 Found 174 hits with Last Name = 'lund' and Initial = 'la'
Found 174 hits with Last Name = 'lund' and Initial = 'la' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182338
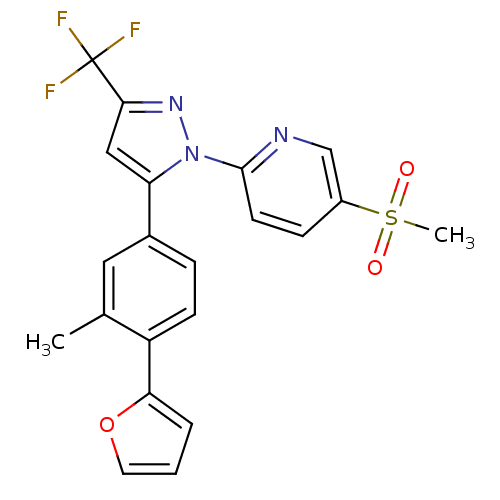
(2-(5-(4-(furan-2-yl)-3-methylphenyl)-3-(trifluorom...)Show SMILES Cc1cc(ccc1-c1ccco1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C21H16F3N3O3S/c1-13-10-14(5-7-16(13)18-4-3-9-30-18)17-11-19(21(22,23)24)26-27(17)20-8-6-15(12-25-20)31(2,28)29/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182352
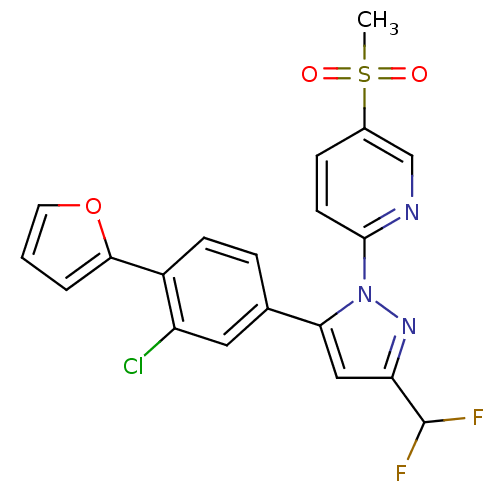
(2-(5-(3-chloro-4-(furan-2-yl)phenyl)-3-(difluorome...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2ccco2)c(Cl)c1)C(F)F Show InChI InChI=1S/C20H14ClF2N3O3S/c1-30(27,28)13-5-7-19(24-11-13)26-17(10-16(25-26)20(22)23)12-4-6-14(15(21)9-12)18-3-2-8-29-18/h2-11,20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182347
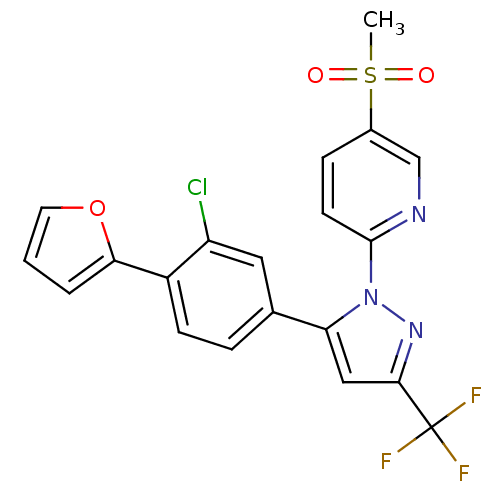
(2-(5-(3-chloro-4-(furan-2-yl)phenyl)-3-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2ccco2)c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C20H13ClF3N3O3S/c1-31(28,29)13-5-7-19(25-11-13)27-16(10-18(26-27)20(22,23)24)12-4-6-14(15(21)9-12)17-3-2-8-30-17/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182339

(2-(furan-2-yl)-5-(1-(5-(methylsulfonyl)pyridin-2-y...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2ccco2)c(c1)C#N)C(F)(F)F Show InChI InChI=1S/C21H13F3N4O3S/c1-32(29,30)15-5-7-20(26-12-15)28-17(10-19(27-28)21(22,23)24)13-4-6-16(14(9-13)11-25)18-3-2-8-31-18/h2-10,12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182353

(2-(3-(difluoromethyl)-5-(3-methyl-4-(thiazol-4-yl)...)Show SMILES Cc1cc(ccc1-c1cscn1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C20H16F2N4O2S2/c1-12-7-13(3-5-15(12)17-10-29-11-24-17)18-8-16(20(21)22)25-26(18)19-6-4-14(9-23-19)30(2,27)28/h3-11,20H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182332
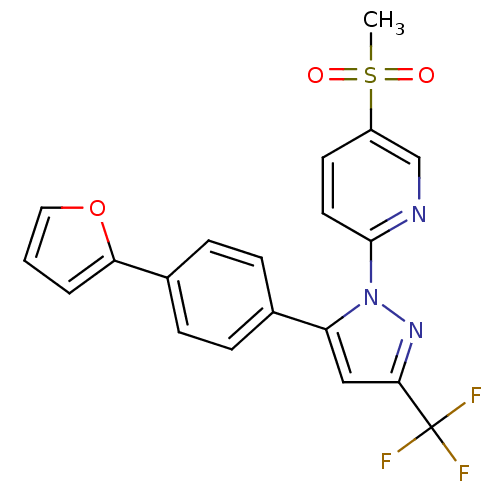
(2-(5-(4-(furan-2-yl)phenyl)-3-(trifluoromethyl)-1H...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(cc1)-c1ccco1)C(F)(F)F Show InChI InChI=1S/C20H14F3N3O3S/c1-30(27,28)15-8-9-19(24-12-15)26-16(11-18(25-26)20(21,22)23)13-4-6-14(7-5-13)17-3-2-10-29-17/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50176545
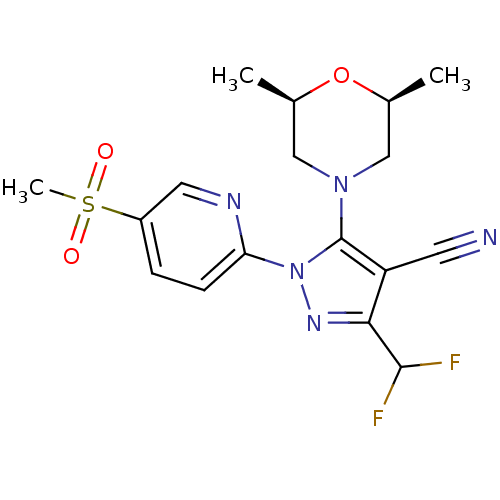
(3-(difluoromethyl)-5-((2R,6S)-2,6-dimethylmorpholi...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H19F2N5O3S/c1-10-8-23(9-11(2)27-10)17-13(6-20)15(16(18)19)22-24(17)14-5-4-12(7-21-14)28(3,25)26/h4-5,7,10-11,16H,8-9H2,1-3H3/t10-,11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in feline whole blood assay |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182345
(2-(5-(3-methyl-4-(oxazol-4-yl)phenyl)-3-(trifluoro...)Show SMILES Cc1cc(ccc1-c1cocn1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C20H15F3N4O3S/c1-12-7-13(3-5-15(12)16-10-30-11-25-16)17-8-18(20(21,22)23)26-27(17)19-6-4-14(9-24-19)31(2,28)29/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176555

(3-(difluoromethyl)-5-(3-methylpiperidin-1-yl)-1-(5...)Show SMILES CC1CCCN(C1)c1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H19F2N5O2S/c1-11-4-3-7-23(10-11)17-13(8-20)15(16(18)19)22-24(17)14-6-5-12(9-21-14)27(2,25)26/h5-6,9,11,16H,3-4,7,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137423
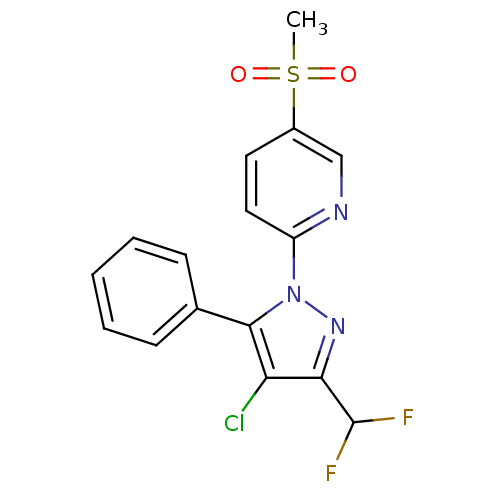
(2-(4-Chloro-3-difluoromethyl-5-phenyl-pyrazol-1-yl...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(C(F)F)c(Cl)c1-c1ccccc1 Show InChI InChI=1S/C16H12ClF2N3O2S/c1-25(23,24)11-7-8-12(20-9-11)22-15(10-5-3-2-4-6-10)13(17)14(21-22)16(18)19/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137438
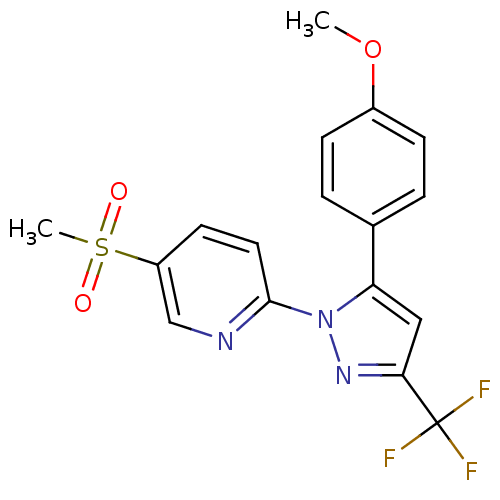
(5-Methanesulfonyl-2-[5-(4-methoxy-phenyl)-3-triflu...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-12-5-3-11(4-6-12)14-9-15(17(18,19)20)22-23(14)16-8-7-13(10-21-16)27(2,24)25/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137442
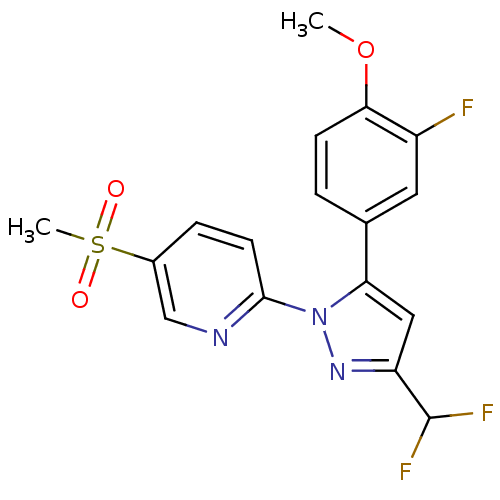
(2-[3-Difluoromethyl-5-(3-fluoro-4-methoxy-phenyl)-...)Show SMILES COc1ccc(cc1F)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H14F3N3O3S/c1-26-15-5-3-10(7-12(15)18)14-8-13(17(19)20)22-23(14)16-6-4-11(9-21-16)27(2,24)25/h3-9,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50137420
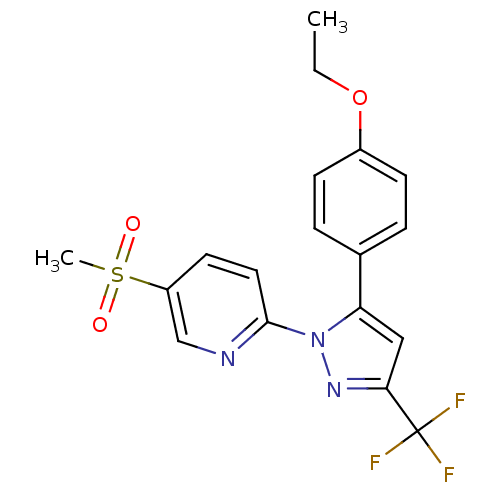
(2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...)Show SMILES CCOc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O3S/c1-3-27-13-6-4-12(5-7-13)15-10-16(18(19,20)21)23-24(15)17-9-8-14(11-22-17)28(2,25)26/h4-11H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182351

(2-(5-(3-chloro-4-(thiazol-4-yl)phenyl)-3-(trifluor...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2cscn2)c(Cl)c1)C(F)(F)F Show InChI InChI=1S/C19H12ClF3N4O2S2/c1-31(28,29)12-3-5-18(24-8-12)27-16(7-17(26-27)19(21,22)23)11-2-4-13(14(20)6-11)15-9-30-10-25-15/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182335
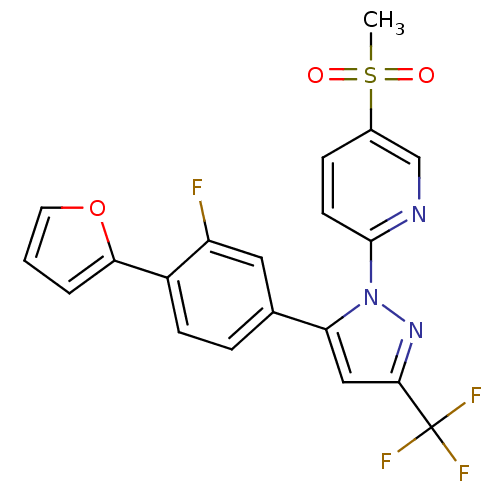
(2-(5-(3-fluoro-4-(furan-2-yl)phenyl)-3-(trifluorom...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2ccco2)c(F)c1)C(F)(F)F Show InChI InChI=1S/C20H13F4N3O3S/c1-31(28,29)13-5-7-19(25-11-13)27-16(10-18(26-27)20(22,23)24)12-4-6-14(15(21)9-12)17-3-2-8-30-17/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137435

(2-(4-Chloro-5-phenyl-3-trifluoromethyl-pyrazol-1-y...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(Cl)c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c1-26(24,25)11-7-8-12(21-9-11)23-14(10-5-3-2-4-6-10)13(17)15(22-23)16(18,19)20/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182334
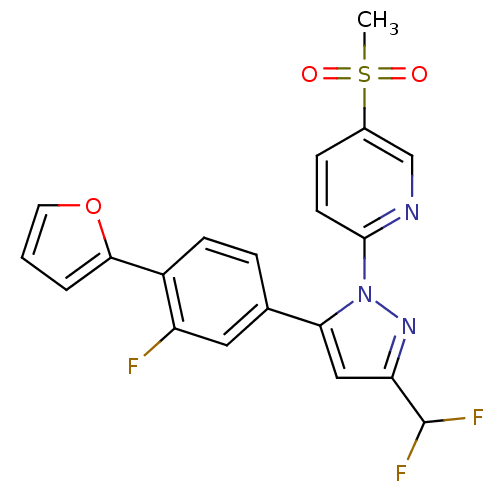
(2-(3-(difluoromethyl)-5-(3-fluoro-4-(furan-2-yl)ph...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2ccco2)c(F)c1)C(F)F Show InChI InChI=1S/C20H14F3N3O3S/c1-30(27,28)13-5-7-19(24-11-13)26-17(10-16(25-26)20(22)23)12-4-6-14(15(21)9-12)18-3-2-8-29-18/h2-11,20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137439

(2-[5-(2-Fluoro-4-methoxy-phenyl)-3-trifluoromethyl...)Show SMILES COc1ccc(-c2cc(nn2-c2ccc(cn2)S(C)(=O)=O)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C17H13F4N3O3S/c1-27-10-3-5-12(13(18)7-10)14-8-15(17(19,20)21)23-24(14)16-6-4-11(9-22-16)28(2,25)26/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137424
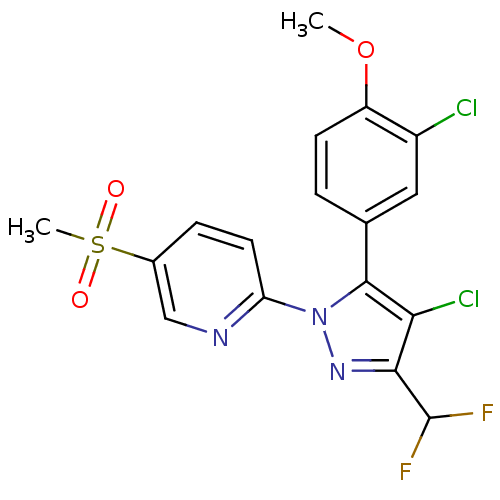
(2-[4-Chloro-5-(3-chloro-4-methoxy-phenyl)-3-difluo...)Show SMILES COc1ccc(cc1Cl)-c1c(Cl)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H13Cl2F2N3O3S/c1-27-12-5-3-9(7-11(12)18)16-14(19)15(17(20)21)23-24(16)13-6-4-10(8-22-13)28(2,25)26/h3-8,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176552

(1-(5-(methylsulfonyl)pyridin-2-yl)-5-((tetrahydrof...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NCC1CCCO1)C(F)(F)F Show InChI InChI=1S/C16H16F3N5O3S/c1-28(25,26)11-4-5-13(21-9-11)24-15(22-8-10-3-2-6-27-10)12(7-20)14(23-24)16(17,18)19/h4-5,9-10,22H,2-3,6,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137411
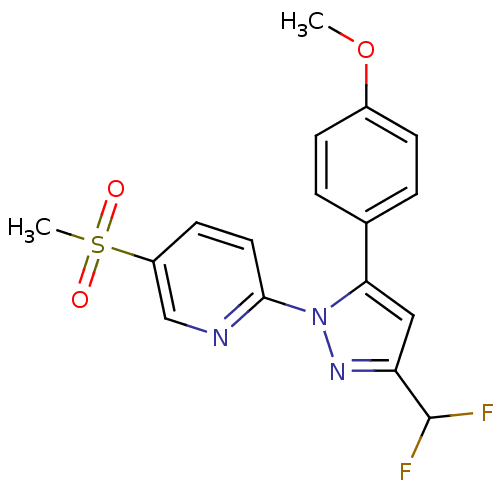
(2-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O3S/c1-25-12-5-3-11(4-6-12)15-9-14(17(18)19)21-22(15)16-8-7-13(10-20-16)26(2,23)24/h3-10,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182346

(2-(5-(3-methyl-4-(oxazol-2-yl)phenyl)-3-(trifluoro...)Show SMILES Cc1cc(ccc1-c1ncco1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C20H15F3N4O3S/c1-12-9-13(3-5-15(12)19-24-7-8-30-19)16-10-17(20(21,22)23)26-27(16)18-6-4-14(11-25-18)31(2,28)29/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137437
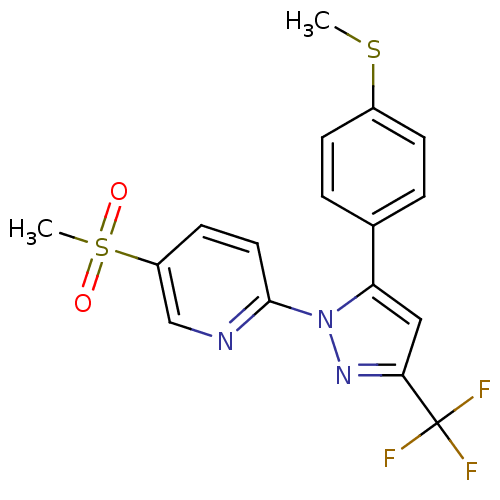
(5-Methanesulfonyl-2-[5-(4-methylsulfanyl-phenyl)-3...)Show SMILES CSc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S2/c1-26-12-5-3-11(4-6-12)14-9-15(17(18,19)20)22-23(14)16-8-7-13(10-21-16)27(2,24)25/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137427

(2-(4-Bromo-3-difluoromethyl-5-phenyl-pyrazol-1-yl)...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(C(F)F)c(Br)c1-c1ccccc1 Show InChI InChI=1S/C16H12BrF2N3O2S/c1-25(23,24)11-7-8-12(20-9-11)22-15(10-5-3-2-4-6-10)13(17)14(21-22)16(18)19/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182333
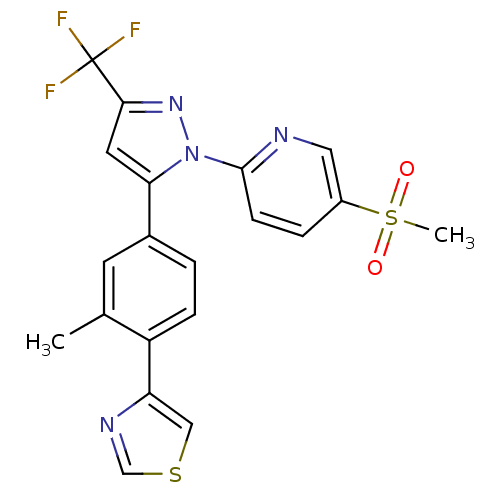
(2-(5-(3-methyl-4-(thiazol-4-yl)phenyl)-3-(trifluor...)Show SMILES Cc1cc(ccc1-c1cscn1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C20H15F3N4O2S2/c1-12-7-13(3-5-15(12)16-10-30-11-25-16)17-8-18(20(21,22)23)26-27(17)19-6-4-14(9-24-19)31(2,28)29/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176557

(5-(3,5-dimethylpiperidin-1-yl)-1-(5-(methylsulfony...)Show SMILES CC1CC(C)CN(C1)c1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H20F3N5O2S/c1-11-6-12(2)10-25(9-11)17-14(7-22)16(18(19,20)21)24-26(17)15-5-4-13(8-23-15)29(3,27)28/h4-5,8,11-12H,6,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176534

(5-(cyclopropylmethylamino)-1-(5-(methylsulfonyl)py...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NCC1CC1)C(F)(F)F Show InChI InChI=1S/C15H14F3N5O2S/c1-26(24,25)10-4-5-12(20-8-10)23-14(21-7-9-2-3-9)11(6-19)13(22-23)15(16,17)18/h4-5,8-9,21H,2-3,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182340

(5-(1-(5-(methylsulfonyl)pyridin-2-yl)-3-(trifluoro...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(-c2nccs2)c(c1)C#N)C(F)(F)F Show InChI InChI=1S/C20H12F3N5O2S2/c1-32(29,30)14-3-5-18(26-11-14)28-16(9-17(27-28)20(21,22)23)12-2-4-15(13(8-12)10-24)19-25-6-7-31-19/h2-9,11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176531

(1-(5-methanesulfonyl-pyridin-2-yl)-5-(4-methyl-ben...)Show SMILES Cc1ccc(CNc2c(C#N)c(nn2-c2ccc(cn2)S(C)(=O)=O)C(F)(F)F)cc1 Show InChI InChI=1S/C19H16F3N5O2S/c1-12-3-5-13(6-4-12)10-25-18-15(9-23)17(19(20,21)22)26-27(18)16-8-7-14(11-24-16)30(2,28)29/h3-8,11,25H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137428
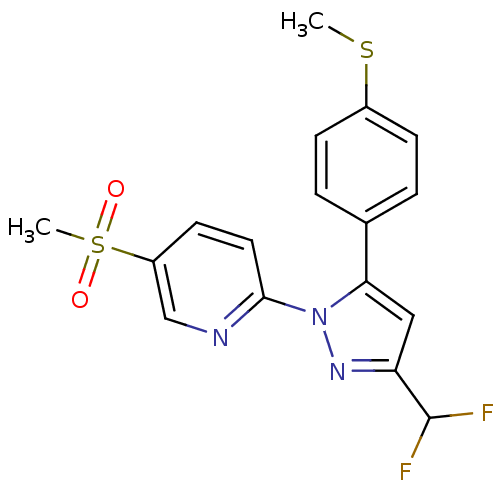
(2-[3-Difluoromethyl-5-(4-methylsulfanyl-phenyl)-py...)Show SMILES CSc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O2S2/c1-25-12-5-3-11(4-6-12)15-9-14(17(18)19)21-22(15)16-8-7-13(10-20-16)26(2,23)24/h3-10,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137440

(CHEMBL176934 | {4-[2-(5-Methanesulfonyl-pyridin-2-...)Show SMILES CN(C)c1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H17F3N4O2S/c1-24(2)13-6-4-12(5-7-13)15-10-16(18(19,20)21)23-25(15)17-9-8-14(11-22-17)28(3,26)27/h4-11H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137414

(2-[5-(4-Chloro-phenyl)-3-difluoromethyl-pyrazol-1-...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)F Show InChI InChI=1S/C16H12ClF2N3O2S/c1-25(23,24)12-6-7-15(20-9-12)22-14(8-13(21-22)16(18)19)10-2-4-11(17)5-3-10/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176533

(1-(5-(methylsulfonyl)pyridin-2-yl)-5-(thiophen-2-y...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NCc1cccs1)C(F)(F)F Show InChI InChI=1S/C16H12F3N5O2S2/c1-28(25,26)11-4-5-13(21-9-11)24-15(22-8-10-3-2-6-27-10)12(7-20)14(23-24)16(17,18)19/h2-6,9,22H,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176554

(5-((1,3-dioxolan-2-yl)methylamino)-1-(5-(methylsul...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NCC1OCCO1)C(F)(F)F Show InChI InChI=1S/C15H14F3N5O4S/c1-28(24,25)9-2-3-11(20-7-9)23-14(21-8-12-26-4-5-27-12)10(6-19)13(22-23)15(16,17)18/h2-3,7,12,21H,4-5,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176544

(5-(azepan-1-yl)-3-(difluoromethyl)-1-(5-(methylsul...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(C(F)F)c(C#N)c1N1CCCCCC1 Show InChI InChI=1S/C17H19F2N5O2S/c1-27(25,26)12-6-7-14(21-11-12)24-17(23-8-4-2-3-5-9-23)13(10-20)15(22-24)16(18)19/h6-7,11,16H,2-5,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176529
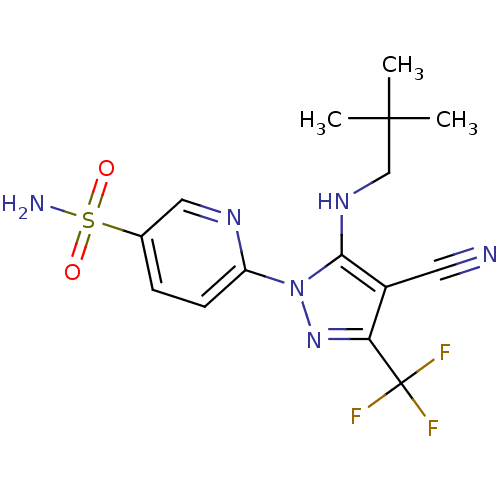
(6-(4-cyano-5-(neopentylamino)-3-(trifluoromethyl)-...)Show SMILES CC(C)(C)CNc1c(C#N)c(nn1-c1ccc(cn1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C15H17F3N6O2S/c1-14(2,3)8-22-13-10(6-19)12(15(16,17)18)23-24(13)11-5-4-9(7-21-11)27(20,25)26/h4-5,7,22H,8H2,1-3H3,(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182343

(2-(3-(difluoromethyl)-5-(4-(furan-2-yl)phenyl)-1H-...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(cc1)-c1ccco1)C(F)F Show InChI InChI=1S/C20H15F2N3O3S/c1-29(26,27)15-8-9-19(23-12-15)25-17(11-16(24-25)20(21)22)13-4-6-14(7-5-13)18-3-2-10-28-18/h2-12,20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137420

(2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...)Show SMILES CCOc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O3S/c1-3-27-13-6-4-12(5-7-13)15-10-16(18(19,20)21)23-24(15)17-9-8-14(11-22-17)28(2,25)26/h4-11H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176547
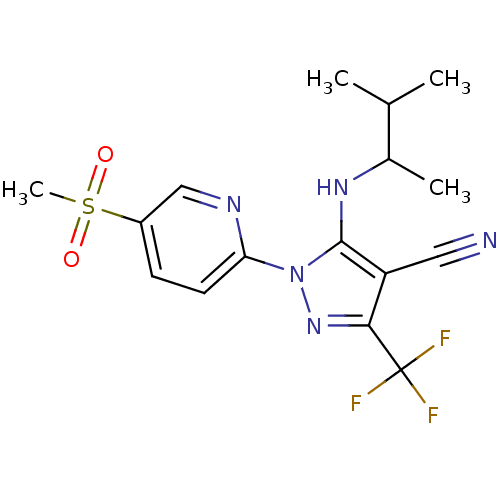
(5-(3-methylbutan-2-ylamino)-1-(5-(methylsulfonyl)p...)Show SMILES CC(C)C(C)Nc1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C16H18F3N5O2S/c1-9(2)10(3)22-15-12(7-20)14(16(17,18)19)23-24(15)13-6-5-11(8-21-13)27(4,25)26/h5-6,8-10,22H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176540

(6-(5-(bicyclo[2.2.1]heptan-2-ylamino)-4-cyano-3-(t...)Show SMILES NS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NC1CC2CCC1C2)C(F)(F)F |TLB:17:18:22.21:24| Show InChI InChI=1S/C17H17F3N6O2S/c18-17(19,20)15-12(7-21)16(24-13-6-9-1-2-10(13)5-9)26(25-15)14-4-3-11(8-23-14)29(22,27)28/h3-4,8-10,13,24H,1-2,5-6H2,(H2,22,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176542

(5-((cis)-2,6-dimethylmorpholino)-1-(5-(methylsulfo...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)c1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H18F3N5O3S/c1-10-8-24(9-11(2)28-10)16-13(6-21)15(17(18,19)20)23-25(16)14-5-4-12(7-22-14)29(3,26)27/h4-5,7,10-11H,8-9H2,1-3H3/t10-,11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176536
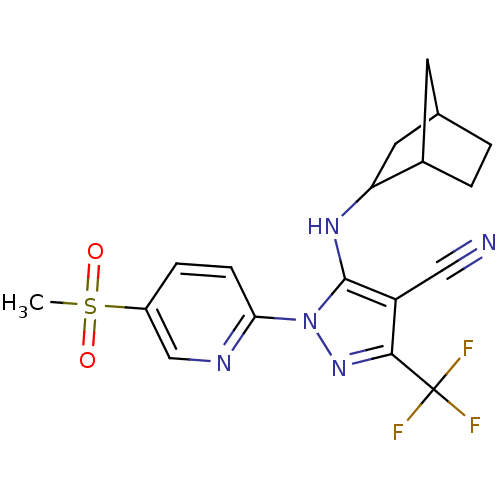
(5-(bicyclo[2.2.1]heptan-2-ylamino)-1-(5-(methylsul...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1NC1CC2CCC1C2)C(F)(F)F |TLB:17:18:22.21:24| Show InChI InChI=1S/C18H18F3N5O2S/c1-29(27,28)12-4-5-15(23-9-12)26-17(13(8-22)16(25-26)18(19,20)21)24-14-7-10-2-3-11(14)6-10/h4-5,9-11,14,24H,2-3,6-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176538

(1-(5-(methylsulfonyl)pyridin-2-yl)-5-(piperidin-1-...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C16H16F3N5O2S/c1-27(25,26)11-5-6-13(21-10-11)24-15(23-7-3-2-4-8-23)12(9-20)14(22-24)16(17,18)19/h5-6,10H,2-4,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182342

(5-(methylsulfonyl)-2-(5-(4-(thiazol-4-yl)phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(cc1)-c1cscn1)C(F)(F)F Show InChI InChI=1S/C19H13F3N4O2S2/c1-30(27,28)14-6-7-18(23-9-14)26-16(8-17(25-26)19(20,21)22)13-4-2-12(3-5-13)15-10-29-11-24-15/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137412

(5-Methanesulfonyl-2-(4-methyl-5-phenyl-3-trifluoro...)Show SMILES Cc1c(nn(c1-c1ccccc1)-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-15(12-6-4-3-5-7-12)23(22-16(11)17(18,19)20)14-9-8-13(10-21-14)26(2,24)25/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137434

(2-[3-Difluoromethyl-5-(4-methoxy-3-methyl-phenyl)-...)Show SMILES COc1ccc(cc1C)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C18H17F2N3O3S/c1-11-8-12(4-6-16(11)26-2)15-9-14(18(19)20)22-23(15)17-7-5-13(10-21-17)27(3,24)25/h4-10,18H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137418

(2-(3-Difluoromethyl-5-p-tolyl-pyrazol-1-yl)-5-meth...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O2S/c1-11-3-5-12(6-4-11)15-9-14(17(18)19)21-22(15)16-8-7-13(10-20-16)25(2,23)24/h3-10,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50182349

(5-(methylsulfonyl)-2-(5-(4-(thiazol-5-yl)phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(cc1-c1ccc(cc1)-c1cncs1)C(F)(F)F Show InChI InChI=1S/C19H13F3N4O2S2/c1-30(27,28)14-6-7-18(24-9-14)26-15(8-17(25-26)19(20,21)22)12-2-4-13(5-3-12)16-10-23-11-29-16/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 2076-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.059
BindingDB Entry DOI: 10.7270/Q28K78PW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50137432
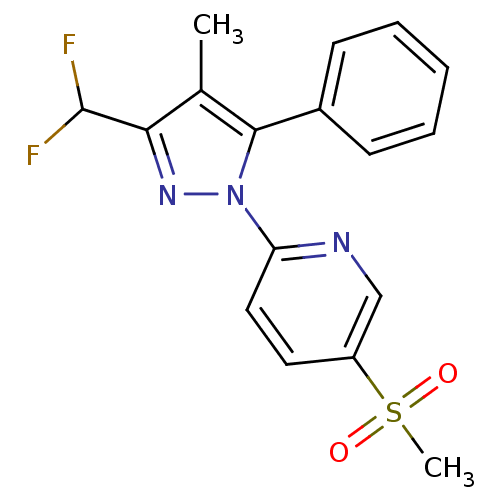
(2-(3-Difluoromethyl-4-methyl-5-phenyl-pyrazol-1-yl...)Show SMILES Cc1c(nn(c1-c1ccccc1)-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O2S/c1-11-15(17(18)19)21-22(16(11)12-6-4-3-5-7-12)14-9-8-13(10-20-14)25(2,23)24/h3-10,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50176541

(6-(4-cyano-5-(2-methylbutylamino)-3-(trifluorometh...)Show SMILES CCC(C)CNc1c(C#N)c(nn1-c1ccc(cn1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C15H17F3N6O2S/c1-3-9(2)7-22-14-11(6-19)13(15(16,17)18)23-24(14)12-5-4-10(8-21-12)27(20,25)26/h4-5,8-9,22H,3,7H2,1-2H3,(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against COX2 in canine whole blood |
Bioorg Med Chem Lett 16: 288-92 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.006
BindingDB Entry DOI: 10.7270/Q2NZ876S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data