 Found 289 hits with Last Name = 'lou' and Initial = 'jp'
Found 289 hits with Last Name = 'lou' and Initial = 'jp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCd |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of ERK |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of EPHB3 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKAP1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205477

((Z)-2-((1R,2S)-2-phenylcyclopropylamino)-5-(quinol...)Show SMILES O=C1N=C(N[C@@H]2C[C@H]2c2ccccc2)SC1=Cc1ccc2ncccc2c1 |w:16.19,t:2| Show InChI InChI=1S/C22H17N3OS/c26-21-20(12-14-8-9-18-16(11-14)7-4-10-23-18)27-22(25-21)24-19-13-17(19)15-5-2-1-3-6-15/h1-12,17,19H,13H2,(H,24,25,26)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of GSKp1 |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of PKCa |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50205468

((Z)-2-amino-5-(quinolin-6-ylmethylene)thiazol-4(5H...)Show SMILES NC1=NC(=O)C(S1)=Cc1ccc2ncccc2c1 |w:7.8,t:1| Show InChI InChI=1S/C13H9N3OS/c14-13-16-12(17)11(18-13)7-8-3-4-10-9(6-8)2-1-5-15-10/h1-7H,(H2,14,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of FYN |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
RAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50205472

((5Z)-5-(quinolin-6-ylmethylidene)-2-[(thiophen-2-y...)Show SMILES O=C1N=C(NCc2cccs2)SC1=Cc1ccc2ncccc2c1 |w:13.15,t:2| Show InChI InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 17: 2134-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.081
BindingDB Entry DOI: 10.7270/Q2BV7G9T |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50317628
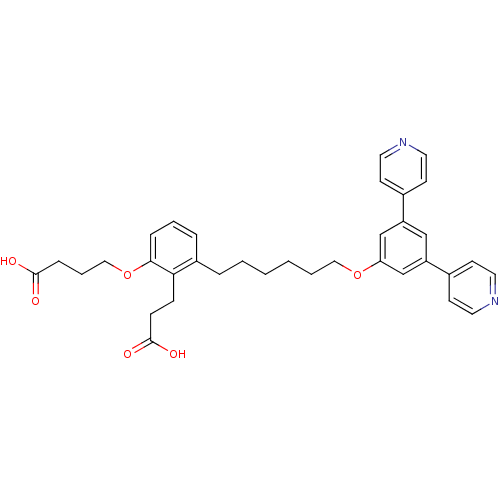
(4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-pyridin-4-yl-p...)Show SMILES OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccncc2)-c2ccncc2)c1CCC(O)=O Show InChI InChI=1S/C35H38N2O6/c38-34(39)10-6-22-43-33-9-5-8-28(32(33)11-12-35(40)41)7-3-1-2-4-21-42-31-24-29(26-13-17-36-18-14-26)23-30(25-31)27-15-19-37-20-16-27/h5,8-9,13-20,23-25H,1-4,6-7,10-12,21-22H2,(H,38,39)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins |
J Med Chem 53: 3502-16 (2010)
Article DOI: 10.1021/jm1001919
BindingDB Entry DOI: 10.7270/Q2KW5G67 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117037
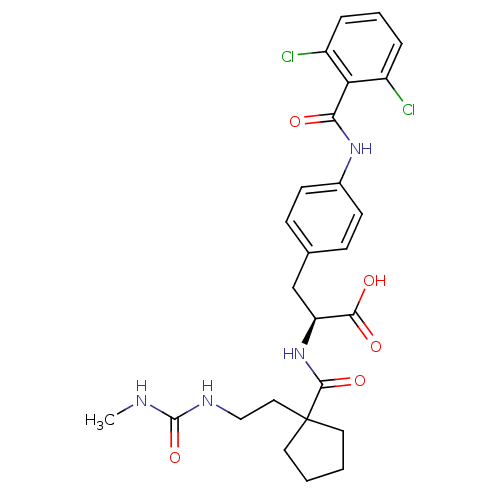
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES CNC(=O)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N4O5/c1-29-25(37)30-14-13-26(11-2-3-12-26)24(36)32-20(23(34)35)15-16-7-9-17(10-8-16)31-22(33)21-18(27)5-4-6-19(21)28/h4-10,20H,2-3,11-15H2,1H3,(H,31,33)(H,32,36)(H,34,35)(H2,29,30,37)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117004
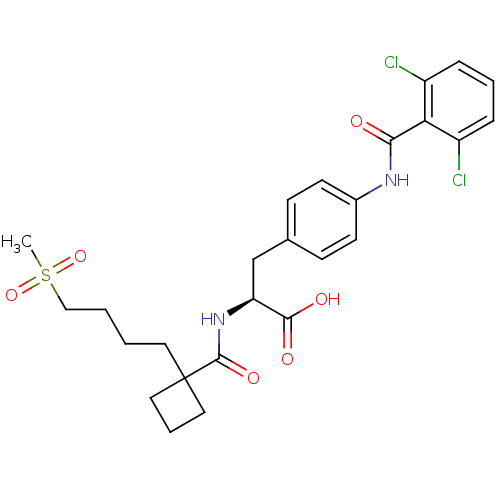
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)(=O)CCCCC1(CCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N2O6S/c1-37(35,36)15-3-2-12-26(13-5-14-26)25(34)30-21(24(32)33)16-17-8-10-18(11-9-17)29-23(31)22-19(27)6-4-7-20(22)28/h4,6-11,21H,2-3,5,12-16H2,1H3,(H,29,31)(H,30,34)(H,32,33)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117041
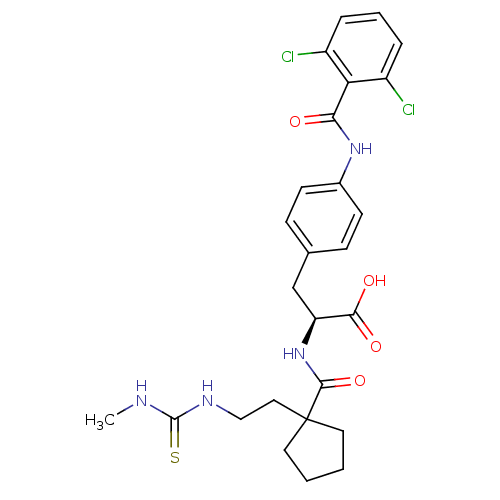
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-({1...)Show SMILES CNC(=S)NCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H30Cl2N4O4S/c1-29-25(37)30-14-13-26(11-2-3-12-26)24(36)32-20(23(34)35)15-16-7-9-17(10-8-16)31-22(33)21-18(27)5-4-6-19(21)28/h4-10,20H,2-3,11-15H2,1H3,(H,31,33)(H,32,36)(H,34,35)(H2,29,30,37)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117036
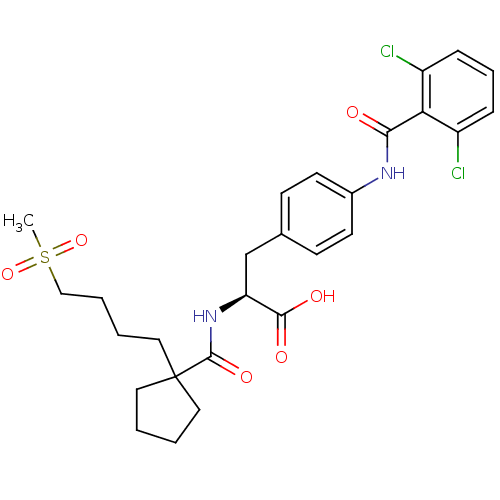
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)(=O)CCCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C27H32Cl2N2O6S/c1-38(36,37)16-5-4-15-27(13-2-3-14-27)26(35)31-22(25(33)34)17-18-9-11-19(12-10-18)30-24(32)23-20(28)7-6-8-21(23)29/h6-12,22H,2-5,13-17H2,1H3,(H,30,32)(H,31,35)(H,33,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50317631
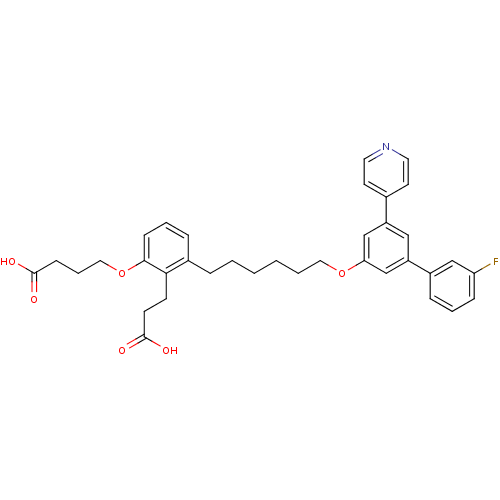
(4-{2-(2-Carboxy-ethyl)-3-[6-(3'-fluoro-5-pyridin-4...)Show SMILES OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2cccc(F)c2)-c2ccncc2)c1CCC(O)=O Show InChI InChI=1S/C36H38FNO6/c37-31-11-5-10-28(23-31)30-22-29(26-16-18-38-19-17-26)24-32(25-30)43-20-4-2-1-3-8-27-9-6-12-34(33(27)14-15-36(41)42)44-21-7-13-35(39)40/h5-6,9-12,16-19,22-25H,1-4,7-8,13-15,20-21H2,(H,39,40)(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins |
J Med Chem 53: 3502-16 (2010)
Article DOI: 10.1021/jm1001919
BindingDB Entry DOI: 10.7270/Q2KW5G67 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117056

((S)-2-(2-Bromo-6-methyl-benzoylamino)-3-[4-(2,6-di...)Show SMILES Cc1cccc(Br)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19BrCl2N2O4/c1-13-4-2-5-16(25)20(13)22(30)29-19(24(32)33)12-14-8-10-15(11-9-14)28-23(31)21-17(26)6-3-7-18(21)27/h2-11,19H,12H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50317632
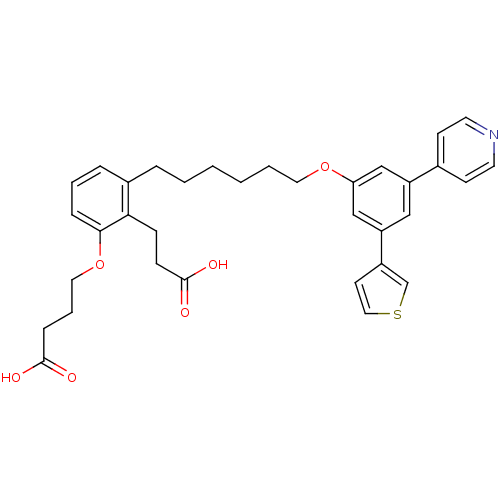
(4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyridin-4-yl-5-thio...)Show SMILES OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccncc2)-c2ccsc2)c1CCC(O)=O Show InChI InChI=1S/C34H37NO6S/c36-33(37)10-6-19-41-32-9-5-8-26(31(32)11-12-34(38)39)7-3-1-2-4-18-40-30-22-28(25-13-16-35-17-14-25)21-29(23-30)27-15-20-42-24-27/h5,8-9,13-17,20-24H,1-4,6-7,10-12,18-19H2,(H,36,37)(H,38,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins |
J Med Chem 53: 3502-16 (2010)
Article DOI: 10.1021/jm1001919
BindingDB Entry DOI: 10.7270/Q2KW5G67 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50317625
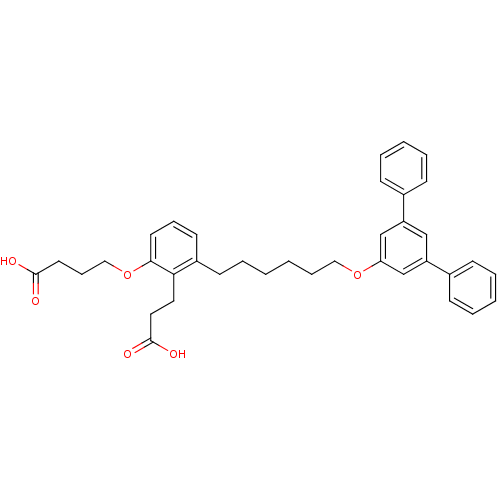
(4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...)Show SMILES OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccccc2)-c2ccccc2)c1CCC(O)=O Show InChI InChI=1S/C37H40O6/c38-36(39)20-12-24-43-35-19-11-18-30(34(35)21-22-37(40)41)17-5-1-2-10-23-42-33-26-31(28-13-6-3-7-14-28)25-32(27-33)29-15-8-4-9-16-29/h3-4,6-9,11,13-16,18-19,25-27H,1-2,5,10,12,17,20-24H2,(H,38,39)(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins |
J Med Chem 53: 3502-16 (2010)
Article DOI: 10.1021/jm1001919
BindingDB Entry DOI: 10.7270/Q2KW5G67 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117016

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CS(=O)CCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O5S/c1-35(34)14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50317633
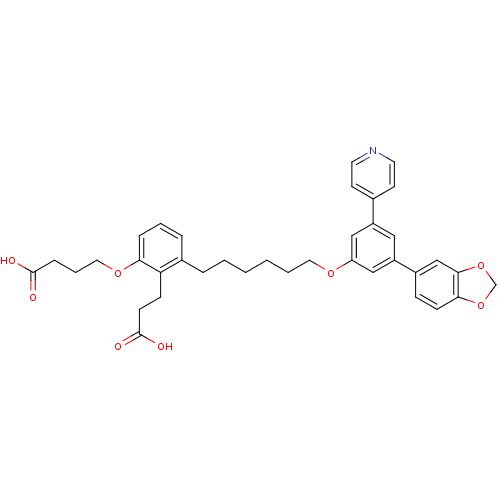
(4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyridin-4-yl-ph...)Show SMILES OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccc3OCOc3c2)-c2ccncc2)c1CCC(O)=O Show InChI InChI=1S/C37H39NO8/c39-36(40)10-6-20-44-33-9-5-8-27(32(33)12-14-37(41)42)7-3-1-2-4-19-43-31-22-29(26-15-17-38-18-16-26)21-30(23-31)28-11-13-34-35(24-28)46-25-45-34/h5,8-9,11,13,15-18,21-24H,1-4,6-7,10,12,14,19-20,25H2,(H,39,40)(H,41,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins |
J Med Chem 53: 3502-16 (2010)
Article DOI: 10.1021/jm1001919
BindingDB Entry DOI: 10.7270/Q2KW5G67 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117073
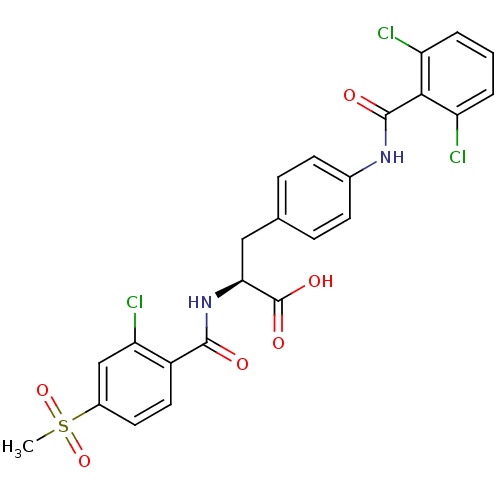
((S)-2-(2-Chloro-4-methanesulfonyl-benzoylamino)-3-...)Show SMILES CS(=O)(=O)c1ccc(C(=O)N[C@@H](Cc2ccc(NC(=O)c3c(Cl)cccc3Cl)cc2)C(O)=O)c(Cl)c1 Show InChI InChI=1S/C24H19Cl3N2O6S/c1-36(34,35)15-9-10-16(19(27)12-15)22(30)29-20(24(32)33)11-13-5-7-14(8-6-13)28-23(31)21-17(25)3-2-4-18(21)26/h2-10,12,20H,11H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117050
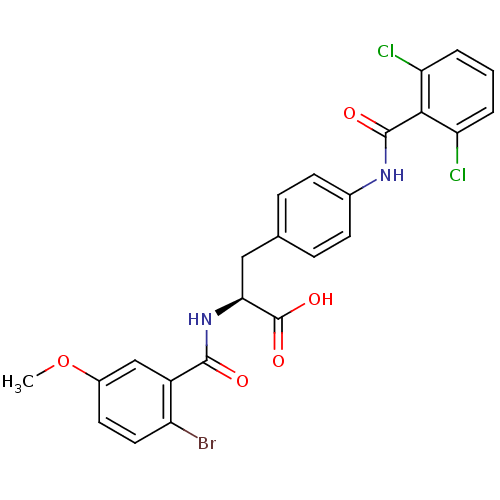
((S)-2-(2-Bromo-5-methoxy-benzoylamino)-3-[4-(2,6-d...)Show SMILES COc1ccc(Br)c(c1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19BrCl2N2O5/c1-34-15-9-10-17(25)16(12-15)22(30)29-20(24(32)33)11-13-5-7-14(8-6-13)28-23(31)21-18(26)3-2-4-19(21)27/h2-10,12,20H,11H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117026
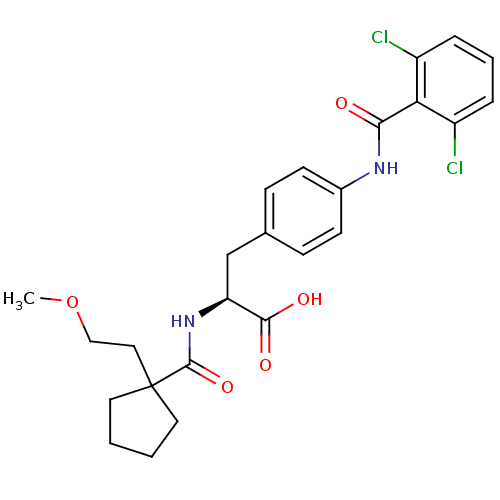
((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES COCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O5/c1-34-14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration determined against VCAM/VLA-4 in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50116998

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-{[1...)Show SMILES CSCCC1(CCCC1)C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C25H28Cl2N2O4S/c1-34-14-13-25(11-2-3-12-25)24(33)29-20(23(31)32)15-16-7-9-17(10-8-16)28-22(30)21-18(26)5-4-6-19(21)27/h4-10,20H,2-3,11-15H2,1H3,(H,28,30)(H,29,33)(H,31,32)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Very late antigen 4/vascular cell adhesion molecule 1 interaction in ELISA |
Bioorg Med Chem Lett 12: 2475-8 (2002)
BindingDB Entry DOI: 10.7270/Q2251HH8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117069
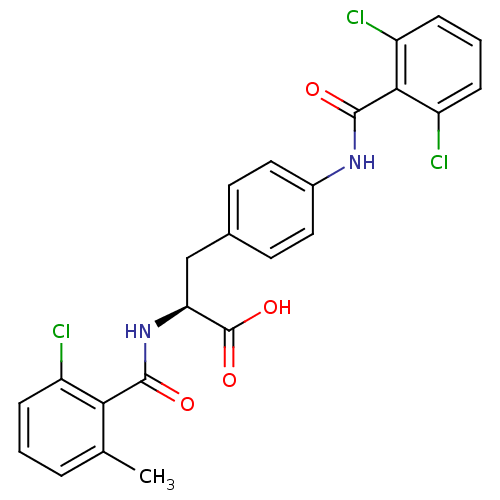
((S)-2-(2-Chloro-6-methyl-benzoylamino)-3-[4-(2,6-d...)Show SMILES Cc1cccc(Cl)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C24H19Cl3N2O4/c1-13-4-2-5-16(25)20(13)22(30)29-19(24(32)33)12-14-8-10-15(11-9-14)28-23(31)21-17(26)6-3-7-18(21)27/h2-11,19H,12H2,1H3,(H,28,31)(H,29,30)(H,32,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50117053

((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(2-...)Show SMILES CCc1cccc(C)c1C(=O)N[C@@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)C(O)=O Show InChI InChI=1S/C26H24Cl2N2O4/c1-3-17-7-4-6-15(2)22(17)24(31)30-21(26(33)34)14-16-10-12-18(13-11-16)29-25(32)23-19(27)8-5-9-20(23)28/h4-13,21H,3,14H2,1-2H3,(H,29,32)(H,30,31)(H,33,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibitory binding concentration against VCAM/VLA-4 in ELISA. |
Bioorg Med Chem Lett 12: 2479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2V989C5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data