 Found 2803 hits with Last Name = 'luo' and Initial = 'j'
Found 2803 hits with Last Name = 'luo' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50258529

(CHEMBL449158 | bryostatin 1)Show SMILES CCC\C=C\C=C\C(=O)O[C@H]1\C(C[C@H]2C[C@@H](OC(=O)C[C@H](O)C[C@@H]3C[C@H](OC(C)=O)C(C)(C)[C@](O)(C[C@@H]4C\C(C[C@@H](O4)\C=C\C(C)(C)[C@]1(O)O2)=C\C(=O)OC)O3)[C@@H](C)O)=C\C(=O)OC |r,t:43| Show InChI InChI=1S/C47H68O17/c1-10-11-12-13-14-15-39(51)62-43-31(22-41(53)58-9)21-34-25-37(28(2)48)61-42(54)24-32(50)23-35-26-38(59-29(3)49)45(6,7)46(55,63-35)27-36-19-30(20-40(52)57-8)18-33(60-36)16-17-44(4,5)47(43,56)64-34/h12-17,20,22,28,32-38,43,48,50,55-56H,10-11,18-19,21,23-27H2,1-9H3/b13-12+,15-14+,17-16+,30-20+,31-22+/t28-,32-,33+,34+,35-,36+,37-,38+,43+,46+,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCdelta expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50252860

((S)-N-((S)-1,6-diamino-1-oxohexan-2-yl)-1-((S)-5-g...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6])-[#6]-c1c(-[#6])cc(-[#8])cc1-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C66H109N21O12/c1-8-38(4)54(62(98)84-48(23-16-28-77-66(73)74)63(99)87-29-17-24-51(87)61(97)81-45(55(68)91)20-12-13-25-67)86-58(94)47(22-15-27-76-65(71)72)82-57(93)46(21-14-26-75-64(69)70)83-59(95)49(30-37(2)3)85-60(96)50(34-42-18-10-9-11-19-42)80-53(90)36-78-52(89)35-79-56(92)41(7)33-44-39(5)31-43(88)32-40(44)6/h9-11,18-19,31-32,37-38,41,45-51,54,88H,8,12-17,20-30,33-36,67H2,1-7H3,(H2,68,91)(H,78,89)(H,79,92)(H,80,90)(H,81,97)(H,82,93)(H,83,95)(H,84,98)(H,85,96)(H,86,94)(H4,69,70,75)(H4,71,72,76)(H4,73,74,77)/t38-,41-,45-,46-,47-,48-,49-,50-,51-,54-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252855

((4S,7S,13S)-13-[(S)-3-(4-Carbamoyl-2,6-dimethyl-ph...)Show SMILES C[C@@H](Cc1c(C)cc(cc1C)C(N)=O)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C30H37N7O8S2/c1-15-8-19(26(31)39)9-16(2)21(15)10-17(3)28(41)36-24-14-47-46-13-23(27(32)40)35-30(43)22(34-25(38)12-33-29(24)42)11-18-4-6-20(7-5-18)37(44)45/h4-9,17,22-24H,10-14H2,1-3H3,(H2,31,39)(H2,32,40)(H,33,42)(H,34,38)(H,35,43)(H,36,41)/t17-,22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Protein ENL
(Homo sapiens) | BDBM50459745

(CHEMBL4210431)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N(C)C |r| Show InChI InChI=1S/C40H70N8O9/c1-12-25(8)33(40(57)48-18-14-15-30(48)35(52)42-27(19-22(2)3)38(55)46(10)11)45-34(51)29(21-49)43-37(54)32(24(6)7)44-36(53)31-16-13-17-47(31)39(56)28(20-23(4)5)41-26(9)50/h22-25,27-33,49H,12-21H2,1-11H3,(H,41,50)(H,42,52)(H,43,54)(H,44,53)(H,45,51)/t25-,27-,28-,29-,30-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50252864

(CHEMBL454035 | antanal 2)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-17-29(44)18-24(2)30(23)22-31(39)38(48)43-16-8-13-34(43)37(47)42-33(20-25-9-4-3-5-10-25)36(46)41-32(35(40)45)21-26-14-15-27-11-6-7-12-28(27)19-26/h3-7,9-12,14-15,17-19,31-34,44H,8,13,16,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32+,33-,34+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity against mu opioid receptor in guinea pig ileum assessed as effect on TAPP-induced response |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252856
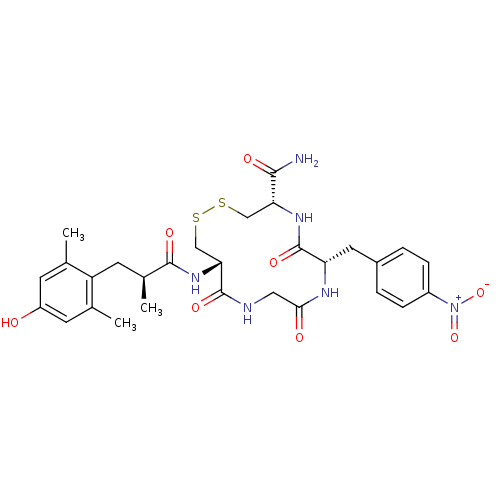
((4S,7S,13S)-13-[(S)-3-(4-Hydroxy-2,6-dimethyl-phen...)Show SMILES C[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C29H36N6O8S2/c1-15-8-20(36)9-16(2)21(15)10-17(3)27(39)34-24-14-45-44-13-23(26(30)38)33-29(41)22(32-25(37)12-31-28(24)40)11-18-4-6-19(7-5-18)35(42)43/h4-9,17,22-24,36H,10-14H2,1-3H3,(H2,30,38)(H,31,40)(H,32,37)(H,33,41)(H,34,39)/t17-,22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252858

((S)-2-({(4R,7S,13S)-7-(4-Fluoro-benzyl)-13-[(S)-3-...)Show SMILES C[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N[C@H]1C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N[C@H](C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C(C)(C)SSC1(C)C |r| Show InChI InChI=1S/C42H52FN5O8S2/c1-23-17-29(49)18-24(2)30(23)19-25(3)36(51)47-34-38(53)44-22-33(50)45-31(20-27-13-15-28(43)16-14-27)37(52)48-35(42(6,7)58-57-41(34,4)5)39(54)46-32(40(55)56)21-26-11-9-8-10-12-26/h8-18,25,31-32,34-35,49H,19-22H2,1-7H3,(H,44,53)(H,45,50)(H,46,54)(H,47,51)(H,48,52)(H,55,56)/t25-,31-,32-,34-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50252863

(CHEMBL507442 | antanal 1)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C40H44N6O5/c1-23-16-29(47)17-24(2)31(23)21-32(41)40(51)46-15-7-12-36(46)39(50)45-35(20-28-22-43-33-11-6-5-10-30(28)33)38(49)44-34(37(42)48)19-25-13-14-26-8-3-4-9-27(26)18-25/h3-6,8-11,13-14,16-18,22,32,34-36,43,47H,7,12,15,19-21,41H2,1-2H3,(H2,42,48)(H,44,49)(H,45,50)/t32-,34+,35-,36+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity against mu opioid receptor in guinea pig ileum assessed as effect on TAPP-induced response |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85671
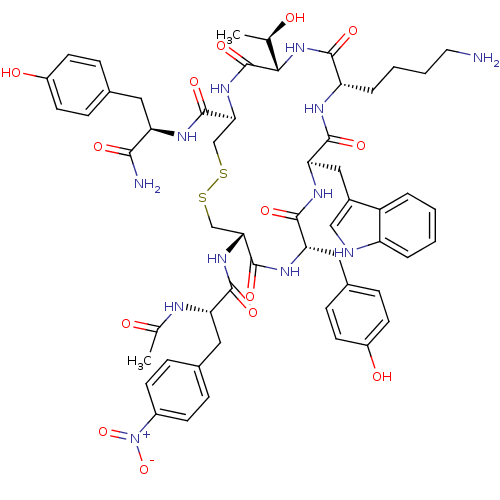
(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252857

((S)-2-{[(4R,7S,13S)-13-[(S)-3-(4-Carbamoyl-2,6-dim...)Show SMILES C[C@@H](Cc1c(C)cc(cc1C)C(N)=O)C(=O)N[C@H]1C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N[C@H](C(=O)N[C@@H](Cc2ccccc2)C(O)=O)C(C)(C)SSC1(C)C |r| Show InChI InChI=1S/C43H53FN6O8S2/c1-23-17-28(36(45)52)18-24(2)30(23)19-25(3)37(53)49-34-39(55)46-22-33(51)47-31(20-27-13-15-29(44)16-14-27)38(54)50-35(43(6,7)60-59-42(34,4)5)40(56)48-32(41(57)58)21-26-11-9-8-10-12-26/h8-18,25,31-32,34-35H,19-22H2,1-7H3,(H2,45,52)(H,46,55)(H,47,51)(H,48,56)(H,49,53)(H,50,54)(H,57,58)/t25-,31-,32-,34-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Protein ENL
(Homo sapiens) | BDBM50606137
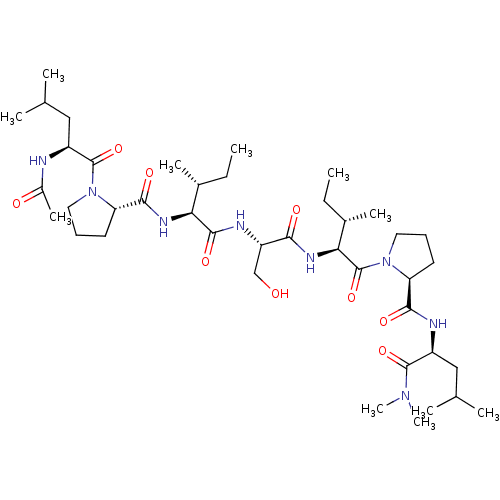
(CHEMBL5187921)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252856

((4S,7S,13S)-13-[(S)-3-(4-Hydroxy-2,6-dimethyl-phen...)Show SMILES C[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C29H36N6O8S2/c1-15-8-20(36)9-16(2)21(15)10-17(3)27(39)34-24-14-45-44-13-23(26(30)38)33-29(41)22(32-25(37)12-31-28(24)40)11-18-4-6-19(7-5-18)35(42)43/h4-9,17,22-24,36H,10-14H2,1-3H3,(H2,30,38)(H,31,40)(H,32,37)(H,33,41)(H,34,39)/t17-,22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human MOR expressed in African green monkey COS1 cell membranes incubated for 1 hr by scintillation counting m... |
Eur J Med Chem 151: 495-507 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.074
BindingDB Entry DOI: 10.7270/Q2NK3HKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 2
(RAT) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50252864

(CHEMBL454035 | antanal 2)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C38H43N5O5/c1-23-17-29(44)18-24(2)30(23)22-31(39)38(48)43-16-8-13-34(43)37(47)42-33(20-25-9-4-3-5-10-25)36(46)41-32(35(40)45)21-26-14-15-27-11-6-7-12-28(27)19-26/h3-7,9-12,14-15,17-19,31-34,44H,8,13,16,20-22,39H2,1-2H3,(H2,40,45)(H,41,46)(H,42,47)/t31-,32+,33-,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity against delta opioid receptor in mouse vas deference assessed as effect on DPDPE-induced response |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(RAT) | BDBM85671

(CAS_183658-72-2 | CYN 154806 | CYN-154806)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CSSC[C@H](NC1=O)C(=O)N[C@H](Cc1ccc(O)cc1)C(N)=O)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O |r| Show InChI InChI=1S/C56H68N12O14S2/c1-30(69)48-56(80)66-47(54(78)62-42(49(58)73)23-33-12-18-37(71)19-13-33)29-84-83-28-46(65-51(75)43(60-31(2)70)24-32-10-16-36(17-11-32)68(81)82)55(79)63-44(25-34-14-20-38(72)21-15-34)52(76)64-45(26-35-27-59-40-8-4-3-7-39(35)40)53(77)61-41(50(74)67-48)9-5-6-22-57/h3-4,7-8,10-21,27,30,41-48,59,69,71-72H,5-6,9,22-26,28-29,57H2,1-2H3,(H2,58,73)(H,60,70)(H,61,77)(H,62,78)(H,63,79)(H,64,76)(H,65,75)(H,66,80)(H,67,74)/t30-,41+,42-,43+,44+,45-,46-,47+,48+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by PDSP Ki Database
| |
Neuropharmacology 39: 1443-50 (2000)
Article DOI: 10.1016/s0028-3908(00)00035-6
BindingDB Entry DOI: 10.7270/Q2QV3K2T |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606139

(CHEMBL5198951)Show SMILES CNC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)CCC(C)C)C(C)C)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606141

(CHEMBL5187149)Show SMILES CC(C)CCC(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606142
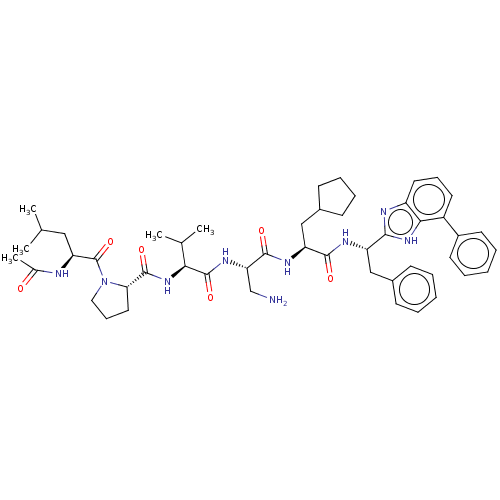
(CHEMBL5197715)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](Cc1ccccc1)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606140
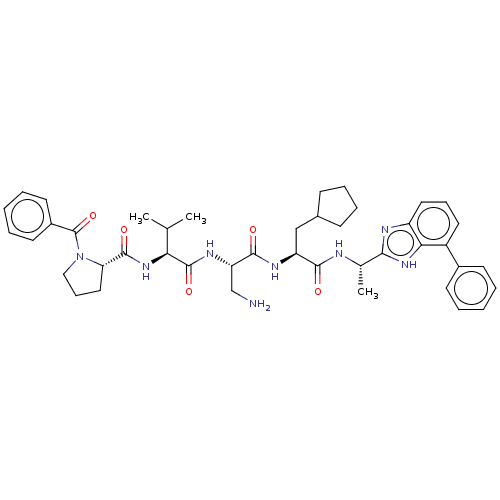
(CHEMBL5184570)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50252862

((5S,8S,14R)-8-Benzyl-14-[(S)-2-[3-(4-hydroxy-2,6-d...)Show SMILES Cc1cc(O)cc(C)c1CCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)NC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O |r| Show InChI InChI=1S/C41H51N7O10/c1-24-18-29(50)19-25(2)30(24)15-16-35(51)45-32(21-27-11-13-28(49)14-12-27)38(54)47-31-10-6-7-17-42-41(58)44-22-34(40(56)57)48-39(55)33(20-26-8-4-3-5-9-26)46-36(52)23-43-37(31)53/h3-5,8-9,11-14,18-19,31-34,49-50H,6-7,10,15-17,20-23H2,1-2H3,(H,43,53)(H,45,51)(H,46,52)(H,47,54)(H,48,55)(H,56,57)(H2,42,44,58)/t31-,32+,33+,34+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity against mu opioid receptor in guinea pig ileum assessed as effect on TAPP-induced response |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606146
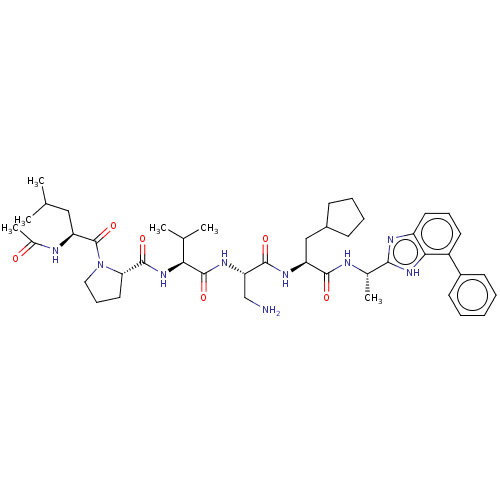
(CHEMBL5206253)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50252863

(CHEMBL507442 | antanal 1)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(N)=O |r| Show InChI InChI=1S/C40H44N6O5/c1-23-16-29(47)17-24(2)31(23)21-32(41)40(51)46-15-7-12-36(46)39(50)45-35(20-28-22-43-33-11-6-5-10-30(28)33)38(49)44-34(37(42)48)19-25-13-14-26-8-3-4-9-27(26)18-25/h3-6,8-11,13-14,16-18,22,32,34-36,43,47H,7,12,15,19-21,41H2,1-2H3,(H2,42,48)(H,44,49)(H,45,50)/t32-,34+,35-,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Antagonist activity against delta opioid receptor in mouse vas deference assessed as effect on DPDPE-induced response |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50211740
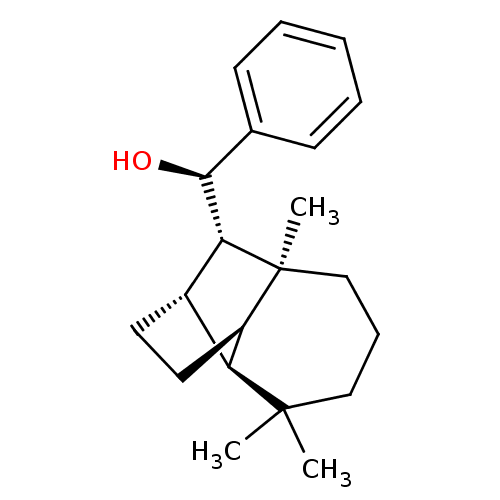
((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...)Show SMILES C[C@]12CCCC(C)(C)[C@H]3[C@H](CC[C@@H]13)[C@@H]2[C@@H](O)c1ccccc1 |TLB:2:1:10.11:8,0:1:10.11:8,THB:5:8:10.11:13.1,14:13:10.11:8| Show InChI InChI=1S/C21H30O/c1-20(2)12-7-13-21(3)16-11-10-15(17(16)20)18(21)19(22)14-8-5-4-6-9-14/h4-6,8-9,15-19,22H,7,10-13H2,1-3H3/t15-,16+,17-,18+,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation |
J Med Chem 50: 2655-64 (2007)
Article DOI: 10.1021/jm061204e
BindingDB Entry DOI: 10.7270/Q23F4QF2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252855

((4S,7S,13S)-13-[(S)-3-(4-Carbamoyl-2,6-dimethyl-ph...)Show SMILES C[C@@H](Cc1c(C)cc(cc1C)C(N)=O)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C30H37N7O8S2/c1-15-8-19(26(31)39)9-16(2)21(15)10-17(3)28(41)36-24-14-47-46-13-23(27(32)40)35-30(43)22(34-25(38)12-33-29(24)42)11-18-4-6-20(7-5-18)37(44)45/h4-9,17,22-24H,10-14H2,1-3H3,(H2,31,39)(H2,32,40)(H,33,42)(H,34,38)(H,35,43)(H,36,41)/t17-,22-,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606144
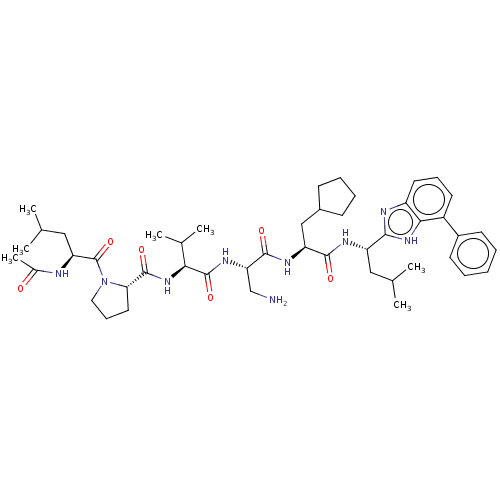
(CHEMBL5199592)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](CC(C)C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606147
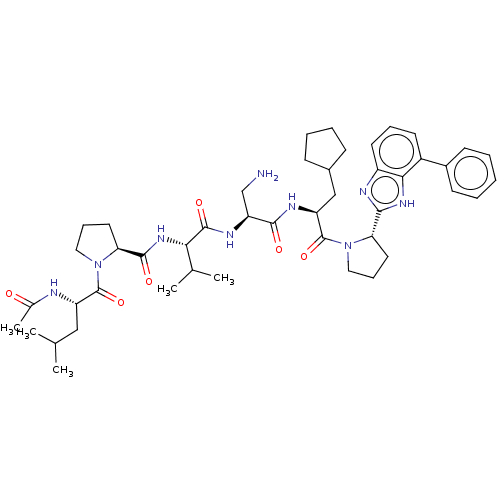
(CHEMBL5203898)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N1CCC[C@H]1c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50459730

(CHEMBL4211714)Show SMILES CC(C)CCC(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N1CCC[C@H]1c1nc(cn1CC1CCCCC1)-c1ccccc1 |r| Show InChI InChI=1S/C47H72N8O5/c1-31(2)23-24-41(56)54-25-14-22-40(54)45(58)52-42(32(3)4)46(59)51-37(28-48)44(57)50-36(27-33-15-11-12-16-33)47(60)55-26-13-21-39(55)43-49-38(35-19-9-6-10-20-35)30-53(43)29-34-17-7-5-8-18-34/h6,9-10,19-20,30-34,36-37,39-40,42H,5,7-8,11-18,21-29,48H2,1-4H3,(H,50,57)(H,51,59)(H,52,58)/t36-,37-,39-,40-,42-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606143

(CHEMBL5208460)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC1CCCC1)NC(=O)[C@H](CN)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(C)C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50252859
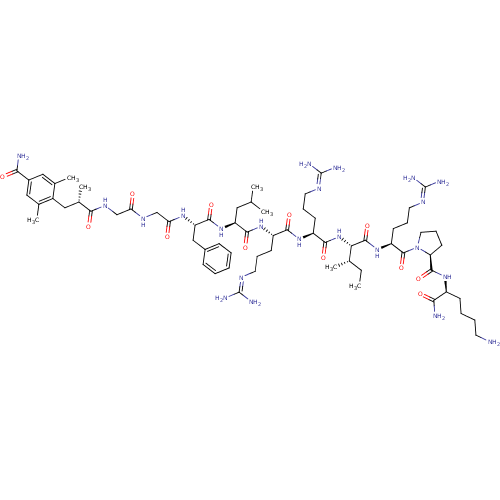
((S)-1-((S)-2-((2S,3S)-2-((S)-2-((S)-2-((S)-2-((S)-...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6])-[#6]-c1c(-[#6])cc(cc1-[#6])-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C67H110N22O12/c1-8-38(4)54(63(100)86-48(23-16-28-79-67(75)76)64(101)89-29-17-24-51(89)62(99)83-45(56(70)93)20-12-13-25-68)88-59(96)47(22-15-27-78-66(73)74)84-58(95)46(21-14-26-77-65(71)72)85-60(97)49(30-37(2)3)87-61(98)50(34-42-18-10-9-11-19-42)82-53(91)36-80-52(90)35-81-57(94)41(7)33-44-39(5)31-43(55(69)92)32-40(44)6/h9-11,18-19,31-32,37-38,41,45-51,54H,8,12-17,20-30,33-36,68H2,1-7H3,(H2,69,92)(H2,70,93)(H,80,90)(H,81,94)(H,82,91)(H,83,99)(H,84,95)(H,85,97)(H,86,100)(H,87,98)(H,88,96)(H4,71,72,77)(H4,73,74,78)(H4,75,76,79)/t38-,41-,45-,46-,47-,48-,49-,50-,51-,54-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252859

((S)-1-((S)-2-((2S,3S)-2-((S)-2-((S)-2-((S)-2-((S)-...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6])-[#6]-c1c(-[#6])cc(cc1-[#6])-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C67H110N22O12/c1-8-38(4)54(63(100)86-48(23-16-28-79-67(75)76)64(101)89-29-17-24-51(89)62(99)83-45(56(70)93)20-12-13-25-68)88-59(96)47(22-15-27-78-66(73)74)84-58(95)46(21-14-26-77-65(71)72)85-60(97)49(30-37(2)3)87-61(98)50(34-42-18-10-9-11-19-42)82-53(91)36-80-52(90)35-81-57(94)41(7)33-44-39(5)31-43(55(69)92)32-40(44)6/h9-11,18-19,31-32,37-38,41,45-51,54H,8,12-17,20-30,33-36,68H2,1-7H3,(H2,69,92)(H2,70,93)(H,80,90)(H,81,94)(H,82,91)(H,83,99)(H,84,95)(H,85,97)(H,86,100)(H,87,98)(H,88,96)(H4,71,72,77)(H4,73,74,78)(H4,75,76,79)/t38-,41-,45-,46-,47-,48-,49-,50-,51-,54-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DSLET from delta opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606145
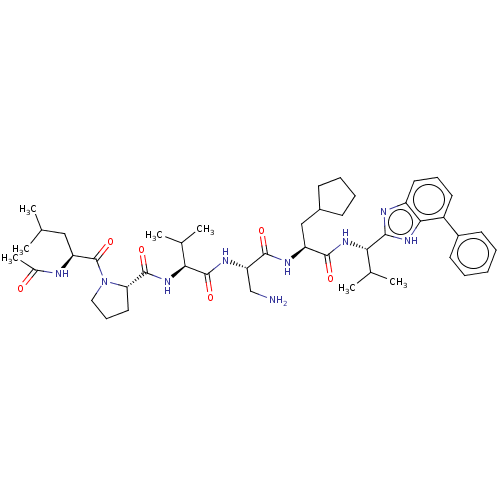
(CHEMBL5190103)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C(C)C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50252859

((S)-1-((S)-2-((2S,3S)-2-((S)-2-((S)-2-((S)-2-((S)-...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#6])-[#6]-c1c(-[#6])cc(cc1-[#6])-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C67H110N22O12/c1-8-38(4)54(63(100)86-48(23-16-28-79-67(75)76)64(101)89-29-17-24-51(89)62(99)83-45(56(70)93)20-12-13-25-68)88-59(96)47(22-15-27-78-66(73)74)84-58(95)46(21-14-26-77-65(71)72)85-60(97)49(30-37(2)3)87-61(98)50(34-42-18-10-9-11-19-42)82-53(91)36-80-52(90)35-81-57(94)41(7)33-44-39(5)31-43(55(69)92)32-40(44)6/h9-11,18-19,31-32,37-38,41,45-51,54H,8,12-17,20-30,33-36,68H2,1-7H3,(H2,69,92)(H2,70,93)(H,80,90)(H,81,94)(H,82,91)(H,83,99)(H,84,95)(H,85,97)(H,86,100)(H,87,98)(H,88,96)(H4,71,72,77)(H4,73,74,78)(H4,75,76,79)/t38-,41-,45-,46-,47-,48-,49-,50-,51-,54-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606138

(CHEMBL5207031)Show SMILES CNC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(C)C)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50015677
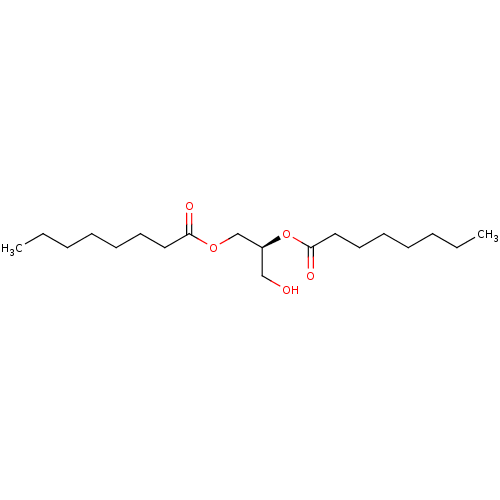
((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...)Show InChI InChI=1S/C19H36O5/c1-3-5-7-9-11-13-18(21)23-16-17(15-20)24-19(22)14-12-10-8-6-4-2/h17,20H,3-16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha expressed in Sf9 cells by liquid scintillation counting |
J Med Chem 52: 3969-81 (2009)
Article DOI: 10.1021/jm900229p
BindingDB Entry DOI: 10.7270/Q2ZK5GJ1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50257823
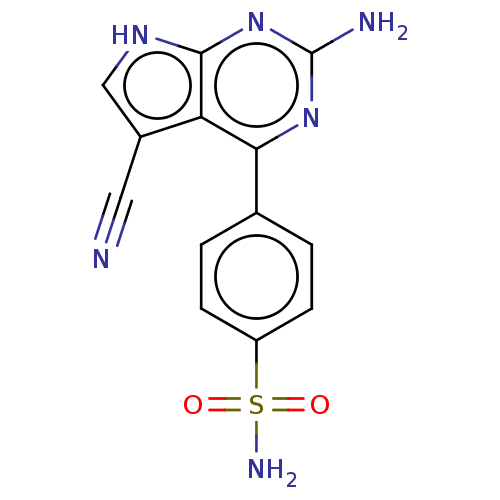
(CHEMBL4089551)Show SMILES Nc1nc(-c2ccc(cc2)S(N)(=O)=O)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C13H10N6O2S/c14-5-8-6-17-12-10(8)11(18-13(15)19-12)7-1-3-9(4-2-7)22(16,20)21/h1-4,6H,(H2,16,20,21)(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde , 161 Cathedral Street, Glasgow G4 0NR, Scotland, United Kingdom.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged IKKalpha (1 to 745 residues) expressed in baculovirus expression system using biotinylated IkBa... |
J Med Chem 60: 7043-7066 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00484
BindingDB Entry DOI: 10.7270/Q2M90C4N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50252856

((4S,7S,13S)-13-[(S)-3-(4-Hydroxy-2,6-dimethyl-phen...)Show SMILES C[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccc(cc2)[N+]([O-])=O)NC(=O)CNC1=O)C(N)=O |r| Show InChI InChI=1S/C29H36N6O8S2/c1-15-8-20(36)9-16(2)21(15)10-17(3)27(39)34-24-14-45-44-13-23(26(30)38)33-29(41)22(32-25(37)12-31-28(24)40)11-18-4-6-19(7-5-18)35(42)43/h4-9,17,22-24,36H,10-14H2,1-3H3,(H2,30,38)(H,31,40)(H,32,37)(H,33,41)(H,34,39)/t17-,22-,23+,24+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor in guinea pig brain membrane |
J Med Chem 51: 5866-70 (2008)
Article DOI: 10.1021/jm8004702
BindingDB Entry DOI: 10.7270/Q20G3K0M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50257884
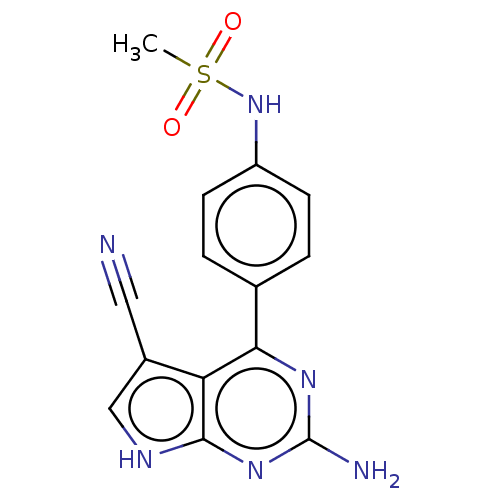
(CHEMBL4065551)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1nc(N)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C14H12N6O2S/c1-23(21,22)20-10-4-2-8(3-5-10)12-11-9(6-15)7-17-13(11)19-14(16)18-12/h2-5,7,20H,1H3,(H3,16,17,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde , 161 Cathedral Street, Glasgow G4 0NR, Scotland, United Kingdom.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human IKKbeta expressed in baculovirus infected sf9 insect cells using biotinylated IkBalpha as substrate after... |
J Med Chem 60: 7043-7066 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00484
BindingDB Entry DOI: 10.7270/Q2M90C4N |
More data for this
Ligand-Target Pair | |
Protein ENL
(Homo sapiens) | BDBM50606139

(CHEMBL5198951)Show SMILES CNC[C@H](NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)CCC(C)C)C(C)C)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein ENL
(Homo sapiens) | BDBM50606141

(CHEMBL5187149)Show SMILES CC(C)CCC(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](C)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein ENL
(Homo sapiens) | BDBM50606142

(CHEMBL5197715)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N[C@@H](Cc1ccccc1)c1nc2cccc(-c3ccccc3)c2[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50606137

(CHEMBL5187921)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50459745

(CHEMBL4210431)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(C)=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N(C)C |r| Show InChI InChI=1S/C40H70N8O9/c1-12-25(8)33(40(57)48-18-14-15-30(48)35(52)42-27(19-22(2)3)38(55)46(10)11)45-34(51)29(21-49)43-37(54)32(24(6)7)44-36(53)31-16-13-17-47(31)39(56)28(20-23(4)5)41-26(9)50/h22-25,27-33,49H,12-21H2,1-11H3,(H,41,50)(H,42,52)(H,43,54)(H,44,53)(H,45,51)/t25-,27-,28-,29-,30-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00083
BindingDB Entry DOI: 10.7270/Q2VT1X6W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data