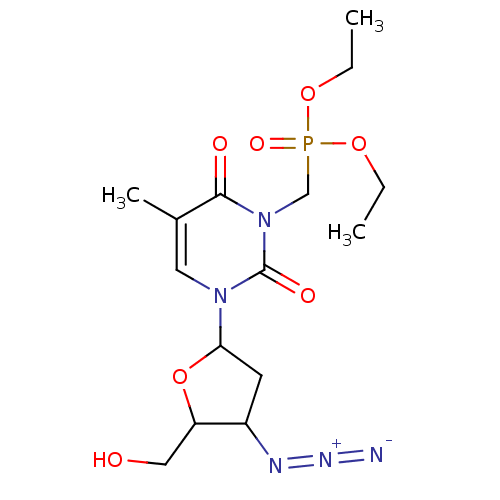 (CHEMBL39945 | [3-(4-Azido-5-hydroxymethyl-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057819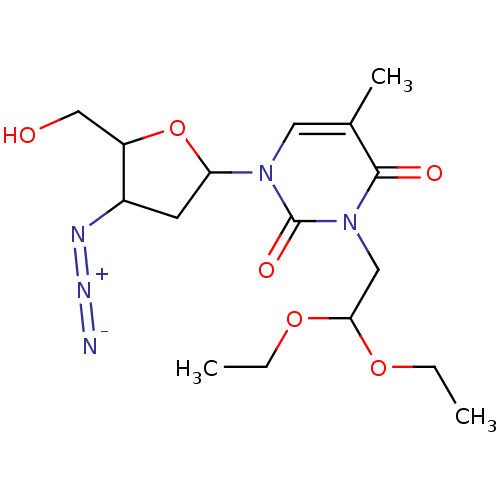 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057822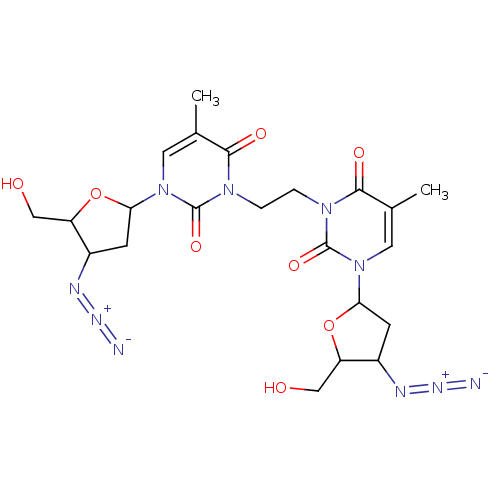 (1-(4-azido-5-hydroxymethyltetrahydro-2-furanyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057809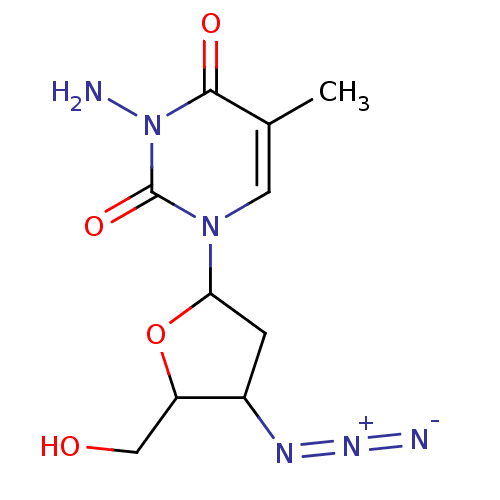 (3-Amino-1-(4-azido-5-hydroxymethyl-tetrahydro-fura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057807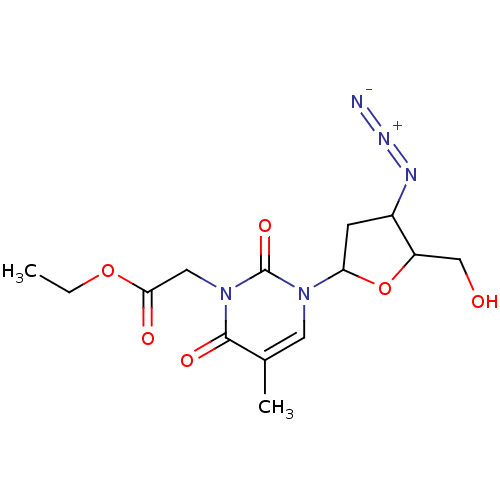 (CHEMBL38606 | [3-(4-Azido-5-hydroxymethyl-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057811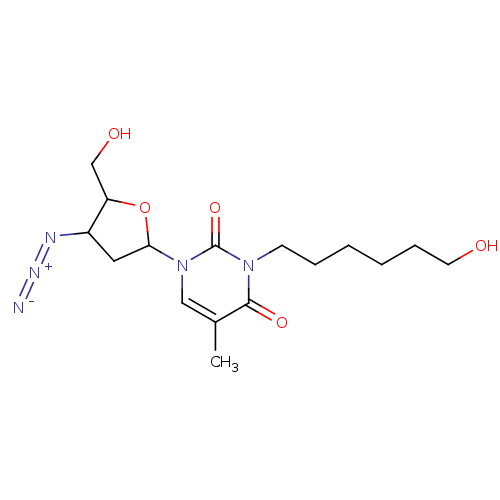 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |