

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50056769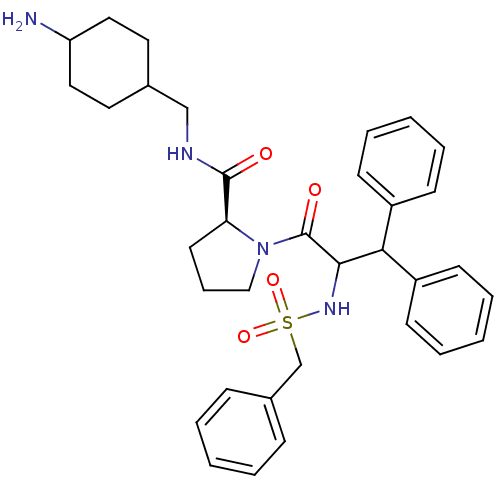 ((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565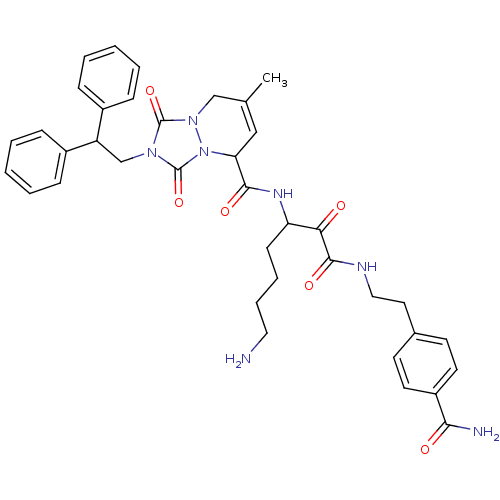 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904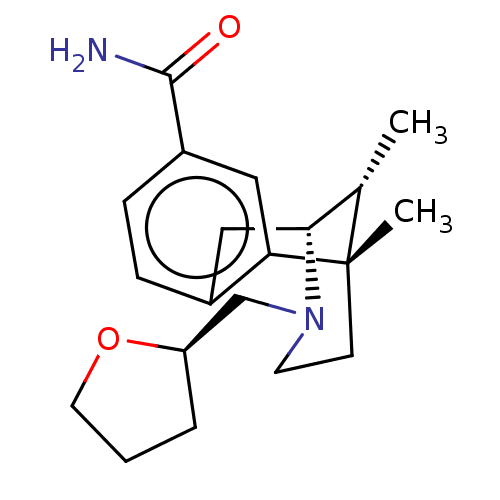 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | 58 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179904 (US10231963, Table C.8 | US10287250, Compound D.8 |...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50397290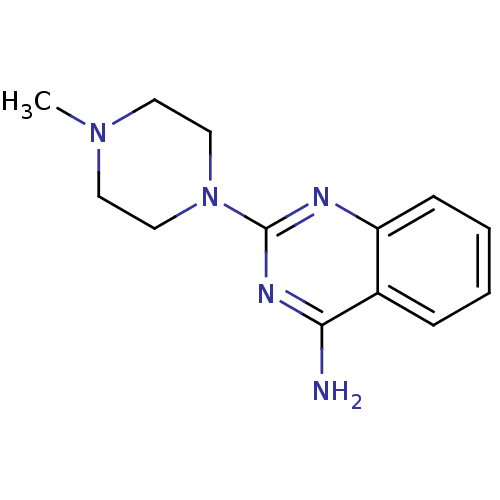 (CHEMBL475331 | VUF-10147) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]granisetron from 5HT3A receptor expressed in HEK293 cells after 24 hrs by scintillation counting in presence of quipazine | J Med Chem 55: 8603-14 (2012) Article DOI: 10.1021/jm300801u BindingDB Entry DOI: 10.7270/Q2RF5W5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057826 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 40: 1565-9 (1997) Article DOI: 10.1021/jm970140s BindingDB Entry DOI: 10.7270/Q2PR7V29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017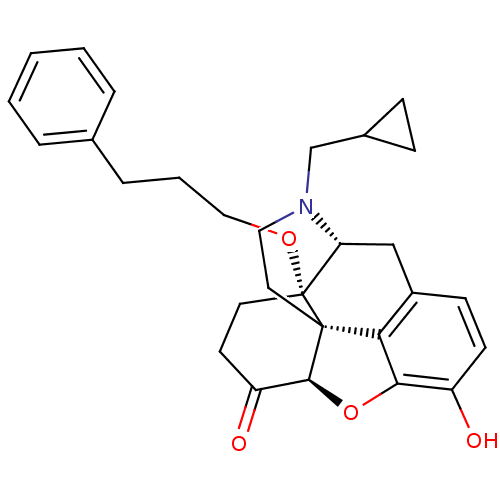 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456904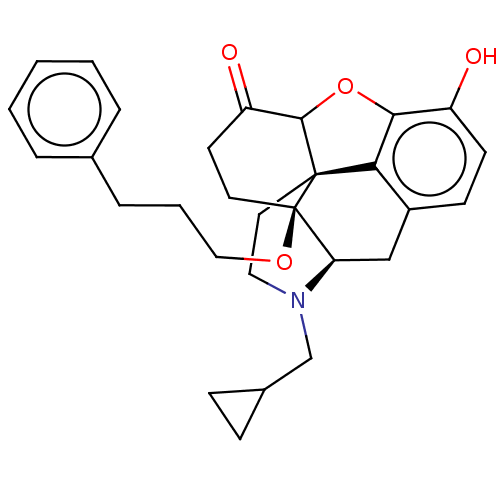 (US10736890, Compound TABLE B.14) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456892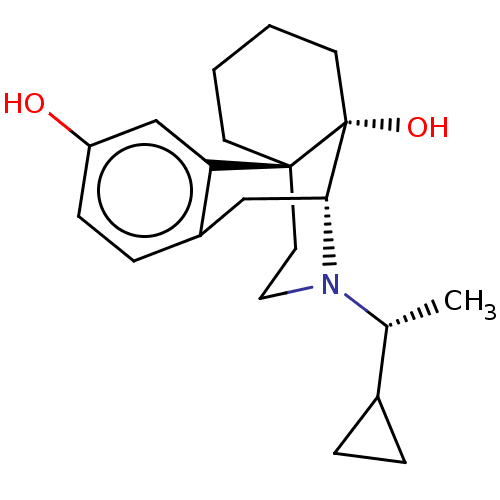 (US10736890, Compound TABLE B.2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309330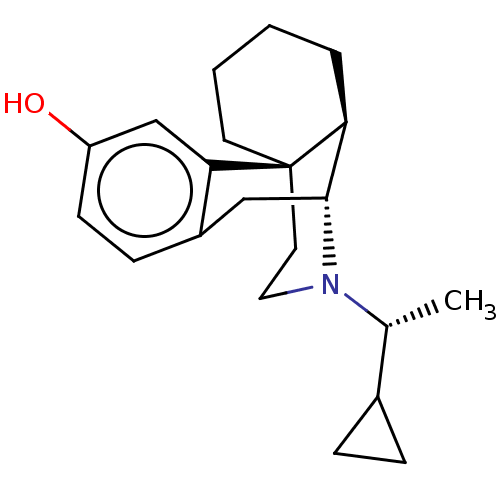 (US10231963, Table B.2 | US11534436, Compound Table...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309330 (US10231963, Table B.2 | US11534436, Compound Table...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309330 (US10231963, Table B.2 | US11534436, Compound Table...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897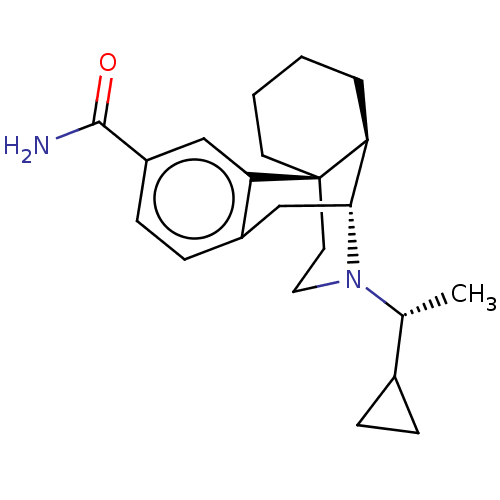 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | -57.5 | 1.70 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179897 (US10287250, Compound 1 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM457874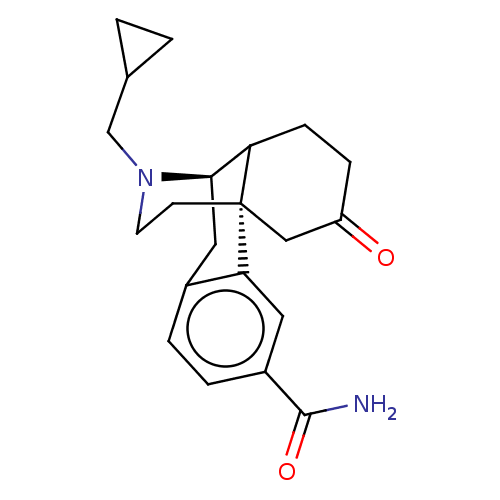 (US10752592, Compound B) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM369489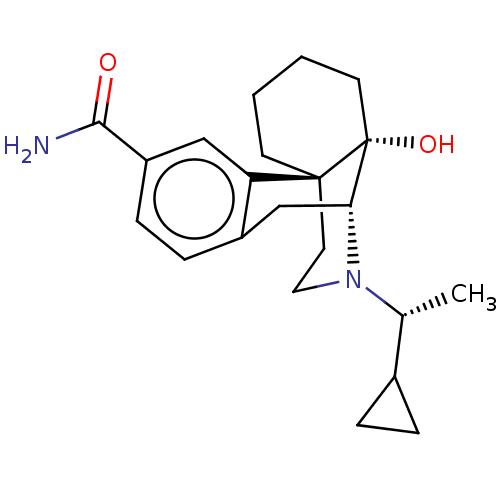 (US10231963, Table C.1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822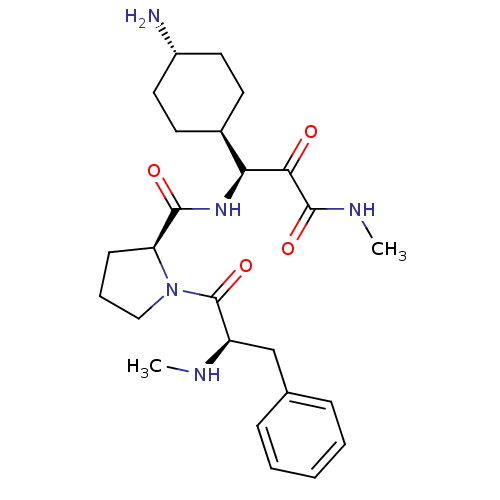 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin | J Med Chem 40: 830-2 (1997) Article DOI: 10.1021/jm960762y BindingDB Entry DOI: 10.7270/Q25H7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877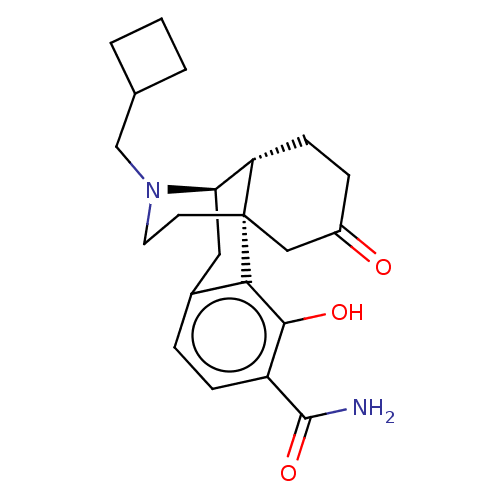 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | -57.2 | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875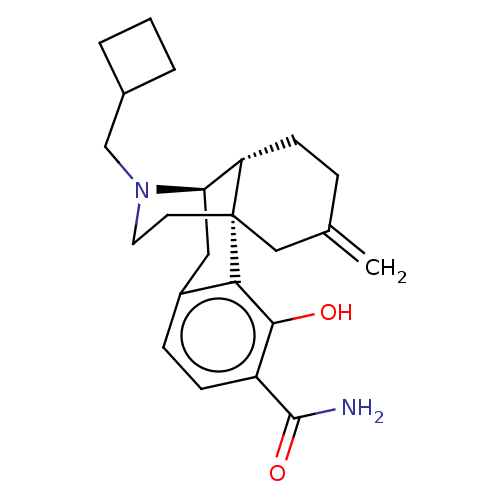 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | -57.2 | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179877 (US10231963, Table B.23 | US10736890, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179875 (US10231963, Table B.21 | US10752592, Compound TABL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50165048 (17-cyclobutylmethyl-3,10-dihydroxy-13-oxo-(1R,9R,1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179874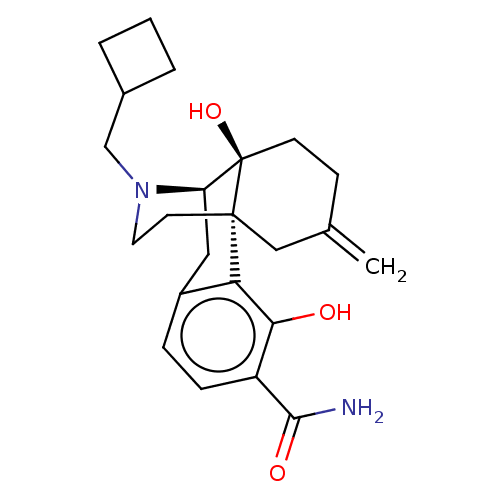 (US10231963, Table B.18 | US10287250, Compound B.3 ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179874 (US10231963, Table B.18 | US10287250, Compound B.3 ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4357 total ) | Next | Last >> |