 Found 4133 hits with Last Name = 'ly' and Initial = 'ks'
Found 4133 hits with Last Name = 'ly' and Initial = 'ks' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
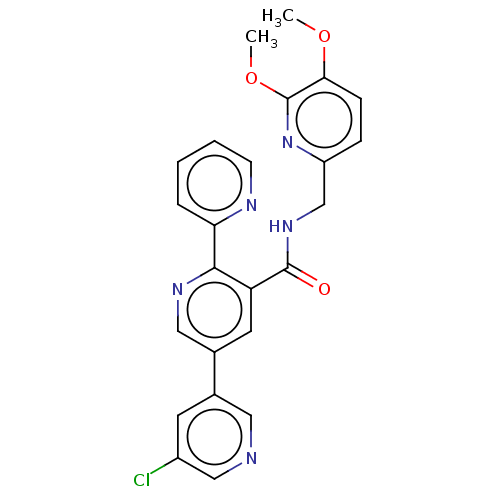
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371305
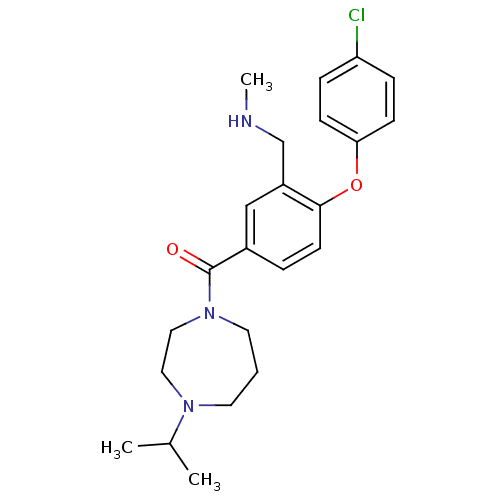
(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589
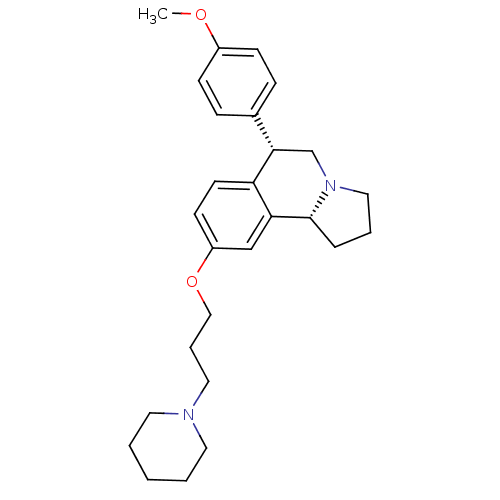
((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371294
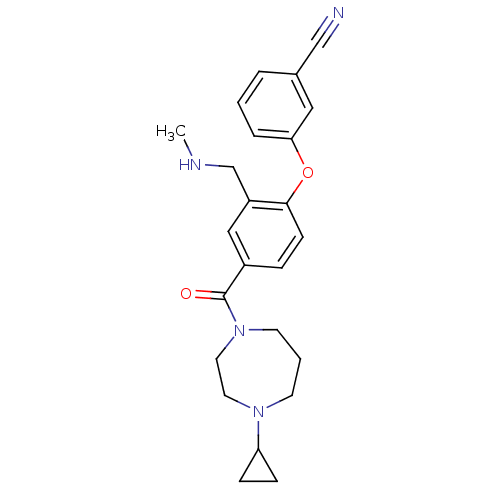
(CHEMBL257208)Show SMILES CNCc1cc(ccc1Oc1cccc(c1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(6-9-23(20)30-22-5-2-4-18(14-22)16-25)24(29)28-11-3-10-27(12-13-28)21-7-8-21/h2,4-6,9,14-15,21,26H,3,7-8,10-13,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089369
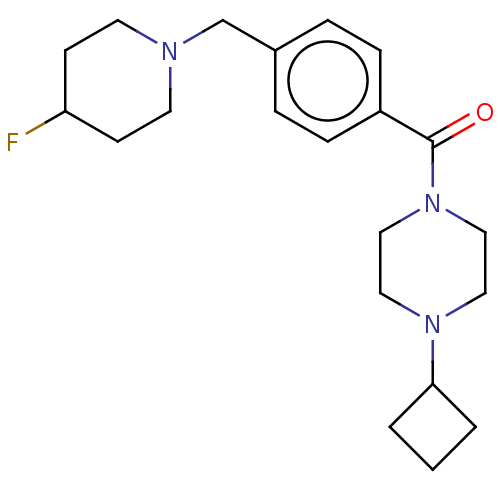
(CHEMBL3577959)Show SMILES FC1CCN(Cc2ccc(cc2)C(=O)N2CCN(CC2)C2CCC2)CC1 Show InChI InChI=1S/C21H30FN3O/c22-19-8-10-23(11-9-19)16-17-4-6-18(7-5-17)21(26)25-14-12-24(13-15-25)20-2-1-3-20/h4-7,19-20H,1-3,8-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to the human histamine H3 receptor |
J Med Chem 46: 3957-60 (2003)
Article DOI: 10.1021/jm0341047
BindingDB Entry DOI: 10.7270/Q2QJ7J1C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198598
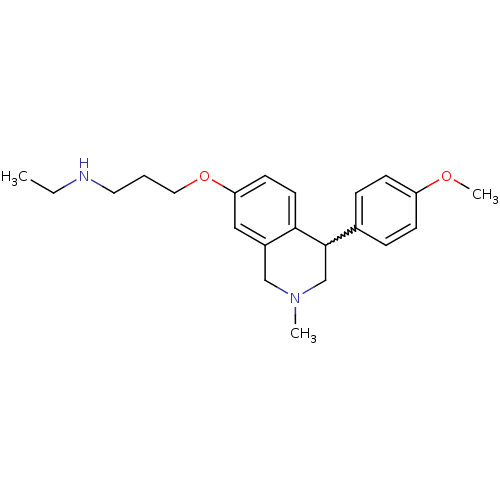
(CHEMBL396945 | N-ethyl-3-(4-(4-methoxyphenyl)-2-me...)Show SMILES CCNCCCOc1ccc2C(CN(C)Cc2c1)c1ccc(OC)cc1 |w:11.19| Show InChI InChI=1S/C22H30N2O2/c1-4-23-12-5-13-26-20-10-11-21-18(14-20)15-24(2)16-22(21)17-6-8-19(25-3)9-7-17/h6-11,14,22-23H,4-5,12-13,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217576

(CHEMBL236046 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C23H28N2O2S/c1-24-18-20-17-19(5-3-4-12-25-13-15-26-16-14-25)6-11-23(20)27-21-7-9-22(28-2)10-8-21/h6-11,17,24H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371290
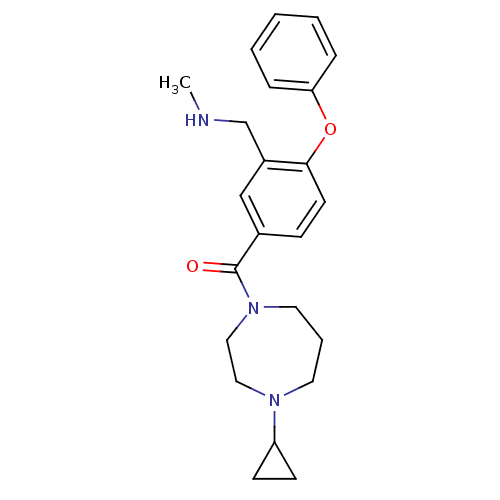
(CHEMBL401683)Show SMILES CNCc1cc(ccc1Oc1ccccc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H29N3O2/c1-24-17-19-16-18(8-11-22(19)28-21-6-3-2-4-7-21)23(27)26-13-5-12-25(14-15-26)20-9-10-20/h2-4,6-8,11,16,20,24H,5,9-10,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371289
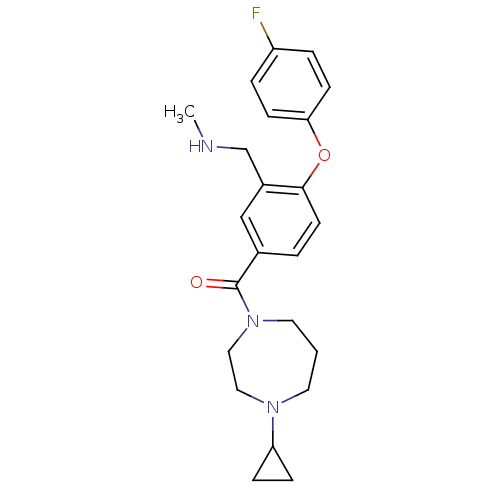
(CHEMBL258349)Show SMILES CNCc1cc(ccc1Oc1ccc(F)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371287
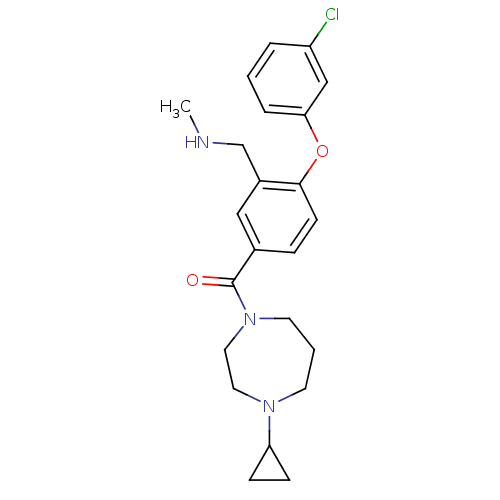
(CHEMBL272699)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28ClN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110
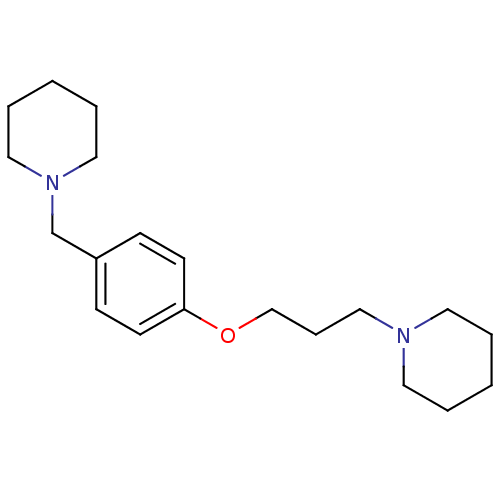
(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells |
Bioorg Med Chem Lett 18: 5796-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.077
BindingDB Entry DOI: 10.7270/Q2154H28 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50255823

((2-((1-isopropylpiperidin-4-yl)methoxy)-1-methyl-1...)Show InChI InChI=1S/C20H27N3O2/c1-15(2)23-11-9-16(10-12-23)14-25-20-21-13-18(22(20)3)19(24)17-7-5-4-6-8-17/h4-8,13,15-16H,9-12,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 19: 903-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.114
BindingDB Entry DOI: 10.7270/Q2X63MTD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371286
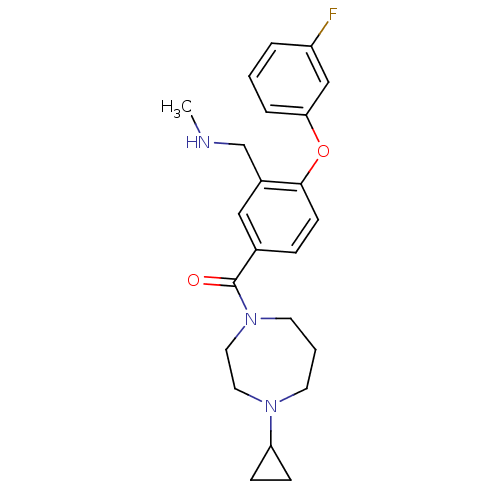
(CHEMBL401867)Show SMILES CNCc1cc(ccc1Oc1cccc(F)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-14-17(6-9-22(18)29-21-5-2-4-19(24)15-21)23(28)27-11-3-10-26(12-13-27)20-7-8-20/h2,4-6,9,14-15,20,25H,3,7-8,10-13,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198609

(7-(3-(azetidin-1-yl)propoxy)-4-(4-methoxyphenyl)-2...)Show SMILES COc1ccc(cc1)C1CN(C)Cc2cc(OCCCN3CCC3)ccc12 |w:8.8| Show InChI InChI=1S/C23H30N2O2/c1-24-16-19-15-21(27-14-4-13-25-11-3-12-25)9-10-22(19)23(17-24)18-5-7-20(26-2)8-6-18/h5-10,15,23H,3-4,11-14,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371280
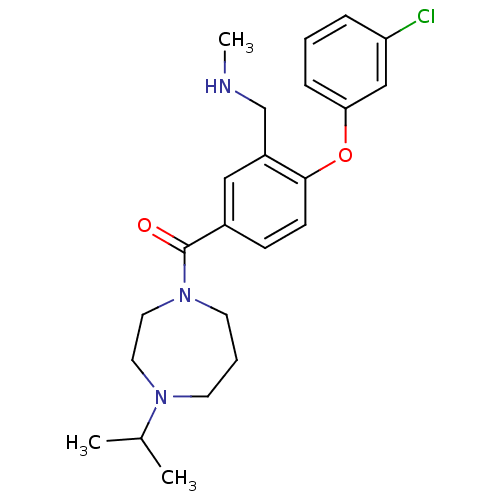
(CHEMBL272289)Show SMILES CNCc1cc(ccc1Oc1cccc(Cl)c1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-10-5-11-27(13-12-26)23(28)18-8-9-22(19(14-18)16-25-3)29-21-7-4-6-20(24)15-21/h4,6-9,14-15,17,25H,5,10-13,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50205656

(4-phenyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tet...)Show SMILES C(COc1cc2CNCC(c3ccccc3)c2cn1)CN1CCCCC1 |w:9.9| Show InChI InChI=1S/C22H29N3O/c1-3-8-18(9-4-1)20-16-23-15-19-14-22(24-17-21(19)20)26-13-7-12-25-10-5-2-6-11-25/h1,3-4,8-9,14,17,20,23H,2,5-7,10-13,15-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Bioorg Med Chem Lett 17: 2566-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.006
BindingDB Entry DOI: 10.7270/Q2TX3F2S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50205667
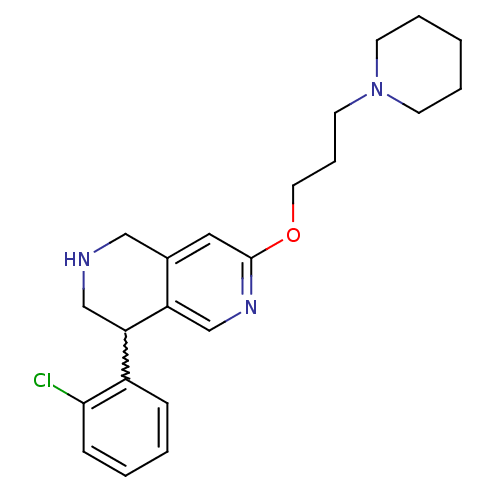
(4-(2-chlorophenyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES Clc1ccccc1C1CNCc2cc(OCCCN3CCCCC3)ncc12 |w:7.7| Show InChI InChI=1S/C22H28ClN3O/c23-21-8-3-2-7-18(21)20-15-24-14-17-13-22(25-16-19(17)20)27-12-6-11-26-9-4-1-5-10-26/h2-3,7-8,13,16,20,24H,1,4-6,9-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Bioorg Med Chem Lett 17: 2566-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.006
BindingDB Entry DOI: 10.7270/Q2TX3F2S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371314

(CHEMBL402949)Show SMILES CNCc1cc(ccc1Oc1ccccc1F)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28FN3O2/c1-25-16-18-15-17(7-10-21(18)29-22-6-3-2-5-20(22)24)23(28)27-12-4-11-26(13-14-27)19-8-9-19/h2-3,5-7,10,15,19,25H,4,8-9,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50371281
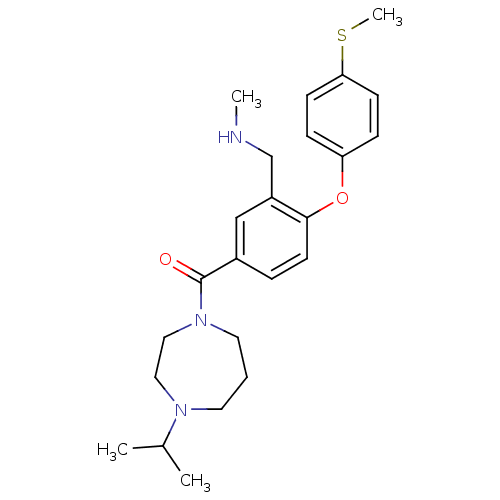
(CHEMBL267267)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C24H33N3O2S/c1-18(2)26-12-5-13-27(15-14-26)24(28)19-6-11-23(20(16-19)17-25-3)29-21-7-9-22(30-4)10-8-21/h6-11,16,18,25H,5,12-15,17H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of rat SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089374
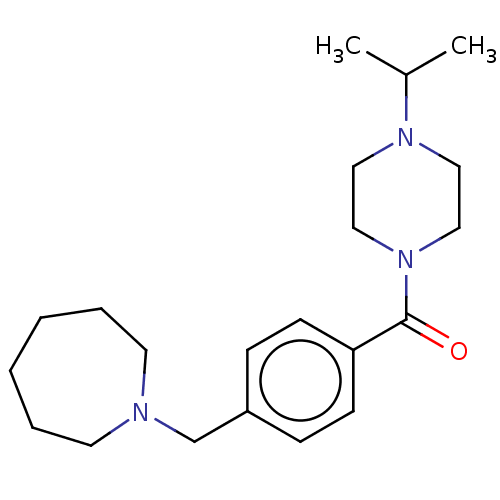
(CHEMBL3577954)Show InChI InChI=1S/C21H33N3O/c1-18(2)23-13-15-24(16-14-23)21(25)20-9-7-19(8-10-20)17-22-11-5-3-4-6-12-22/h7-10,18H,3-6,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371291

(CHEMBL257009)Show SMILES CNCc1cc(ccc1Oc1ccccc1C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-18(7-10-23(20)30-22-6-3-2-5-19(22)16-25)24(29)28-12-4-11-27(13-14-28)21-8-9-21/h2-3,5-7,10,15,21,26H,4,8-9,11-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089375
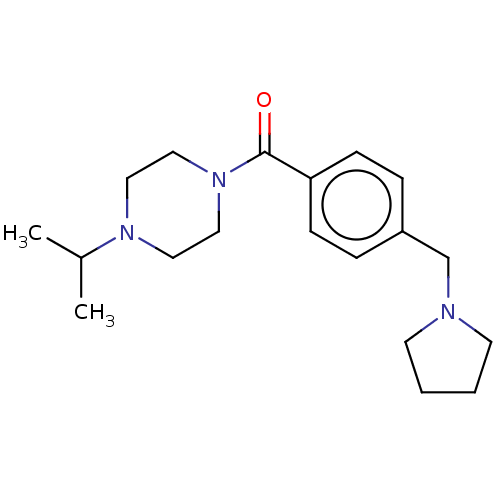
(CHEMBL3577953)Show InChI InChI=1S/C19H29N3O/c1-16(2)21-11-13-22(14-12-21)19(23)18-7-5-17(6-8-18)15-20-9-3-4-10-20/h5-8,16H,3-4,9-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50321465
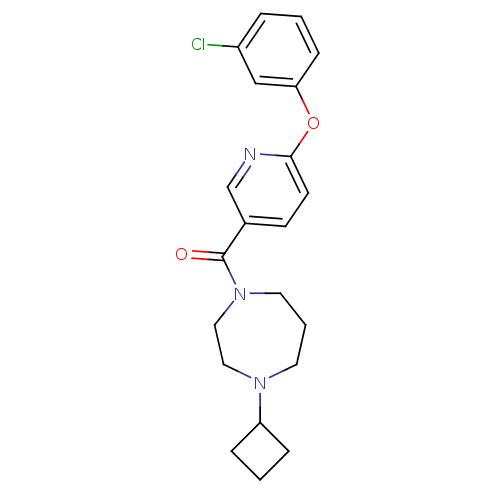
((6-(3-chlorophenoxy)pyridin-3-yl)(4-cyclobutyl-1,4...)Show SMILES Clc1cccc(Oc2ccc(cn2)C(=O)N2CCCN(CC2)C2CCC2)c1 Show InChI InChI=1S/C21H24ClN3O2/c22-17-4-1-7-19(14-17)27-20-9-8-16(15-23-20)21(26)25-11-3-10-24(12-13-25)18-5-2-6-18/h1,4,7-9,14-15,18H,2-3,5-6,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin... |
Bioorg Med Chem Lett 20: 4210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.041
BindingDB Entry DOI: 10.7270/Q29G5NS9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50255824
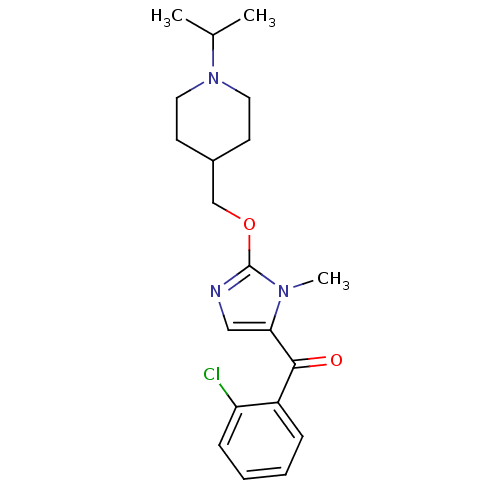
((2-chlorophenyl)(2-((1-isopropylpiperidin-4-yl)met...)Show InChI InChI=1S/C20H26ClN3O2/c1-14(2)24-10-8-15(9-11-24)13-26-20-22-12-18(23(20)3)19(25)16-6-4-5-7-17(16)21/h4-7,12,14-15H,8-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 19: 903-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.114
BindingDB Entry DOI: 10.7270/Q2X63MTD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371315

(CHEMBL273136)Show SMILES CNCc1cc(ccc1Oc1cccc(OC)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H31N3O3/c1-25-17-19-15-18(7-10-23(19)30-22-6-3-5-21(16-22)29-2)24(28)27-12-4-11-26(13-14-27)20-8-9-20/h3,5-7,10,15-16,20,25H,4,8-9,11-14,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371300
(CHEMBL403148)Show SMILES CNCc1cc(ccc1Oc1cccc(OC)c1)C(=O)N1CCN(CC1)C(C)C Show InChI InChI=1S/C23H31N3O3/c1-17(2)25-10-12-26(13-11-25)23(27)18-8-9-22(19(14-18)16-24-3)29-21-7-5-6-20(15-21)28-4/h5-9,14-15,17,24H,10-13,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371281

(CHEMBL267267)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C24H33N3O2S/c1-18(2)26-12-5-13-27(15-14-26)24(28)19-6-11-23(20(16-19)17-25-3)29-21-7-9-22(30-4)10-8-21/h6-11,16,18,25H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
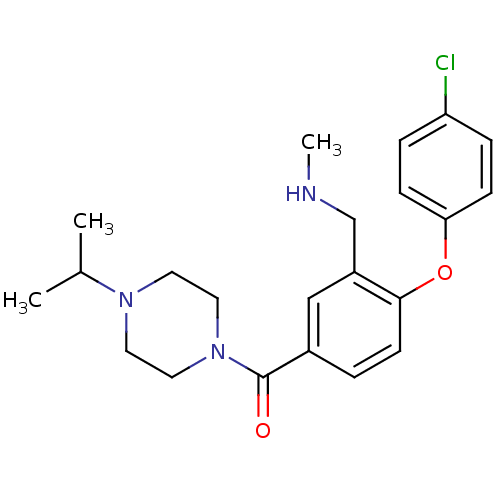
(Homo sapiens (Human)) | BDBM50371311
(CHEMBL256044)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCN(CC1)C(C)C Show InChI InChI=1S/C22H28ClN3O2/c1-16(2)25-10-12-26(13-11-25)22(27)17-4-9-21(18(14-17)15-24-3)28-20-7-5-19(23)6-8-20/h4-9,14,16,24H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371284
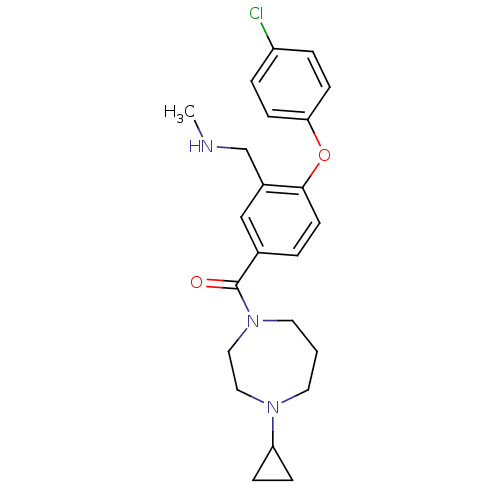
(CHEMBL257909)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H28ClN3O2/c1-25-16-18-15-17(3-10-22(18)29-21-8-4-19(24)5-9-21)23(28)27-12-2-11-26(13-14-27)20-6-7-20/h3-5,8-10,15,20,25H,2,6-7,11-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371297
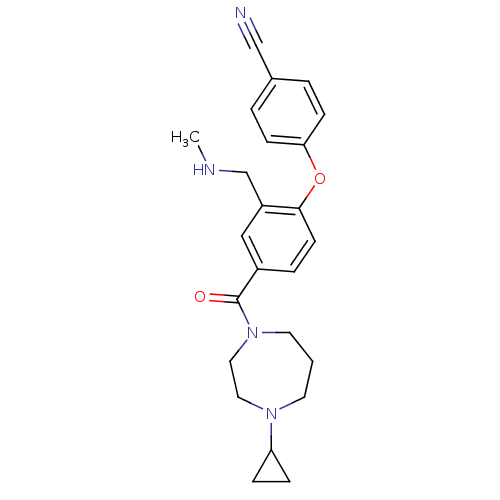
(CHEMBL404572)Show SMILES CNCc1cc(ccc1Oc1ccc(cc1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(5-10-23(20)30-22-8-3-18(16-25)4-9-22)24(29)28-12-2-11-27(13-14-28)21-6-7-21/h3-5,8-10,15,21,26H,2,6-7,11-14,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317720

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES CS(=O)(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C21H30FN3O4S/c1-30(27,28)25-15-19(29-18-8-6-16(22)7-9-18)14-20(25)21(26)24-11-3-10-23(12-13-24)17-4-2-5-17/h6-9,17,19-20H,2-5,10-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371282
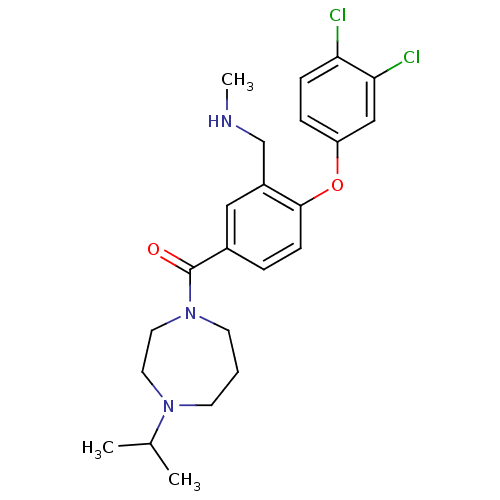
(CHEMBL272503)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)c(Cl)c1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H29Cl2N3O2/c1-16(2)27-9-4-10-28(12-11-27)23(29)17-5-8-22(18(13-17)15-26-3)30-19-6-7-20(24)21(25)14-19/h5-8,13-14,16,26H,4,9-12,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317711

(((2R,4S)-4-(4-chloro-3-methylphenoxy)pyrrolidin-2-...)Show SMILES Cc1cc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)ccc1Cl |r| Show InChI InChI=1S/C21H30ClN3O2/c1-15-12-17(6-7-19(15)22)27-18-13-20(23-14-18)21(26)25-9-3-8-24(10-11-25)16-4-2-5-16/h6-7,12,16,18,20,23H,2-5,8-11,13-14H2,1H3/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089372
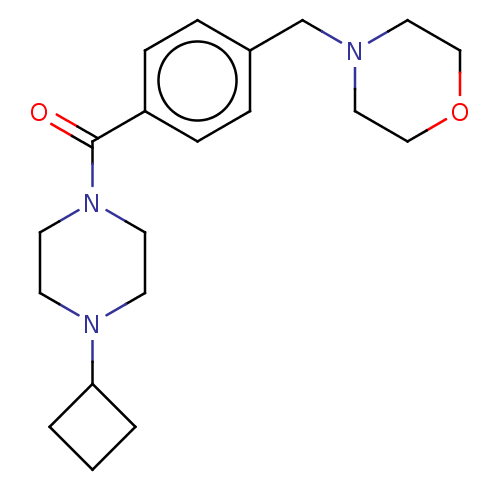
(CHEMBL3577956)Show InChI InChI=1S/C20H29N3O2/c24-20(23-10-8-22(9-11-23)19-2-1-3-19)18-6-4-17(5-7-18)16-21-12-14-25-15-13-21/h4-7,19H,1-3,8-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346208
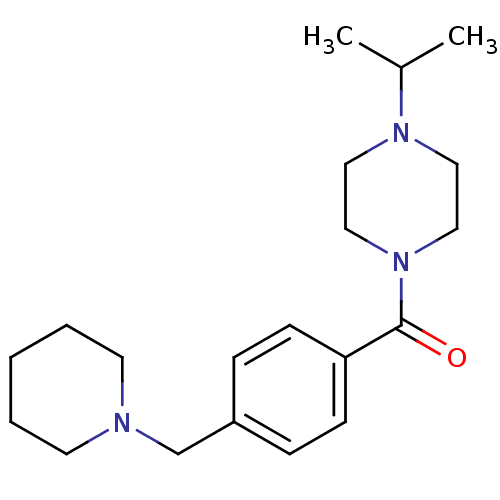
((1-isopropylpiperidin-4-yl)(4-(piperidin-1-ylmethy...)Show InChI InChI=1S/C20H31N3O/c1-17(2)22-12-14-23(15-13-22)20(24)19-8-6-18(7-9-19)16-21-10-4-3-5-11-21/h6-9,17H,3-5,10-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198585
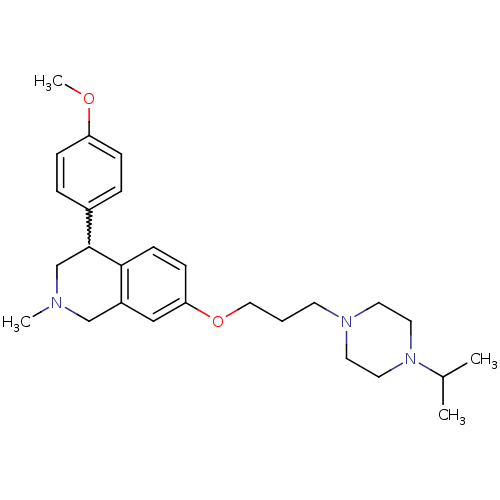
(7-(3-(4-isopropylpiperazin-1-yl)propoxy)-4-(4-meth...)Show SMILES COc1ccc(cc1)C1CN(C)Cc2cc(OCCCN3CCN(CC3)C(C)C)ccc12 |w:8.8| Show InChI InChI=1S/C27H39N3O2/c1-21(2)30-15-13-29(14-16-30)12-5-17-32-25-10-11-26-23(18-25)19-28(3)20-27(26)22-6-8-24(31-4)9-7-22/h6-11,18,21,27H,5,12-17,19-20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371292
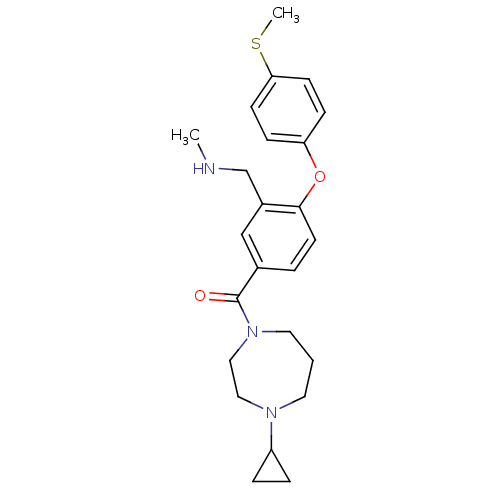
(CHEMBL257434)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H31N3O2S/c1-25-17-19-16-18(4-11-23(19)29-21-7-9-22(30-2)10-8-21)24(28)27-13-3-12-26(14-15-27)20-5-6-20/h4,7-11,16,20,25H,3,5-6,12-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50371305

(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of rat SERT |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50321467
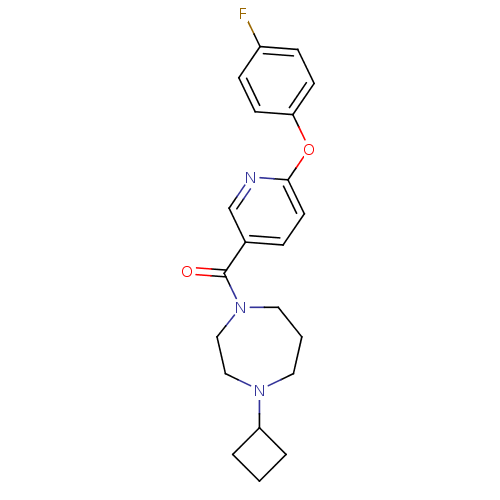
((4-cyclobutyl-1,4-diazepan-1-yl)(6-(4-fluorophenox...)Show SMILES Fc1ccc(Oc2ccc(cn2)C(=O)N2CCCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C21H24FN3O2/c22-17-6-8-19(9-7-17)27-20-10-5-16(15-23-20)21(26)25-12-2-11-24(13-14-25)18-3-1-4-18/h5-10,15,18H,1-4,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin... |
Bioorg Med Chem Lett 20: 4210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.041
BindingDB Entry DOI: 10.7270/Q29G5NS9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371288

(CHEMBL272698)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1F)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H27ClFN3O2/c1-26-15-17-13-16(3-7-21(17)30-22-8-4-18(24)14-20(22)25)23(29)28-10-2-9-27(11-12-28)19-5-6-19/h3-4,7-8,13-14,19,26H,2,5-6,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198599

(1-(3-(4-(4-methoxyphenyl)-2-methyl-1,2,3,4-tetrahy...)Show SMILES COc1ccc(cc1)C1CN(C)Cc2cc(OCCCN3CCC(O)CC3)ccc12 |w:8.8| Show InChI InChI=1S/C25H34N2O3/c1-26-17-20-16-23(30-15-3-12-27-13-10-21(28)11-14-27)8-9-24(20)25(18-26)19-4-6-22(29-2)7-5-19/h4-9,16,21,25,28H,3,10-15,17-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371285
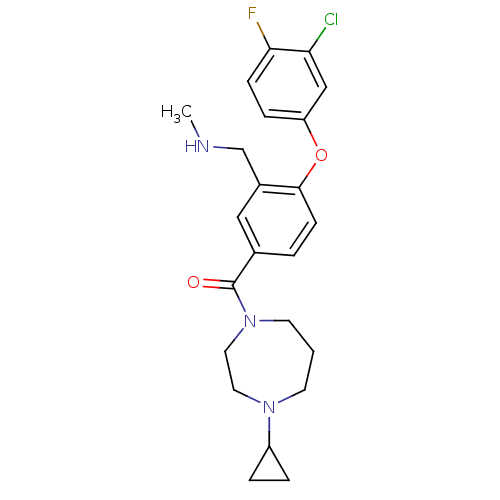
(CHEMBL272917)Show SMILES CNCc1cc(ccc1Oc1ccc(F)c(Cl)c1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H27ClFN3O2/c1-26-15-17-13-16(3-8-22(17)30-19-6-7-21(25)20(24)14-19)23(29)28-10-2-9-27(11-12-28)18-4-5-18/h3,6-8,13-14,18,26H,2,4-5,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089370

(CHEMBL3577958)Show InChI InChI=1S/C20H30FN3O/c1-16(2)23-11-13-24(14-12-23)20(25)18-5-3-17(4-6-18)15-22-9-7-19(21)8-10-22/h3-6,16,19H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50321503

((5-(3,4-dichlorophenoxy)pyridin-2-yl)(4-isopropyl-...)Show SMILES CC(C)N1CCCN(CC1)C(=O)c1ccc(Oc2ccc(Cl)c(Cl)c2)cn1 Show InChI InChI=1S/C20H23Cl2N3O2/c1-14(2)24-8-3-9-25(11-10-24)20(26)19-7-5-16(13-23-19)27-15-4-6-17(21)18(22)12-15/h4-7,12-14H,3,8-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by scintillation countin... |
Bioorg Med Chem Lett 20: 4210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.041
BindingDB Entry DOI: 10.7270/Q29G5NS9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198607

(7-(3-(4-ethylpiperazin-1-yl)propoxy)-4-(4-methoxyp...)Show SMILES CCN1CCN(CCCOc2ccc3C(CN(C)Cc3c2)c2ccc(OC)cc2)CC1 |w:14.22| Show InChI InChI=1S/C26H37N3O2/c1-4-28-13-15-29(16-14-28)12-5-17-31-24-10-11-25-22(18-24)19-27(2)20-26(25)21-6-8-23(30-3)9-7-21/h6-11,18,26H,4-5,12-17,19-20H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317706

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-6-17(23)7-9-19)14-21(26)22(28)25-11-3-10-24(12-13-25)18-4-2-5-18/h6-9,18,20-21H,2-5,10-15H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317698
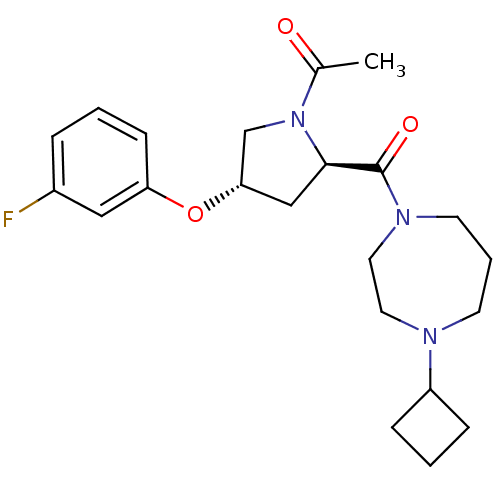
(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198593
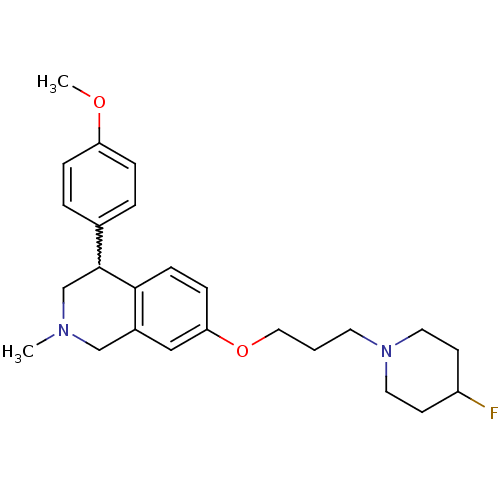
(7-(3-(4-fluoropiperidin-1-yl)propoxy)-4-(4-methoxy...)Show SMILES COc1ccc(cc1)C1CN(C)Cc2cc(OCCCN3CCC(F)CC3)ccc12 |w:8.8| Show InChI InChI=1S/C25H33FN2O2/c1-27-17-20-16-23(30-15-3-12-28-13-10-21(26)11-14-28)8-9-24(20)25(18-27)19-4-6-22(29-2)7-5-19/h4-9,16,21,25H,3,10-15,17-18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity at rat SERT |
Bioorg Med Chem Lett 17: 1047-51 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.036
BindingDB Entry DOI: 10.7270/Q2VM4BXB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data