 Found 270 hits with Last Name = 'low' and Initial = 'cm'
Found 270 hits with Last Name = 'low' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471066
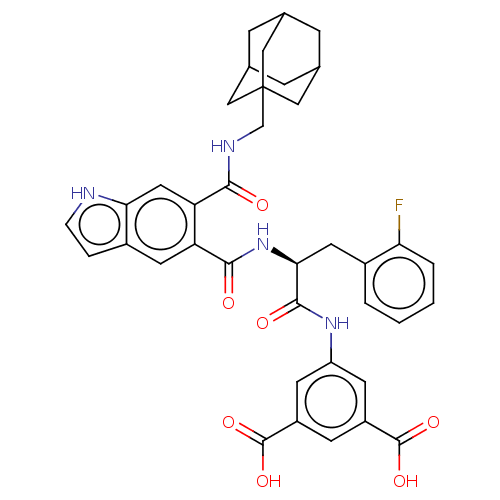
(CHEMBL415936)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:36:43:40.42.39,41:40:43:36.35.37,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C38H37FN4O7/c39-30-4-2-1-3-23(30)14-32(35(46)42-27-11-25(36(47)48)10-26(12-27)37(49)50)43-34(45)28-13-24-5-6-40-31(24)15-29(28)33(44)41-19-38-16-20-7-21(17-38)9-22(8-20)18-38/h1-6,10-13,15,20-22,32,40H,7-9,14,16-19H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t20?,21?,22?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002926
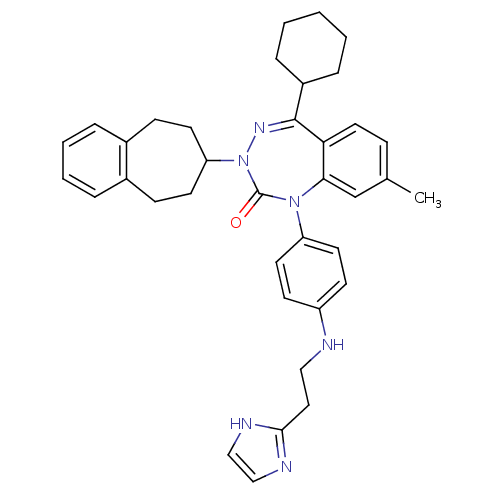
(CHEMBL244325)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(N=C2C1CCCCC1)C1CCc2ccccc2CC1 |c:28| Show InChI InChI=1S/C37H42N6O/c1-26-11-20-33-34(25-26)42(31-18-14-30(15-19-31)38-22-21-35-39-23-24-40-35)37(44)43(41-36(33)29-9-3-2-4-10-29)32-16-12-27-7-5-6-8-28(27)13-17-32/h5-8,11,14-15,18-20,23-25,29,32,38H,2-4,9-10,12-13,16-17,21-22H2,1H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845
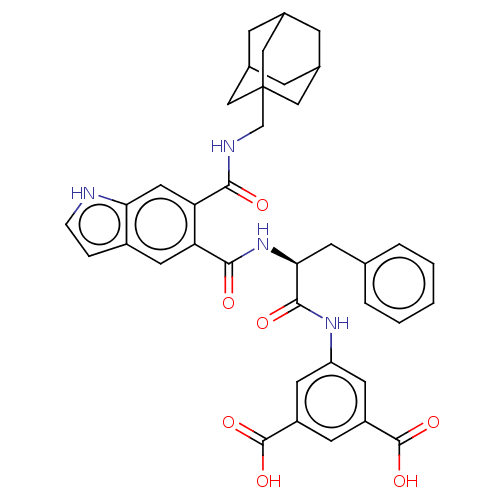
(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845

(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of binding of [125I]CCK-8S to Cholecystokinin type B receptor in mouse cerebral cortex homogenates |
J Med Chem 43: 3518-29 (2000)
Article DOI: 10.1021/jm000960w
BindingDB Entry DOI: 10.7270/Q2KS6V9G |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50213845

(CHEMBL14557)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:40:35:42:39.41.38,40:39:42:35.34.36,THB:38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471075
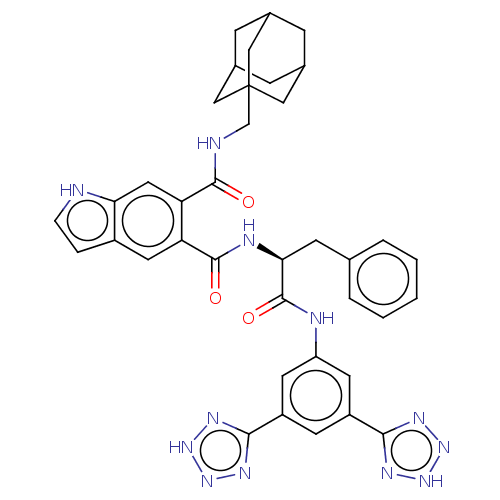
(CHEMBL299540)Show SMILES O=C(Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1)[C@H](Cc1ccccc1)NC(=O)c1cc2cc[nH]c2cc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:46:47:51:45.44.50,50:49:52:45.44.46,50:45:52:49.51.48,THB:46:45:51:47.52.48| Show InChI InChI=1S/C38H38N12O3/c51-35(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-31-25(6-7-39-31)15-29(30)36(52)42-32(11-21-4-2-1-3-5-21)37(53)41-28-13-26(33-43-47-48-44-33)12-27(14-28)34-45-49-50-46-34/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,51)(H,41,53)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471067
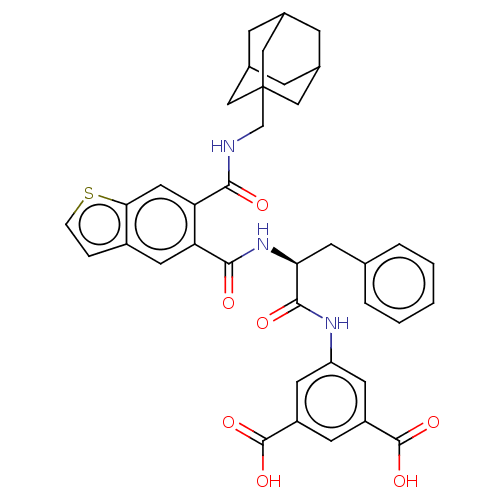
(CHEMBL298521)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccsc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H37N3O7S/c42-33(39-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-32-25(6-7-49-32)15-29(30)34(43)41-31(11-21-4-2-1-3-5-21)35(44)40-28-13-26(36(45)46)12-27(14-28)37(47)48/h1-7,12-16,22-24,31H,8-11,17-20H2,(H,39,42)(H,40,44)(H,41,43)(H,45,46)(H,47,48)/t22?,23?,24?,31-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002913
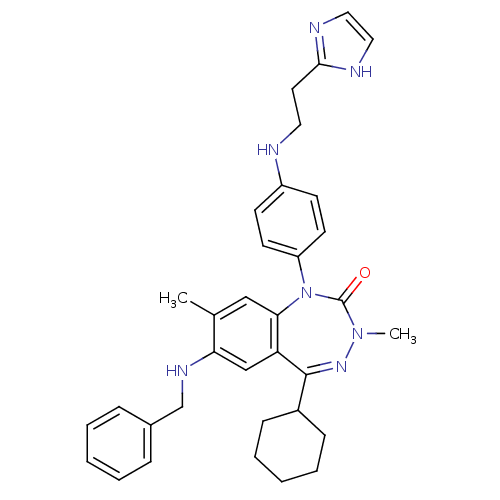
(CHEMBL389783)Show SMILES CN1N=C(C2CCCCC2)c2cc(NCc3ccccc3)c(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C34H39N7O/c1-24-21-31-29(22-30(24)38-23-25-9-5-3-6-10-25)33(26-11-7-4-8-12-26)39-40(2)34(42)41(31)28-15-13-27(14-16-28)35-18-17-32-36-19-20-37-32/h3,5-6,9-10,13-16,19-22,26,35,38H,4,7-8,11-12,17-18,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629
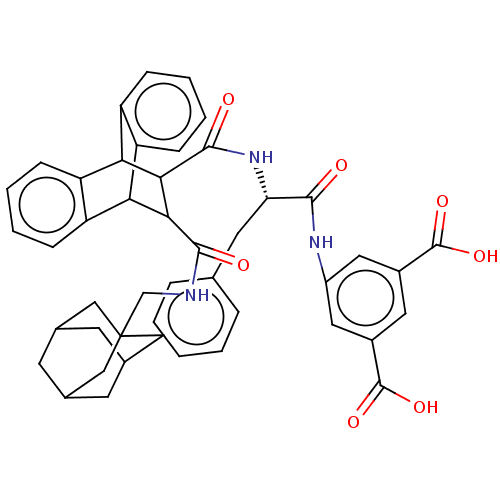
(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471065
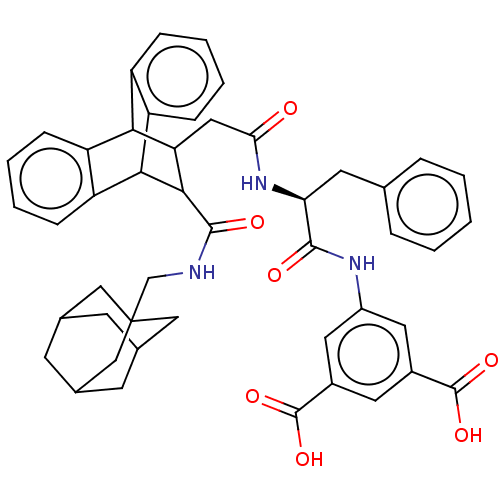
(CHEMBL296167)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)CC2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:44:45:49:43.42.48,37:22:29.24:36.31,28:29:22.21:36.31,35:36:22.21:29.24,25:24:22.21:36.31,THB:20:21:29.24:36.31,44:43:49:45.50.46,46:45:42:47.49.48,46:47:42:45.50.44,32:31:22.21:29.24,(8.01,-6.63,;9.37,-5.87,;10.71,-6.64,;9.37,-4.31,;8.05,-3.54,;8.05,-1.98,;6.95,-.88,;5.47,-.48,;5.23,1.05,;4.2,-1.35,;4.05,-2.9,;5.21,-3.96,;6.55,-3.18,;7.89,-3.96,;7.89,-5.51,;6.52,-6.29,;5.21,-5.51,;2.83,-.57,;1.51,-1.35,;1.51,-2.9,;1.18,-1.33,;-.3,-.93,;.88,-.85,;.17,-1.58,;-1.64,-1.11,;-1.36,.19,;-2.44,1.1,;-3.8,.71,;-4.09,-.59,;-3,-1.49,;-1.14,-1.86,;-.53,-3.49,;-.89,-4.71,;.13,-5.65,;1.47,-5.34,;1.8,-4.1,;.81,-3.2,;1.28,.65,;.76,2.13,;2.78,.35,;3.8,1.53,;5.02,2.46,;6.24,1.69,;7.4,2.63,;9.16,2.49,;7.89,3.4,;7.47,4.87,;5.82,4.86,;6.97,3.98,;4.6,3.92,;6.64,2.4,;9.39,-1.21,;10.74,-1.98,;10.74,-3.54,;12.08,-1.22,;12.1,.33,;13.42,-2.01,)| Show InChI InChI=1S/C47H47N3O7/c51-39(50-38(17-26-8-2-1-3-9-26)43(52)49-32-19-30(45(54)55)18-31(20-32)46(56)57)21-37-40-33-10-4-6-12-35(33)41(36-13-7-5-11-34(36)40)42(37)44(53)48-25-47-22-27-14-28(23-47)16-29(15-27)24-47/h1-13,18-20,27-29,37-38,40-42H,14-17,21-25H2,(H,48,53)(H,49,52)(H,50,51)(H,54,55)(H,56,57)/t27?,28?,29?,37?,38-,40?,41?,42?,47?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002915
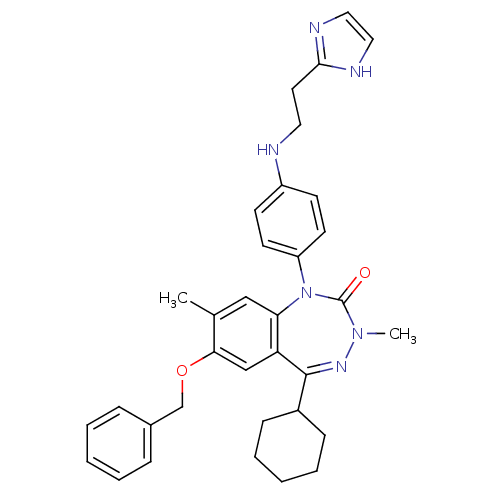
(CHEMBL389787)Show SMILES CN1N=C(C2CCCCC2)c2cc(OCc3ccccc3)c(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C34H38N6O2/c1-24-21-30-29(22-31(24)42-23-25-9-5-3-6-10-25)33(26-11-7-4-8-12-26)38-39(2)34(41)40(30)28-15-13-27(14-16-28)35-18-17-32-36-19-20-37-32/h3,5-6,9-10,13-16,19-22,26,35H,4,7-8,11-12,17-18,23H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470612
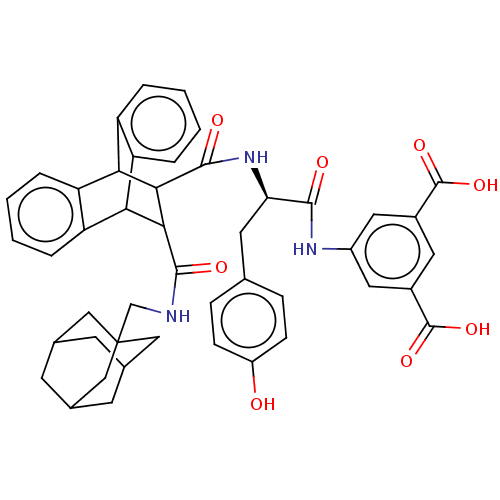
(CHEMBL436209)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,48:43:50:47.49.46,48:47:50:43.42.44,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,28:29:21.22:31.36,(19.63,-7.77,;18.28,-7,;18.28,-5.44,;16.94,-7.79,;15.59,-7.01,;14.26,-7.77,;12.94,-7,;11.38,-7.01,;10.98,-5.49,;10.28,-8.1,;10.69,-9.6,;11.77,-10.69,;13.25,-10.3,;14.33,-11.38,;13.94,-12.87,;15.04,-13.97,;12.45,-13.26,;11.37,-12.18,;8.79,-7.71,;7.29,-7.3,;7.75,-5.77,;2.67,-8.1,;3.67,-7.07,;3.67,-8.95,;5.65,-9.56,;7.45,-9.56,;8.72,-10.82,;8.07,-12.08,;6.09,-12.08,;4.93,-10.77,;2.69,-10.25,;.79,-10.83,;-.77,-11.41,;-2.3,-10.93,;-1.97,-9.88,;-.32,-9.21,;1.37,-9.5,;4.32,-5.93,;3.37,-5.02,;5.45,-5.26,;5.42,-3.95,;6.55,-3.28,;7.75,-3.57,;8.89,-3.15,;9.91,-4.25,;8.63,-3.89,;8.63,-2.53,;7.75,-1.49,;8.91,-1.89,;6.55,-1.98,;7.43,-4.37,;14.26,-9.33,;15.59,-10.11,;16.94,-9.34,;16.36,-11.44,;15.65,-13,;17.91,-11.45,)| Show InChI InChI=1S/C46H45N3O8/c50-31-11-9-24(10-12-31)16-36(41(51)48-30-18-28(44(54)55)17-29(19-30)45(56)57)49-43(53)40-38-34-7-3-1-5-32(34)37(33-6-2-4-8-35(33)38)39(40)42(52)47-23-46-20-25-13-26(21-46)15-27(14-25)22-46/h1-12,17-19,25-27,36-40,50H,13-16,20-23H2,(H,47,52)(H,48,51)(H,49,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470623
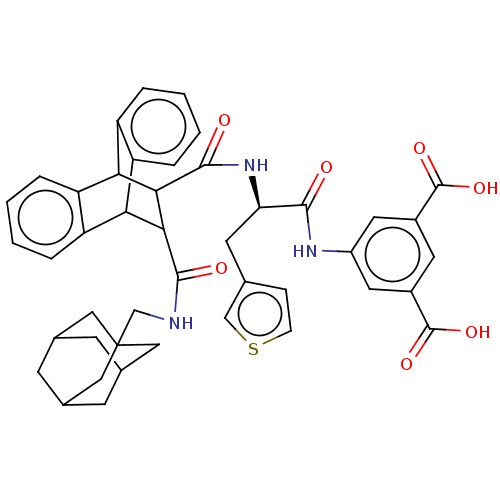
(CHEMBL140179)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccsc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:33:34:19.20:27.22,23:22:19.20:34.29,46:41:48:45.47.44,46:45:48:41.40.42,THB:30:29:19.20:27.22,17:19:27.22:34.29,35:20:27.22:34.29,44:43:40:45.47.46,44:45:40:43.48.42,(14.85,-1.43,;13.52,-.66,;13.5,.89,;12.18,-1.43,;10.84,-.66,;9.52,-1.43,;8.17,-.66,;6.63,-.66,;6.24,.84,;5.55,-1.75,;5.95,-3.26,;7.27,-4.03,;7.43,-5.56,;8.94,-5.88,;9.71,-4.55,;8.68,-3.4,;4.04,-1.37,;2.56,-.97,;3.01,.57,;-2.05,-1.75,;-1.03,-.74,;-1.05,-2.59,;-3.32,-3.16,;-5.02,-2.87,;-6.67,-3.53,;-6.99,-4.56,;-5.45,-5.07,;-3.93,-4.46,;-2.02,-3.9,;.2,-4.4,;1.37,-5.72,;3.33,-5.72,;4,-4.46,;2.72,-3.21,;.91,-3.21,;-.41,.41,;-1.34,1.31,;.72,1.06,;.71,2.38,;1.84,3.05,;2.71,1.96,;3.88,2.44,;3.9,3.8,;3.01,4.83,;4.17,4.44,;4.16,3.16,;5.17,2.09,;3.03,2.74,;1.82,4.35,;9.5,-2.98,;10.84,-3.75,;12.18,-2.98,;11.6,-5.08,;10.89,-6.63,;13.14,-5.08,)| Show InChI InChI=1S/C44H43N3O7S/c48-39(46-29-16-27(42(51)52)15-28(17-29)43(53)54)34(14-23-9-10-55-21-23)47-41(50)38-36-32-7-3-1-5-30(32)35(31-6-2-4-8-33(31)36)37(38)40(49)45-22-44-18-24-11-25(19-44)13-26(12-24)20-44/h1-10,15-17,21,24-26,34-38H,11-14,18-20,22H2,(H,45,49)(H,46,48)(H,47,50)(H,51,52)(H,53,54)/t24?,25?,26?,34-,35?,36?,37?,38?,44?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470617
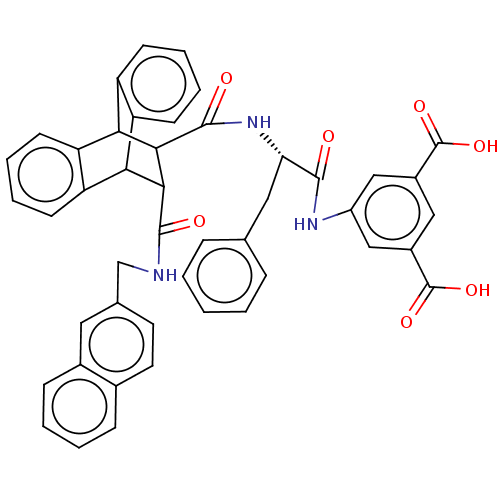
(CHEMBL137180)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCc2ccc3ccccc3c2)cc(c1)C(O)=O |wD:9.9,TLB:18:20:30.35:23.28,34:35:20.21:23.28,24:23:20.21:30.35,36:21:30.35:23.28,THB:27:28:20.21:30.35,(18.12,-9.94,;16.79,-9.16,;16.76,-7.61,;15.44,-9.96,;14.1,-9.19,;12.78,-9.96,;11.43,-9.19,;9.88,-9.19,;9.49,-7.68,;8.8,-10.28,;9.2,-11.78,;10.52,-12.56,;10.51,-14.1,;11.85,-14.88,;13.17,-14.11,;13.17,-12.56,;11.85,-11.78,;7.29,-9.88,;5.8,-9.49,;6.25,-7.93,;1.18,-10.28,;2.2,-9.26,;2.18,-11.13,;4.15,-11.72,;5.96,-11.72,;7.24,-13,;6.58,-14.24,;4.6,-14.24,;3.44,-12.94,;1.21,-12.43,;-.7,-13,;-2.23,-13.59,;-3.77,-13.1,;-3.45,-12.06,;-1.8,-11.4,;-.1,-11.69,;2.82,-8.12,;1.89,-7.19,;3.96,-7.45,;3.95,-6.12,;5.08,-5.47,;6.21,-6.12,;7.34,-5.47,;7.34,-4.15,;8.47,-3.49,;8.45,-2.18,;7.31,-1.53,;6.19,-2.2,;6.19,-3.5,;5.06,-4.15,;12.75,-11.49,;14.1,-12.27,;15.44,-11.49,;14.86,-13.62,;14.14,-15.17,;16.41,-13.62,)| Show InChI InChI=1S/C46H37N3O7/c50-42(48-32-23-30(45(53)54)22-31(24-32)46(55)56)37(21-26-10-2-1-3-11-26)49-44(52)41-39-35-16-8-6-14-33(35)38(34-15-7-9-17-36(34)39)40(41)43(51)47-25-27-18-19-28-12-4-5-13-29(28)20-27/h1-20,22-24,37-41H,21,25H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t37-,38?,39?,40?,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470636
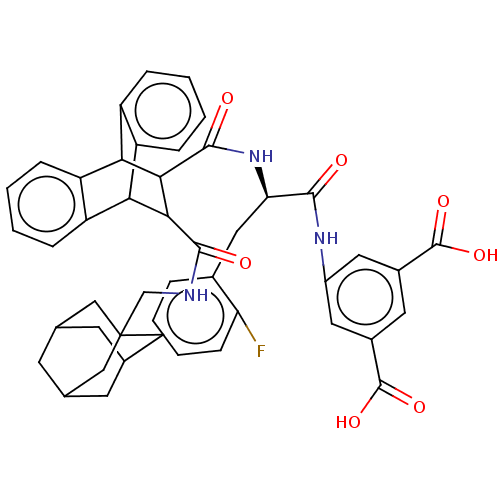
(CHEMBL343367)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccccc2F)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:35:36:21.22:29.24,48:43:50:47.49.46,48:47:50:43.42.44,25:24:21.22:36.31,THB:19:21:29.24:36.31,46:45:42:47.49.48,46:47:42:45.50.44,37:22:29.24:36.31,32:31:21.22:29.24,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;9.61,-5.02,;8.52,-3.96,;8.39,-2.37,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;-3.33,-3.17,;-5.03,-2.88,;-6.68,-3.54,;-7.01,-4.57,;-5.47,-5.08,;-3.93,-4.47,;-2.03,-3.91,;.2,-4.41,;1.37,-5.74,;3.34,-5.74,;4.01,-4.47,;2.73,-3.21,;.92,-3.21,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;2.72,1.96,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.04,2.75,;1.82,4.36,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44FN3O7/c47-35-12-6-1-7-27(35)19-36(41(51)49-30-17-28(44(54)55)16-29(18-30)45(56)57)50-43(53)40-38-33-10-4-2-8-31(33)37(32-9-3-5-11-34(32)38)39(40)42(52)48-23-46-20-24-13-25(21-46)15-26(14-24)22-46/h1-12,16-18,24-26,36-40H,13-15,19-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t24?,25?,26?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002923
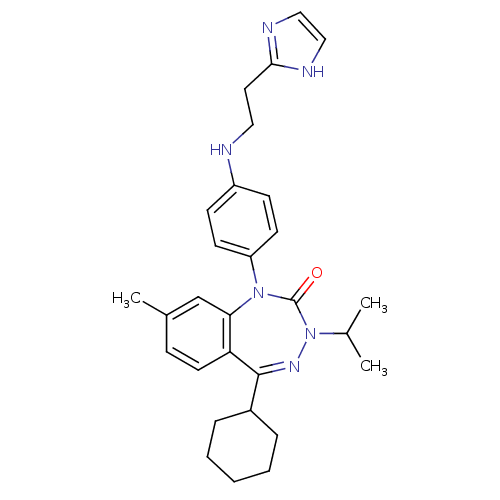
(CHEMBL243698)Show SMILES CC(C)N1N=C(C2CCCCC2)c2ccc(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:4| Show InChI InChI=1S/C29H36N6O/c1-20(2)35-29(36)34(24-12-10-23(11-13-24)30-16-15-27-31-17-18-32-27)26-19-21(3)9-14-25(26)28(33-35)22-7-5-4-6-8-22/h9-14,17-20,22,30H,4-8,15-16H2,1-3H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Tested for its receptor affinity from competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homo... |
J Med Chem 37: 3671-3 (1994)
Article DOI: 10.1021/jm00048a001
BindingDB Entry DOI: 10.7270/Q28918MZ |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002924
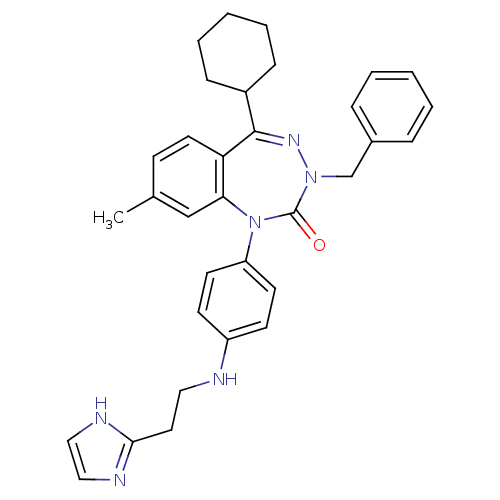
(CHEMBL242567)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(Cc1ccccc1)N=C2C1CCCCC1 |c:36| Show InChI InChI=1S/C33H36N6O/c1-24-12-17-29-30(22-24)39(28-15-13-27(14-16-28)34-19-18-31-35-20-21-36-31)33(40)38(23-25-8-4-2-5-9-25)37-32(29)26-10-6-3-7-11-26/h2,4-5,8-9,12-17,20-22,26,34H,3,6-7,10-11,18-19,23H2,1H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471076
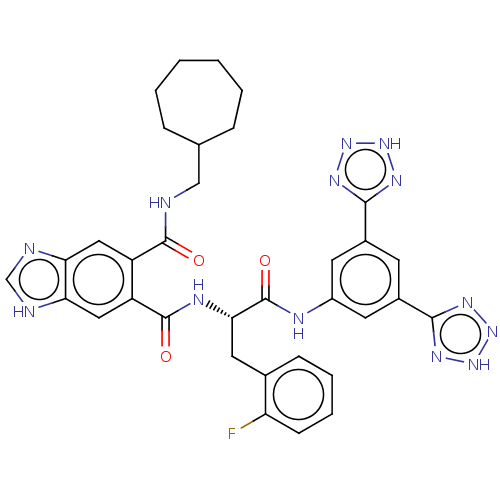
(CHEMBL299387)Show SMILES Fc1ccccc1C[C@H](NC(=O)c1cc2[nH]cnc2cc1C(=O)NCC1CCCCCC1)C(=O)Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1 Show InChI InChI=1S/C34H34FN13O3/c35-26-10-6-5-9-20(26)14-29(34(51)39-23-12-21(30-41-45-46-42-30)11-22(13-23)31-43-47-48-44-31)40-33(50)25-16-28-27(37-18-38-28)15-24(25)32(49)36-17-19-7-3-1-2-4-8-19/h5-6,9-13,15-16,18-19,29H,1-4,7-8,14,17H2,(H,36,49)(H,37,38)(H,39,51)(H,40,50)(H,41,42,45,46)(H,43,44,47,48)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470639
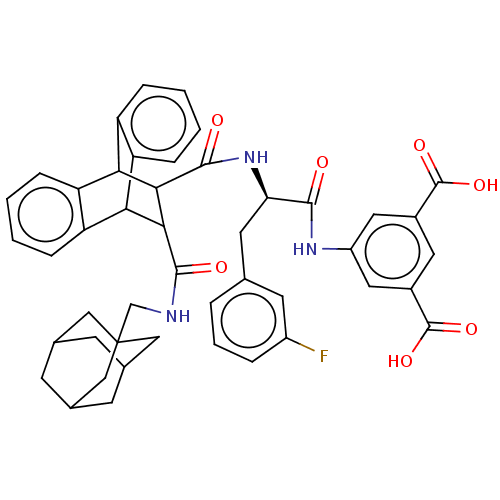
(CHEMBL433656)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2cccc(F)c2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,46:47:42:45.50.44,46:45:42:47.49.48,28:29:21.22:31.36,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;8.52,-3.96,;9.61,-5.02,;9.2,-6.53,;7.71,-6.92,;7.32,-8.42,;6.64,-5.83,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;.92,-3.21,;2.73,-3.21,;4.01,-4.47,;3.34,-5.74,;1.37,-5.74,;.2,-4.41,;-2.03,-3.91,;-3.93,-4.47,;-5.47,-5.08,;-7.01,-4.57,;-6.68,-3.54,;-5.03,-2.88,;-3.33,-3.17,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;1.82,4.36,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.89,2.45,;3.91,3.81,;2.72,1.96,;3.04,2.75,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44FN3O7/c47-30-7-5-6-24(15-30)16-36(41(51)49-31-18-28(44(54)55)17-29(19-31)45(56)57)50-43(53)40-38-34-10-3-1-8-32(34)37(33-9-2-4-11-35(33)38)39(40)42(52)48-23-46-20-25-12-26(21-46)14-27(13-25)22-46/h1-11,15,17-19,25-27,36-40H,12-14,16,20-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of binding of [125I]CCK-8S to Cholecystokinin type B receptor in mouse cerebral cortex homogenates |
J Med Chem 43: 3518-29 (2000)
Article DOI: 10.1021/jm000960w
BindingDB Entry DOI: 10.7270/Q2KS6V9G |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471072
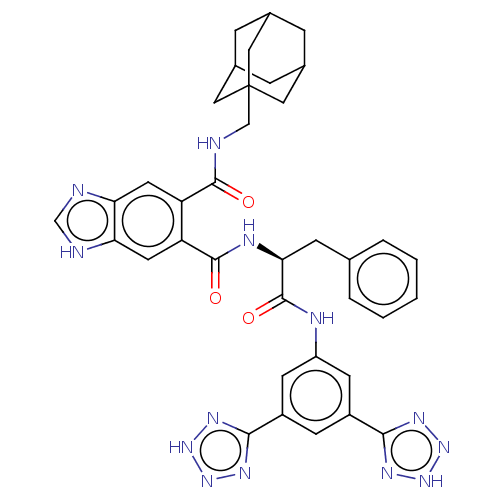
(CHEMBL53237)Show SMILES O=C(Nc1cc(cc(c1)-c1nn[nH]n1)-c1nn[nH]n1)[C@H](Cc1ccccc1)NC(=O)c1cc2[nH]cnc2cc1C(=O)NCC12CC3CC(CC(C3)C1)C2 |TLB:46:47:51:45.44.50,50:49:52:45.44.46,50:45:52:49.51.48,THB:46:45:51:47.52.48| Show InChI InChI=1S/C37H37N13O3/c51-34(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)35(52)42-31(9-20-4-2-1-3-5-20)36(53)41-26-11-24(32-43-47-48-44-32)10-25(12-26)33-45-49-50-46-33/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,51)(H,39,40)(H,41,53)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470624
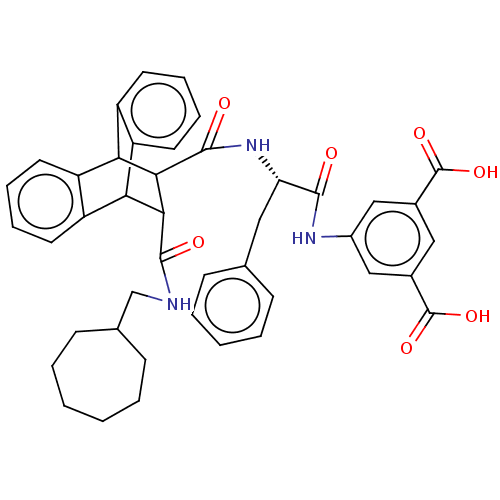
(CHEMBL337673)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC2CCCCCC2)cc(c1)C(O)=O |wD:9.9,TLB:24:23:20.21:35.30,34:35:20.21:28.23,THB:31:30:20.21:28.23,18:20:28.23:35.30,36:21:28.23:35.30,(18.07,-9.91,;16.74,-9.14,;16.72,-7.59,;15.39,-9.93,;14.06,-9.16,;12.74,-9.93,;11.39,-9.16,;9.85,-9.16,;9.46,-7.65,;8.77,-10.26,;9.17,-11.75,;10.49,-12.52,;11.81,-11.75,;13.14,-12.52,;13.14,-14.07,;11.81,-14.83,;10.48,-14.06,;7.27,-9.85,;5.78,-9.46,;6.23,-7.91,;1.17,-10.26,;2.19,-9.23,;2.17,-11.09,;-.1,-11.65,;-1.8,-11.36,;-3.44,-12.03,;-3.76,-13.06,;-2.23,-13.56,;-.7,-12.96,;1.2,-12.39,;3.43,-12.9,;4.59,-14.2,;6.56,-14.2,;7.22,-12.96,;5.94,-11.68,;4.14,-11.68,;2.82,-8.09,;1.88,-7.17,;3.95,-7.43,;3.93,-6.11,;5.06,-5.45,;4.94,-4.14,;5.9,-3.26,;7.2,-3.43,;7.85,-4.56,;7.38,-5.78,;6.14,-6.17,;12.72,-11.46,;14.06,-12.23,;15.39,-11.46,;14.81,-13.58,;14.1,-15.12,;16.36,-13.58,)| Show InChI InChI=1S/C43H43N3O7/c47-39(45-29-22-27(42(50)51)21-28(23-29)43(52)53)34(20-25-12-6-3-7-13-25)46-41(49)38-36-32-18-10-8-16-30(32)35(31-17-9-11-19-33(31)36)37(38)40(48)44-24-26-14-4-1-2-5-15-26/h3,6-13,16-19,21-23,26,34-38H,1-2,4-5,14-15,20,24H2,(H,44,48)(H,45,47)(H,46,49)(H,50,51)(H,52,53)/t34-,35?,36?,37?,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471068
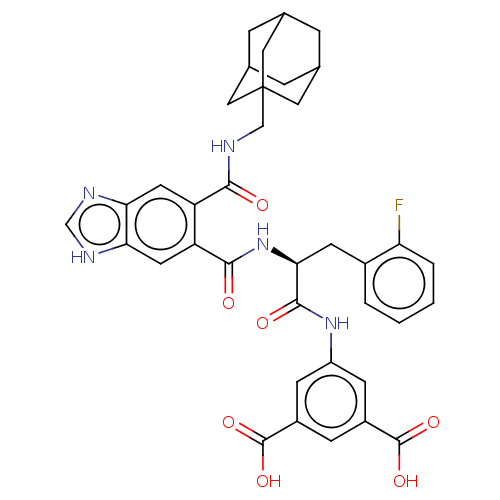
(CHEMBL298551)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C37H36FN5O7/c38-28-4-2-1-3-22(28)11-31(34(46)42-25-9-23(35(47)48)8-24(10-25)36(49)50)43-33(45)27-13-30-29(40-18-41-30)12-26(27)32(44)39-17-37-14-19-5-20(15-37)7-21(6-19)16-37/h1-4,8-10,12-13,18-21,31H,5-7,11,14-17H2,(H,39,44)(H,40,41)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t19?,20?,21?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50421390

(CHEMBL24313)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C37H37N5O7/c43-32(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)33(44)42-31(9-20-4-2-1-3-5-20)34(45)41-26-11-24(35(46)47)10-25(12-26)36(48)49/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,43)(H,39,40)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50421390

(CHEMBL24313)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C37H37N5O7/c43-32(38-18-37-15-21-6-22(16-37)8-23(7-21)17-37)27-13-29-30(40-19-39-29)14-28(27)33(44)42-31(9-20-4-2-1-3-5-20)34(45)41-26-11-24(35(46)47)10-25(12-26)36(48)49/h1-5,10-14,19,21-23,31H,6-9,15-18H2,(H,38,43)(H,39,40)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t21?,22?,23?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471071
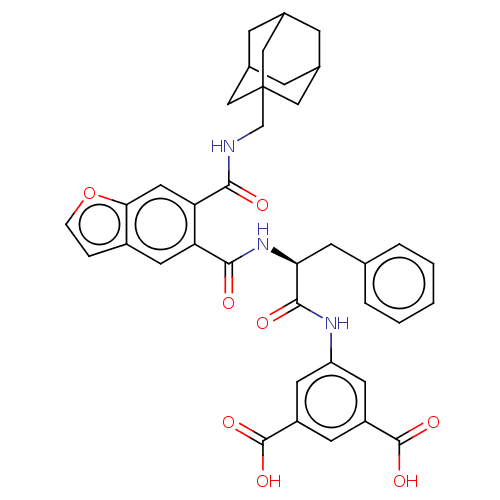
(CHEMBL297480)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccoc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,THB:36:35:41:37.42.38,38:37:34:39.41.40,38:39:34:37.42.36| Show InChI InChI=1S/C38H37N3O8/c42-33(39-20-38-17-22-8-23(18-38)10-24(9-22)19-38)30-16-32-25(6-7-49-32)15-29(30)34(43)41-31(11-21-4-2-1-3-5-21)35(44)40-28-13-26(36(45)46)12-27(14-28)37(47)48/h1-7,12-16,22-24,31H,8-11,17-20H2,(H,39,42)(H,40,44)(H,41,43)(H,45,46)(H,47,48)/t22?,23?,24?,31-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002914
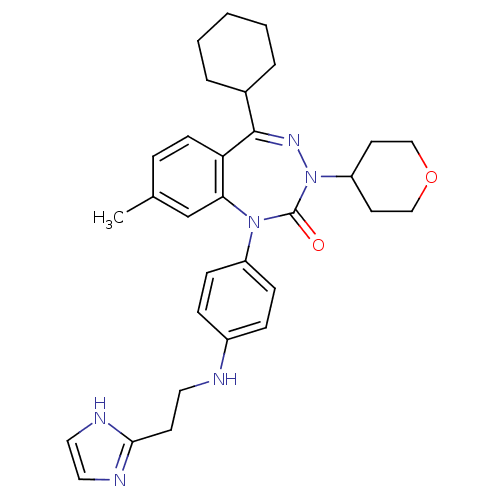
(CHEMBL244956)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(N=C2C1CCCCC1)C1CCOCC1 |c:28| Show InChI InChI=1S/C31H38N6O2/c1-22-7-12-27-28(21-22)36(25-10-8-24(9-11-25)32-16-13-29-33-17-18-34-29)31(38)37(26-14-19-39-20-15-26)35-30(27)23-5-3-2-4-6-23/h7-12,17-18,21,23,26,32H,2-6,13-16,19-20H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002925
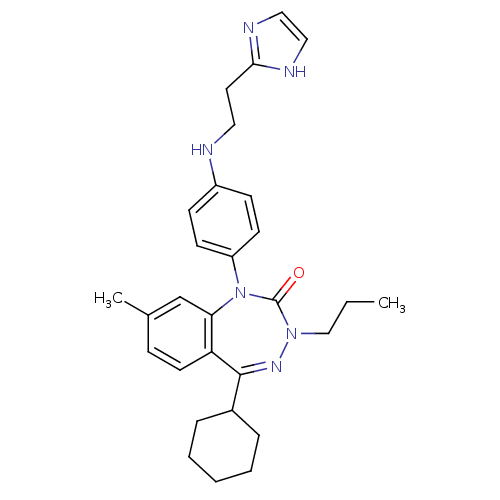
(CHEMBL244074)Show SMILES CCCN1N=C(C2CCCCC2)c2ccc(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:4| Show InChI InChI=1S/C29H36N6O/c1-3-19-34-29(36)35(24-12-10-23(11-13-24)30-16-15-27-31-17-18-32-27)26-20-21(2)9-14-25(26)28(33-34)22-7-5-4-6-8-22/h9-14,17-18,20,22,30H,3-8,15-16,19H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002922
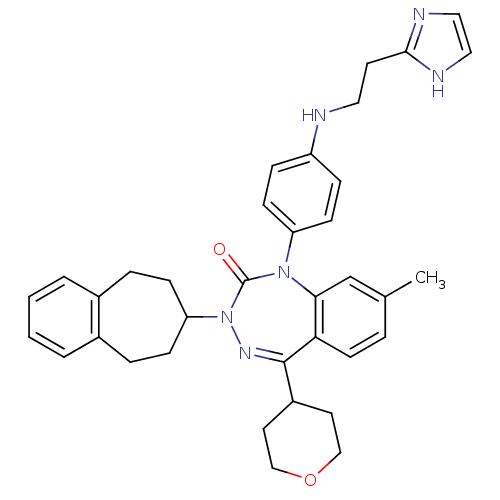
(CHEMBL224729)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(N=C2C1CCOCC1)C1CCc2ccccc2CC1 |c:28| Show InChI InChI=1S/C36H40N6O2/c1-25-6-15-32-33(24-25)41(30-13-9-29(10-14-30)37-19-16-34-38-20-21-39-34)36(43)42(40-35(32)28-17-22-44-23-18-28)31-11-7-26-4-2-3-5-27(26)8-12-31/h2-6,9-10,13-15,20-21,24,28,31,37H,7-8,11-12,16-19,22-23H2,1H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470625
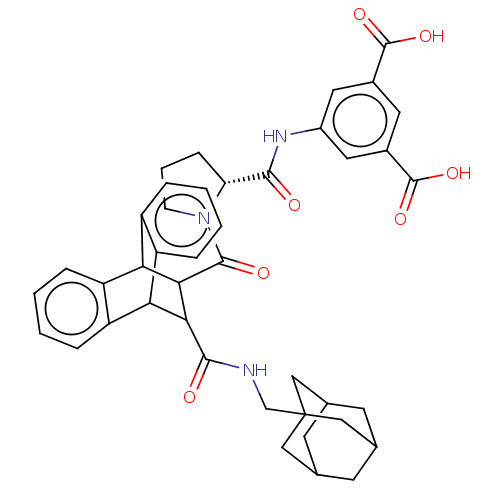
(CHEMBL335587)Show SMILES OC(=O)c1cc(NC(=O)[C@H]2CCCN2C(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.8,TLB:14:16:19.24:31.26,43:38:45:42.44.41,43:42:45:38.37.39,30:31:16.17:19.24,32:17:19.24:31.26,20:19:16.17:31.26,THB:41:42:37:40.45.39,41:40:37:42.44.43,23:24:16.17:31.26,(21.09,-9.48,;21.07,-7.94,;22.4,-7.17,;19.74,-7.19,;18.4,-7.97,;17.08,-7.2,;15.74,-7.99,;14.41,-7.23,;14.39,-5.69,;13.07,-8.02,;14.38,-8.86,;13.97,-10.35,;12.43,-10.41,;11.89,-9,;10.4,-8.6,;10.86,-7.07,;5.82,-9.38,;6.82,-8.38,;6.82,-10.22,;8.77,-10.83,;10.56,-10.83,;11.82,-12.08,;11.17,-13.33,;9.21,-13.33,;8.07,-12.02,;5.84,-11.52,;3.96,-12.08,;2.42,-12.68,;.9,-12.18,;1.23,-11.15,;2.86,-10.5,;4.54,-10.76,;7.46,-7.23,;6.52,-6.33,;8.58,-6.58,;8.55,-5.27,;9.67,-4.6,;10.54,-5.69,;11.73,-5.21,;11.73,-3.86,;10.86,-2.84,;12,-3.22,;11.98,-4.49,;13,-5.56,;10.86,-4.91,;9.67,-3.32,;17.06,-5.68,;18.37,-4.89,;19.72,-5.65,;18.34,-3.35,;19.68,-2.57,;16.99,-2.6,)| Show InChI InChI=1S/C42H43N3O7/c46-37(44-27-16-25(40(49)50)15-26(17-27)41(51)52)32-10-5-11-45(32)39(48)36-34-30-8-3-1-6-28(30)33(29-7-2-4-9-31(29)34)35(36)38(47)43-21-42-18-22-12-23(19-42)14-24(13-22)20-42/h1-4,6-9,15-17,22-24,32-36H,5,10-14,18-21H2,(H,43,47)(H,44,46)(H,49,50)(H,51,52)/t22?,23?,24?,32-,33?,34?,35?,36?,42?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471073

(CHEMBL301777)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2F)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC2CCCCCC2)cc(c1)C(O)=O Show InChI InChI=1S/C34H34FN5O7/c35-26-10-6-5-9-20(26)14-29(32(43)39-23-12-21(33(44)45)11-22(13-23)34(46)47)40-31(42)25-16-28-27(37-18-38-28)15-24(25)30(41)36-17-19-7-3-1-2-4-8-19/h5-6,9-13,15-16,18-19,29H,1-4,7-8,14,17H2,(H,36,41)(H,37,38)(H,39,43)(H,40,42)(H,44,45)(H,46,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629

(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470629

(CHEMBL342616)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wD:9.9,TLB:43:44:48:42.41.47,47:46:49:42.41.43,47:42:49:46.48.45,24:23:20.21:35.30,THB:18:20:35.30:28.23,43:42:48:44.49.45,34:35:20.21:28.23,27:28:20.21:35.30,31:30:20.21:28.23,(16.62,-7.02,;16.63,-5.48,;15.29,-4.71,;17.97,-4.72,;17.97,-3.18,;19.29,-2.43,;19.31,-.88,;20.64,-.12,;21.97,-.9,;20.64,1.42,;19.31,2.19,;17.98,2.97,;16.63,2.21,;15.31,2.97,;15.33,4.52,;16.65,5.3,;17.99,4.5,;21.97,2.19,;21.97,3.73,;20.64,4.51,;23.44,4.41,;24.65,3.71,;25.05,5.21,;27.84,6.65,;29.36,6.44,;30.3,7.66,;29.71,9.1,;28.18,9.3,;27.25,8.09,;23.52,5.95,;22.2,7.73,;21.67,9.18,;22.67,10.37,;24.18,10.1,;24.71,8.66,;23.73,7.47,;24.66,2.18,;25.98,2.96,;25.99,1.41,;27.32,2.18,;28.66,1.41,;30.24,1.7,;31.62,.9,;31.41,-.6,;29.78,-.84,;30.79,.2,;31.02,1.59,;32.73,1.99,;29.75,2.35,;28.46,-.09,;20.63,-3.19,;20.63,-4.73,;19.3,-5.51,;21.96,-5.51,;21.97,-7.04,;23.29,-4.74,)| Show InChI InChI=1S/C46H45N3O7/c50-41(48-31-19-29(44(53)54)18-30(20-31)45(55)56)36(17-25-8-2-1-3-9-25)49-43(52)40-38-34-12-6-4-10-32(34)37(33-11-5-7-13-35(33)38)39(40)42(51)47-24-46-21-26-14-27(22-46)16-28(15-26)23-46/h1-13,18-20,26-28,36-40H,14-17,21-24H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t26?,27?,28?,36-,37?,38?,39?,40?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002912
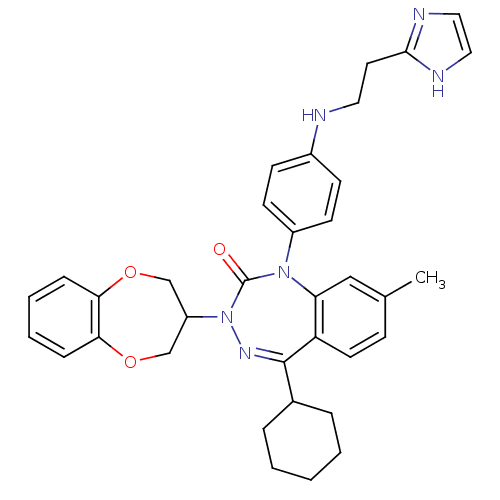
(CHEMBL388911)Show SMILES Cc1ccc2c(c1)N(c1ccc(NCCc3ncc[nH]3)cc1)C(=O)N(N=C2C1CCCCC1)C1COc2ccccc2OC1 |c:28| Show InChI InChI=1S/C35H38N6O3/c1-24-11-16-29-30(21-24)40(27-14-12-26(13-15-27)36-18-17-33-37-19-20-38-33)35(42)41(39-34(29)25-7-3-2-4-8-25)28-22-43-31-9-5-6-10-32(31)44-23-28/h5-6,9-16,19-21,25,28,36H,2-4,7-8,17-18,22-23H2,1H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470634
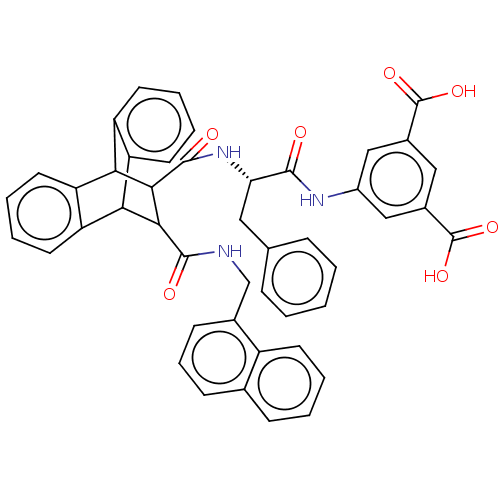
(CHEMBL135350)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCc2cccc3ccccc23)cc(c1)C(O)=O |wD:9.9,TLB:24:23:20.21:30.35,18:20:30.35:23.28,34:35:20.21:23.28,36:21:30.35:23.28,THB:27:28:20.21:30.35,(18.12,-9.94,;16.79,-9.16,;16.76,-7.61,;15.44,-9.96,;14.1,-9.19,;12.78,-9.96,;11.43,-9.19,;9.88,-9.19,;9.49,-7.68,;8.8,-10.28,;9.2,-11.78,;10.52,-12.56,;10.51,-14.1,;11.85,-14.88,;13.17,-14.11,;13.17,-12.56,;11.85,-11.78,;7.29,-9.88,;5.8,-9.49,;6.25,-7.93,;1.18,-10.28,;2.2,-9.26,;2.18,-11.13,;4.15,-11.72,;5.96,-11.72,;7.24,-13,;6.58,-14.24,;4.6,-14.24,;3.44,-12.94,;1.21,-12.43,;-.7,-13,;-2.23,-13.59,;-3.77,-13.1,;-3.45,-12.06,;-1.8,-11.4,;-.1,-11.69,;2.82,-8.12,;1.89,-7.19,;3.96,-7.45,;3.95,-6.12,;5.08,-5.47,;6.21,-6.12,;7.34,-5.47,;7.34,-4.15,;6.19,-3.5,;6.19,-2.21,;5.06,-1.56,;3.92,-2.23,;3.95,-3.53,;5.06,-4.15,;12.75,-11.49,;14.1,-12.27,;15.44,-11.49,;14.86,-13.62,;14.14,-15.17,;16.41,-13.62,)| Show InChI InChI=1S/C46H37N3O7/c50-42(48-31-23-29(45(53)54)22-30(24-31)46(55)56)37(21-26-11-2-1-3-12-26)49-44(52)41-39-35-19-8-6-17-33(35)38(34-18-7-9-20-36(34)39)40(41)43(51)47-25-28-15-10-14-27-13-4-5-16-32(27)28/h1-20,22-24,37-41H,21,25H2,(H,47,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t37-,38?,39?,40?,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470642

(CHEMBL421921)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccccc2Cl)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:35:36:21.22:29.24,48:43:50:47.49.46,48:47:50:43.42.44,25:24:21.22:36.31,THB:19:21:29.24:36.31,46:45:42:47.49.48,46:47:42:45.50.44,37:22:29.24:36.31,32:31:21.22:29.24,(10.91,-6.64,;11.62,-5.09,;13.17,-5.09,;10.87,-3.76,;9.52,-2.99,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;9.61,-5.02,;8.52,-3.96,;8.39,-2.37,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;-3.33,-3.17,;-5.03,-2.88,;-6.68,-3.54,;-7.01,-4.57,;-5.47,-5.08,;-3.93,-4.47,;-2.03,-3.91,;.2,-4.41,;1.37,-5.74,;3.34,-5.74,;4.01,-4.47,;2.73,-3.21,;.92,-3.21,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;2.72,1.96,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;4.17,3.17,;5.19,2.09,;3.04,2.75,;1.82,4.36,;10.87,-.66,;12.2,-1.43,;12.2,-2.99,;13.55,-.66,;14.89,-1.43,;13.53,.89,)| Show InChI InChI=1S/C46H44ClN3O7/c47-35-12-6-1-7-27(35)19-36(41(51)49-30-17-28(44(54)55)16-29(18-30)45(56)57)50-43(53)40-38-33-10-4-2-8-31(33)37(32-9-3-5-11-34(32)38)39(40)42(52)48-23-46-20-24-13-25(21-46)15-26(14-24)22-46/h1-12,16-18,24-26,36-40H,13-15,19-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t24?,25?,26?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
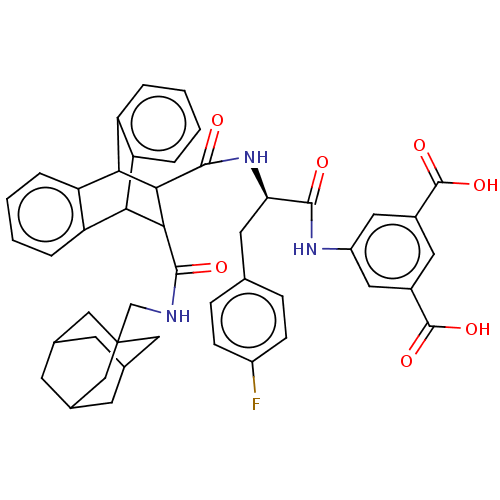
(MOUSE) | BDBM50470641
(CHEMBL334714)Show SMILES OC(=O)c1cc(NC(=O)[C@@H](Cc2ccc(F)cc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |wU:9.9,TLB:19:21:31.36:24.29,44:45:49:43.42.48,48:43:50:47.49.46,48:47:50:43.42.44,37:22:31.36:24.29,25:24:21.22:31.36,35:36:21.22:24.29,THB:44:43:49:45.50.46,28:29:21.22:31.36,(14.89,-1.43,;13.55,-.66,;13.53,.89,;12.2,-1.43,;10.87,-.66,;9.54,-1.43,;8.19,-.66,;6.64,-.66,;6.25,.84,;5.56,-1.76,;5.96,-3.27,;7.05,-4.34,;6.64,-5.83,;7.71,-6.92,;9.2,-6.53,;10.3,-7.61,;9.61,-5.02,;8.52,-3.96,;4.05,-1.37,;2.57,-.97,;3.02,.57,;-2.06,-1.76,;-1.03,-.74,;-1.06,-2.6,;.92,-3.21,;2.73,-3.21,;4.01,-4.47,;3.34,-5.74,;1.37,-5.74,;.2,-4.41,;-2.03,-3.91,;-3.93,-4.47,;-5.47,-5.08,;-7.01,-4.57,;-6.68,-3.54,;-5.03,-2.88,;-3.33,-3.17,;-.41,.41,;-1.35,1.32,;.72,1.07,;.71,2.38,;1.84,3.06,;3.04,2.75,;4.17,3.17,;5.19,2.09,;3.89,2.45,;3.91,3.81,;3.02,4.84,;4.18,4.45,;1.82,4.36,;2.72,1.96,;9.52,-2.99,;10.87,-3.76,;12.2,-2.99,;11.62,-5.09,;10.91,-6.64,;13.17,-5.09,)| Show InChI InChI=1S/C46H44FN3O7/c47-30-11-9-24(10-12-30)16-36(41(51)49-31-18-28(44(54)55)17-29(19-31)45(56)57)50-43(53)40-38-34-7-3-1-5-32(34)37(33-6-2-4-8-35(33)38)39(40)42(52)48-23-46-20-25-13-26(21-46)15-27(14-25)22-46/h1-12,17-19,25-27,36-40H,13-16,20-23H2,(H,48,52)(H,49,51)(H,50,53)(H,54,55)(H,56,57)/t25?,26?,27?,36-,37?,38?,39?,40?,46?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471064
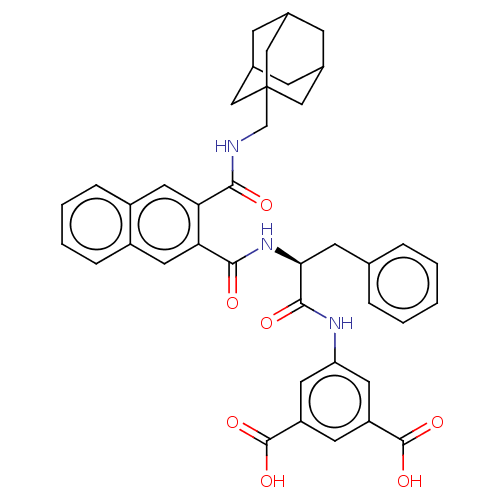
(CHEMBL301810)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C40H39N3O7/c44-35(41-22-40-19-24-10-25(20-40)12-26(11-24)21-40)32-17-27-8-4-5-9-28(27)18-33(32)36(45)43-34(13-23-6-2-1-3-7-23)37(46)42-31-15-29(38(47)48)14-30(16-31)39(49)50/h1-9,14-18,24-26,34H,10-13,19-22H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t24?,25?,26?,34-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50411364
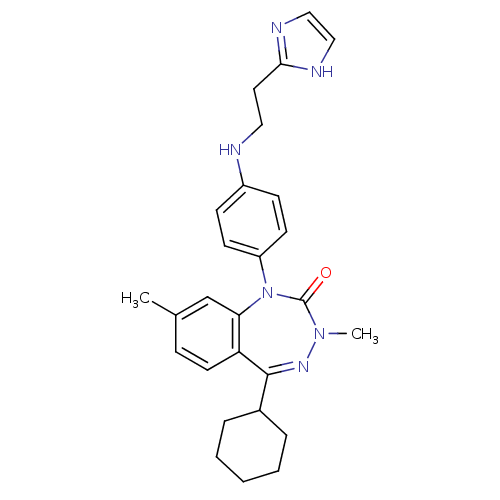
(CHEMBL242784)Show SMILES CN1N=C(C2CCCCC2)c2ccc(C)cc2N(c2ccc(NCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C27H32N6O/c1-19-8-13-23-24(18-19)33(27(34)32(2)31-26(23)20-6-4-3-5-7-20)22-11-9-21(10-12-22)28-15-14-25-29-16-17-30-25/h8-13,16-18,20,28H,3-7,14-15H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471077
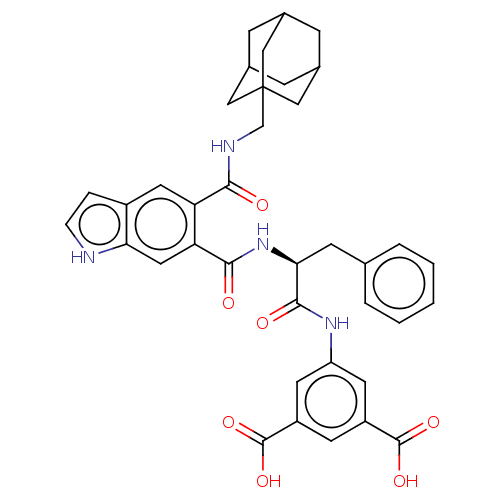
(CHEMBL280248)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3[nH]ccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:36:37:41:35.34.40,40:39:42:35.34.36,40:35:42:39.41.38,THB:36:35:41:37.42.38| Show InChI InChI=1S/C38H38N4O7/c43-33(40-20-38-17-22-8-23(18-38)10-24(9-22)19-38)29-15-25-6-7-39-31(25)16-30(29)34(44)42-32(11-21-4-2-1-3-5-21)35(45)41-28-13-26(36(46)47)12-27(14-28)37(48)49/h1-7,12-16,22-24,32,39H,8-11,17-20H2,(H,40,43)(H,41,45)(H,42,44)(H,46,47)(H,48,49)/t22?,23?,24?,32-,38?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471064

(CHEMBL301810)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3ccccc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:41:40:43:36.35.37,41:36:43:40.42.39,THB:39:38:35:40.42.41,39:40:35:38.43.37| Show InChI InChI=1S/C40H39N3O7/c44-35(41-22-40-19-24-10-25(20-40)12-26(11-24)21-40)32-17-27-8-4-5-9-28(27)18-33(32)36(45)43-34(13-23-6-2-1-3-7-23)37(46)42-31-15-29(38(47)48)14-30(16-31)39(49)50/h1-9,14-18,24-26,34H,10-13,19-22H2,(H,41,44)(H,42,46)(H,43,45)(H,47,48)(H,49,50)/t24?,25?,26?,34-,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471070

(CHEMBL298861)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)c2cc3cc[nH]c3cc2C(=O)NCC2CCCCCC2)cc(c1)C(O)=O Show InChI InChI=1S/C35H36N4O7/c40-31(37-20-22-10-4-1-2-5-11-22)28-19-29-23(12-13-36-29)18-27(28)32(41)39-30(14-21-8-6-3-7-9-21)33(42)38-26-16-24(34(43)44)15-25(17-26)35(45)46/h3,6-9,12-13,15-19,22,30,36H,1-2,4-5,10-11,14,20H2,(H,37,40)(H,38,42)(H,39,41)(H,43,44)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470640
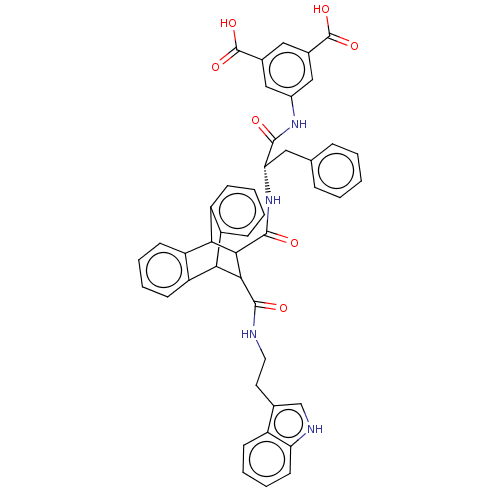
(CHEMBL443028)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCCc2c[nH]c3ccccc23)cc(c1)C(O)=O |wD:9.9,TLB:18:20:30.35:23.28,34:35:20.21:23.28,24:23:20.21:30.35,36:21:30.35:23.28,THB:27:28:20.21:30.35,(14.13,-15.15,;14.84,-13.6,;16.39,-13.6,;14.09,-12.26,;12.74,-11.48,;12.76,-9.95,;11.42,-9.18,;9.87,-9.18,;9.48,-7.67,;8.79,-10.27,;9.19,-11.77,;10.51,-12.55,;10.5,-14.09,;11.84,-14.86,;13.16,-14.1,;13.16,-12.55,;11.84,-11.77,;7.28,-9.87,;5.79,-9.48,;6.25,-7.93,;1.17,-10.27,;2.2,-9.25,;2.18,-11.11,;4.15,-11.71,;5.96,-11.71,;7.24,-12.99,;6.57,-14.23,;4.6,-14.23,;3.44,-12.92,;1.21,-12.42,;-.7,-12.99,;-2.23,-13.58,;-3.77,-13.09,;-3.45,-12.05,;-1.8,-11.38,;-.1,-11.67,;2.82,-8.11,;1.88,-7.18,;3.95,-7.44,;3.94,-6.12,;5.07,-5.46,;5.05,-4.15,;6.11,-3.39,;5.7,-2.14,;4.39,-2.16,;3.52,-1.21,;2.24,-1.49,;1.85,-2.72,;2.72,-3.68,;3.98,-3.4,;14.09,-9.18,;15.42,-9.95,;15.42,-11.48,;16.77,-9.15,;18.1,-9.93,;16.75,-7.6,)| Show InChI InChI=1S/C45H38N4O7/c50-41(48-29-22-27(44(53)54)21-28(23-29)45(55)56)36(20-25-10-2-1-3-11-25)49-43(52)40-38-33-15-6-4-13-31(33)37(32-14-5-7-16-34(32)38)39(40)42(51)46-19-18-26-24-47-35-17-9-8-12-30(26)35/h1-17,21-24,36-40,47H,18-20H2,(H,46,51)(H,48,50)(H,49,52)(H,53,54)(H,55,56)/t36-,37?,38?,39?,40?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50475510

(CHEMBL2067967)Show SMILES CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO.CN(CC12CC3CC(CC(C3)C1)C2)C(=O)c1cc2[nH]cnc2cc1C(=O)N[C@@H](Cc1ccccc1)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O |r,TLB:23:22:25:18.17.19,23:18:25:22.24.21,THB:21:22:17:20.25.19,21:20:17:22.24.23| Show InChI InChI=1S/C38H39N5O7.C7H17NO5/c1-43(19-38-16-22-7-23(17-38)9-24(8-22)18-38)35(46)29-15-31-30(39-20-40-31)14-28(29)33(44)42-32(10-21-5-3-2-4-6-21)34(45)41-27-12-25(36(47)48)11-26(13-27)37(49)50;1-8-2-4(10)6(12)7(13)5(11)3-9/h2-6,11-15,20,22-24,32H,7-10,16-19H2,1H3,(H,39,40)(H,41,45)(H,42,44)(H,47,48)(H,49,50);4-13H,2-3H2,1H3/t22?,23?,24?,32-,38?;4-,5+,6+,7+/m00/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor |
J Med Chem 48: 6790-802 (2005)
Article DOI: 10.1021/jm049069y
BindingDB Entry DOI: 10.7270/Q2BC428F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50471062
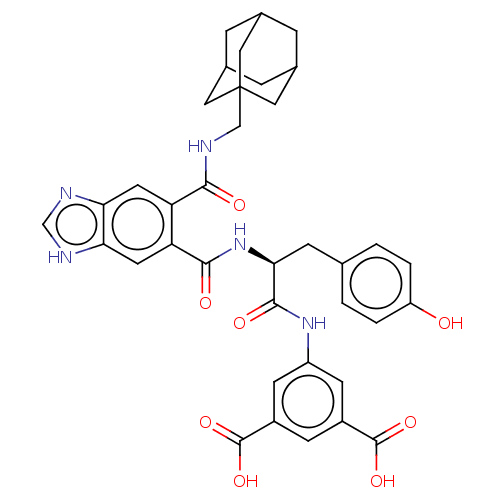
(CHEMBL300456)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)c2cc3[nH]cnc3cc2C(=O)NCC23CC4CC(CC(C4)C2)C3)cc(c1)C(O)=O |TLB:37:38:42:36.35.41,41:36:43:40.42.39,41:40:43:36.35.37,THB:37:36:42:38.43.39| Show InChI InChI=1S/C37H37N5O8/c43-26-3-1-19(2-4-26)8-31(34(46)41-25-10-23(35(47)48)9-24(11-25)36(49)50)42-33(45)28-13-30-29(39-18-40-30)12-27(28)32(44)38-17-37-14-20-5-21(15-37)7-22(6-20)16-37/h1-4,9-13,18,20-22,31,43H,5-8,14-17H2,(H,38,44)(H,39,40)(H,41,46)(H,42,45)(H,47,48)(H,49,50)/t20?,21?,22?,31-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Cholecystokinin type B receptor using [125I]BH-CCK-8S as radioligand in mouse cortical membranes |
J Med Chem 39: 1806-15 (1996)
Article DOI: 10.1021/jm9508907
BindingDB Entry DOI: 10.7270/Q2571FR6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470613
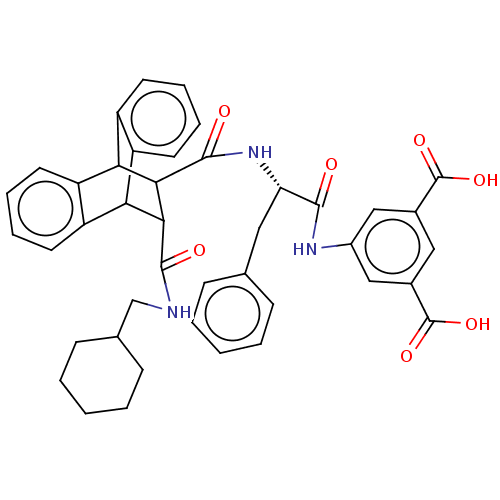
(CHEMBL343515)Show SMILES OC(=O)c1cc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC2CCCCC2)cc(c1)C(O)=O |wD:9.9,TLB:24:23:20.21:35.30,34:35:20.21:28.23,THB:31:30:20.21:28.23,18:20:28.23:35.30,36:21:28.23:35.30,(18.05,-9.9,;16.72,-9.13,;16.7,-7.58,;15.38,-9.92,;14.05,-9.15,;12.73,-9.92,;11.38,-9.15,;9.84,-9.15,;9.45,-7.65,;8.76,-10.25,;9.16,-11.74,;10.48,-12.51,;11.8,-11.74,;13.12,-12.51,;13.12,-14.06,;11.8,-14.82,;10.47,-14.05,;7.26,-9.84,;5.78,-9.45,;6.23,-7.9,;1.17,-10.25,;2.19,-9.23,;2.17,-11.08,;-.1,-11.64,;-1.79,-11.35,;-3.44,-12.02,;-3.76,-13.05,;-2.22,-13.54,;-.7,-12.95,;1.2,-12.38,;3.43,-12.89,;4.59,-14.19,;6.55,-14.19,;7.22,-12.95,;5.94,-11.67,;4.13,-11.67,;2.81,-8.09,;1.88,-7.16,;3.94,-7.42,;3.93,-6.1,;5.06,-5.44,;6.19,-6.1,;7.31,-5.44,;7.31,-4.13,;6.16,-3.49,;5.04,-4.13,;12.71,-11.45,;14.05,-12.22,;15.38,-11.45,;14.8,-13.56,;14.09,-15.11,;16.35,-13.56,)| Show InChI InChI=1S/C42H41N3O7/c46-38(44-28-21-26(41(49)50)20-27(22-28)42(51)52)33(19-24-11-3-1-4-12-24)45-40(48)37-35-31-17-9-7-15-29(31)34(30-16-8-10-18-32(30)35)36(37)39(47)43-23-25-13-5-2-6-14-25/h1,3-4,7-12,15-18,20-22,25,33-37H,2,5-6,13-14,19,23H2,(H,43,47)(H,44,46)(H,45,48)(H,49,50)(H,51,52)/t33-,34?,35?,36?,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470633
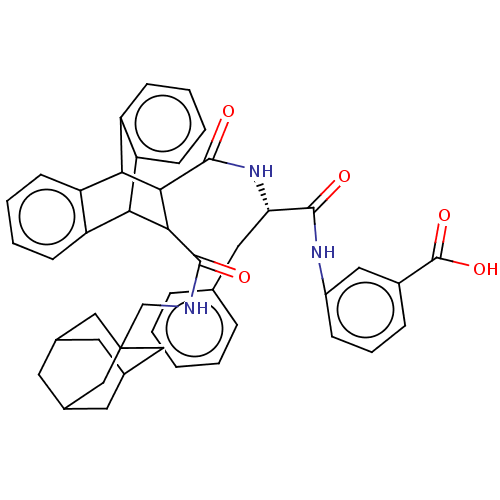
(CHEMBL135658)Show SMILES OC(=O)c1cccc(NC(=O)[C@H](Cc2ccccc2)NC(=O)C2C(C3c4ccccc4C2c2ccccc32)C(=O)NCC23CC4CC(CC(C4)C2)C3)c1 |wD:11.11,TLB:45:46:50:44.43.49,36:37:22.23:30.25,26:25:22.23:37.32,THB:45:44:50:46.51.47,47:48:43:46.51.45,47:46:43:48.50.49,20:22:30.25:37.32,33:32:22.23:30.25,38:23:30.25:37.32,(21.3,-8.13,;19.95,-7.36,;19.95,-5.81,;18.62,-8.13,;18.62,-9.68,;17.28,-10.45,;15.96,-9.68,;15.96,-8.13,;14.64,-7.36,;13.09,-7.36,;12.69,-5.86,;12,-8.45,;12.4,-9.96,;13.74,-10.73,;13.7,-12.25,;15.05,-13.03,;16.38,-12.28,;16.38,-10.73,;15.06,-9.97,;10.51,-8.07,;9.02,-7.67,;9.48,-6.13,;4.42,-8.45,;5.42,-7.44,;5.42,-9.29,;3.14,-9.86,;1.45,-9.57,;-.18,-10.22,;-.52,-11.26,;1.01,-11.76,;2.56,-11.16,;4.45,-10.6,;6.68,-11.09,;7.83,-12.42,;9.79,-12.42,;10.45,-11.16,;9.18,-9.9,;7.39,-9.9,;6.07,-6.29,;5.12,-5.39,;7.2,-5.64,;7.16,-4.33,;8.29,-3.65,;8.29,-2.36,;9.48,-1.88,;10.63,-2.27,;10.61,-3.54,;11.63,-4.62,;10.35,-4.26,;10.35,-2.91,;9.16,-4.75,;9.48,-3.96,;17.28,-7.36,)| Show InChI InChI=1S/C45H45N3O5/c49-41(47-31-12-8-11-30(21-31)44(52)53)36(20-26-9-2-1-3-10-26)48-43(51)40-38-34-15-6-4-13-32(34)37(33-14-5-7-16-35(33)38)39(40)42(50)46-25-45-22-27-17-28(23-45)19-29(18-27)24-45/h1-16,21,27-29,36-40H,17-20,22-25H2,(H,46,50)(H,47,49)(H,48,51)(H,52,53)/t27?,28?,29?,36-,37?,38?,39?,40?,45?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50470630
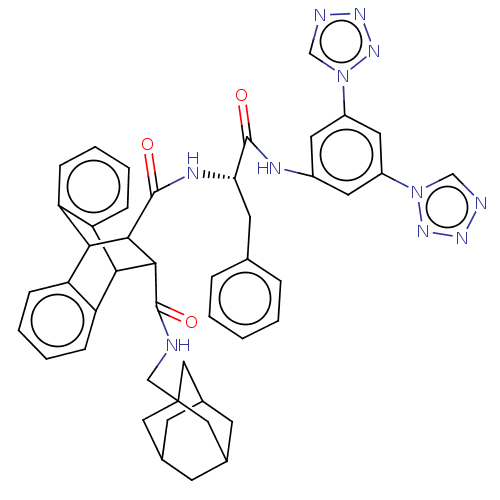
(CHEMBL436187)Show SMILES O=C(Nc1cc(cc(c1)-n1cnnn1)-n1cnnn1)[C@H](Cc1ccccc1)NC(=O)C1C(C2c3ccccc3C1c1ccccc21)C(=O)NCC12CC3CC(CC(C3)C1)C2 |wD:19.22,TLB:34:33:30.31:40.45,28:30:33.38:40.45,46:31:33.38:40.45,53:54:58:52.51.57,57:56:59:52.51.53,57:52:59:56.58.55,44:45:30.31:33.38,THB:37:38:30.31:40.45,53:52:58:54.59.55,(12.72,-5.87,;13.12,-7.37,;14.66,-7.37,;15.99,-8.14,;17.31,-7.37,;18.66,-8.14,;18.66,-9.69,;17.31,-10.47,;15.99,-9.69,;17.72,-11.95,;16.75,-13.15,;17.59,-14.46,;19.08,-14.05,;19.15,-12.5,;19.99,-7.37,;21.41,-7.98,;22.44,-6.84,;21.67,-5.5,;20.15,-5.82,;12.02,-8.47,;12.43,-9.97,;13.76,-10.75,;15.08,-9.98,;16.41,-10.75,;16.41,-12.3,;15.07,-13.05,;13.73,-12.28,;10.53,-8.08,;9.04,-7.68,;9.5,-6.14,;4.43,-8.47,;5.43,-7.46,;5.43,-9.31,;7.4,-9.92,;9.2,-9.92,;10.47,-11.18,;9.81,-12.44,;7.84,-12.44,;6.69,-11.11,;4.45,-10.62,;2.56,-11.18,;1.01,-11.78,;-.52,-11.28,;-.18,-10.24,;1.45,-9.59,;3.14,-9.88,;6.08,-6.3,;5.13,-5.4,;7.21,-5.65,;7.18,-4.34,;8.31,-3.66,;9.5,-3.97,;10.63,-3.55,;11.65,-4.63,;10.37,-4.27,;10.37,-2.92,;9.5,-1.88,;10.65,-2.27,;8.31,-2.37,;9.18,-4.76,)| Show InChI InChI=1S/C46H45N11O3/c58-43(50-31-18-32(56-25-48-52-54-56)20-33(19-31)57-26-49-53-55-57)38(17-27-8-2-1-3-9-27)51-45(60)42-40-36-12-6-4-10-34(36)39(35-11-5-7-13-37(35)40)41(42)44(59)47-24-46-21-28-14-29(22-46)16-30(15-28)23-46/h1-13,18-20,25-26,28-30,38-42H,14-17,21-24H2,(H,47,59)(H,50,58)(H,51,60)/t28?,29?,30?,38-,39?,40?,41?,42?,46?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Competition with 20 pM [125I]BH-CCK-8S for Cholecystokinin type B receptor binding sites in mouse cortical homogenates |
J Med Chem 38: 4294-302 (1995)
Article DOI: 10.1021/jm00021a019
BindingDB Entry DOI: 10.7270/Q2930WWV |
More data for this
Ligand-Target Pair | |
Parathyroid hormone/parathyroid hormone-related peptide receptor
(Homo sapiens (Human)) | BDBM50002929
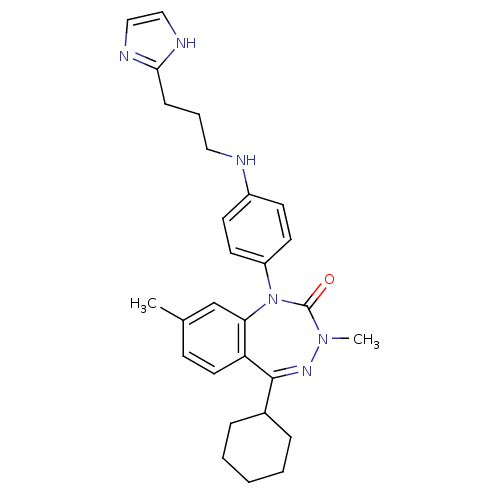
(CHEMBL390628)Show SMILES CN1N=C(C2CCCCC2)c2ccc(C)cc2N(c2ccc(NCCCc3ncc[nH]3)cc2)C1=O |t:2| Show InChI InChI=1S/C28H34N6O/c1-20-10-15-24-25(19-20)34(28(35)33(2)32-27(24)21-7-4-3-5-8-21)23-13-11-22(12-14-23)29-16-6-9-26-30-17-18-31-26/h10-15,17-19,21,29H,3-9,16H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle,8,18 Tyr34]-hPTH(1-34) from human recombinant PTH1R expressed in HEK293 cells |
J Med Chem 50: 4789-92 (2007)
Checked by Author
Article DOI: 10.1021/jm0707626
BindingDB Entry DOI: 10.7270/Q2Z039C3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data