

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81731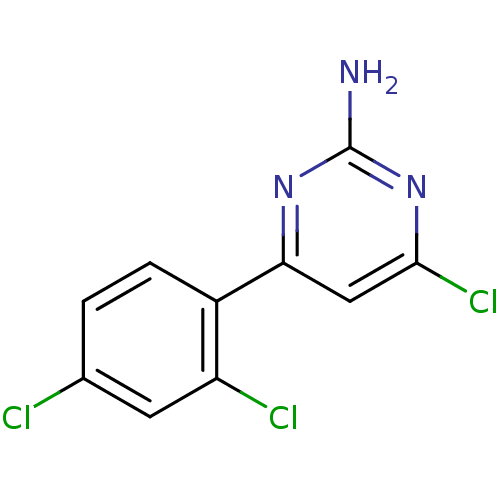 (HSP90 Inhibitor, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81730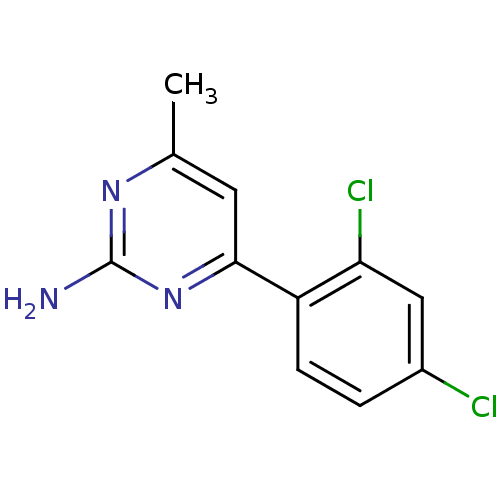 (HSP90 Inhibitor, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81729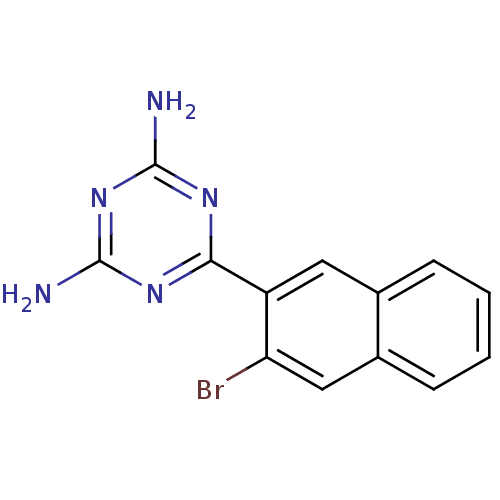 (HSP90 Inhibitor, 1 | hsp90_125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 320 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81732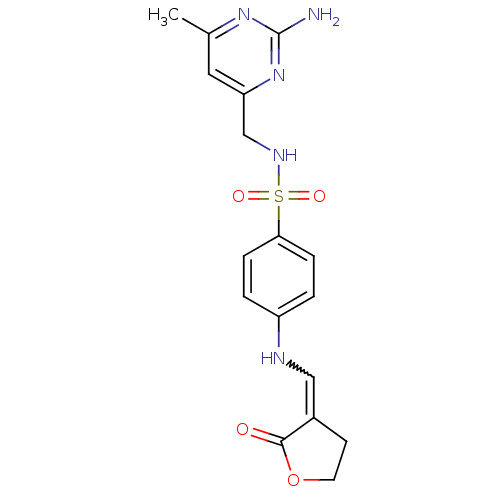 (HSP90 Inhibitor, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM81733 (HSP90 Inhibitor, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-alpha (Homo sapiens (Human)) | BDBM50270588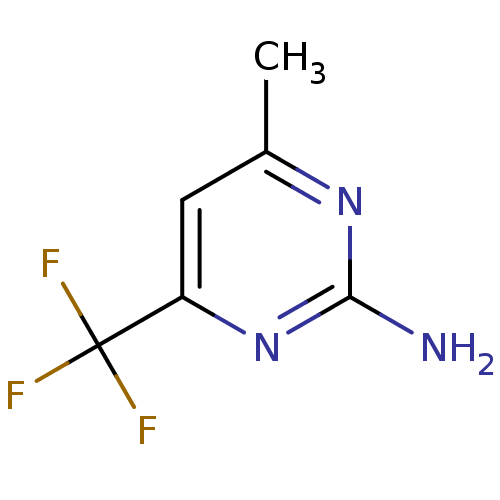 (4-METHYL-6-(TRIFLUOROMETHYL)PYRIMIDIN-2-AMINE | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 1.80E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Abbott Laboratories | Assay Description HSP90 inhibitors identified using Fluorescence resonance energy transfer assay. | Chem Biol Drug Des 70: 1-12 (2007) Article DOI: 10.1111/j.1747-0285.2007.00535.x BindingDB Entry DOI: 10.7270/Q25X27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM553804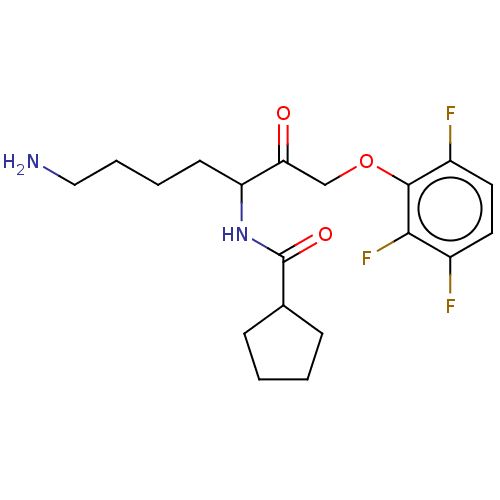 (US11325884, Compound 1a-non-racemic | US11325884, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM553804 (US11325884, Compound 1a-non-racemic | US11325884, ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453323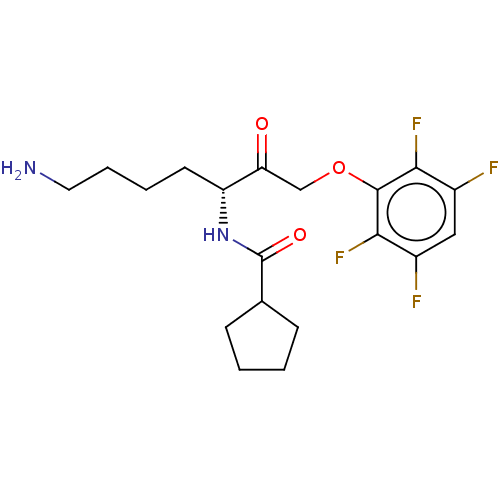 (BDBM553828 | US10730826, Compound 74) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453289 (US10730826, Compound 2a | US11325884, Compound 2a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453323 (BDBM553828 | US10730826, Compound 74) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM397464 (US10676470, Compound 43 | US10730826, Compound 73 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453275 (BDBM453327 | US10730826, Compound 1a-non-racemic |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121838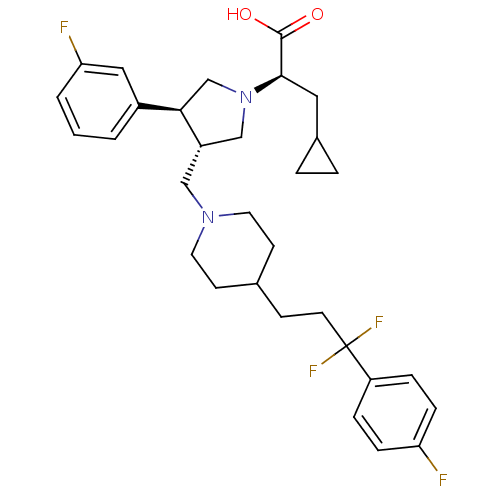 ((R)-3-cyclopropyl-2-((3S,4S)-3-((4-(3,3-difluoro-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453352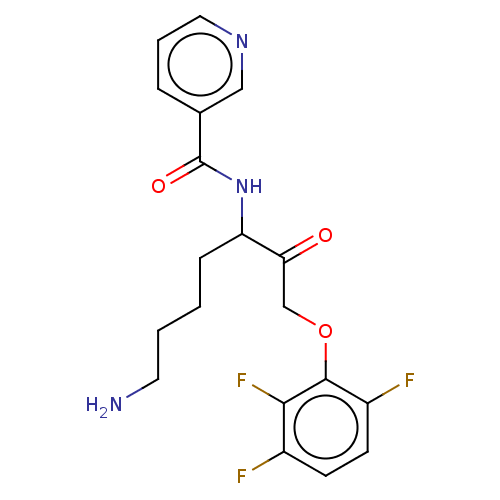 (BDBM553856 | US10730826, Compound 42a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453359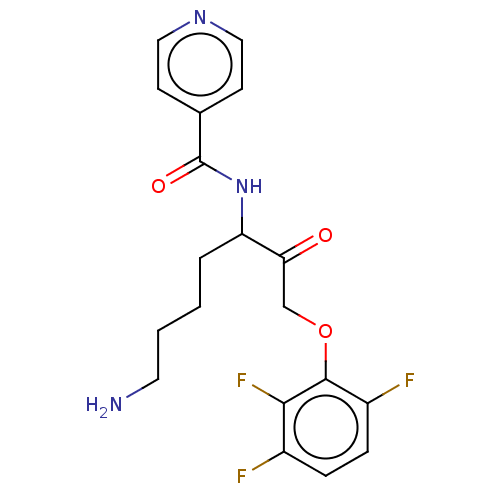 (BDBM553862 | US10730826, Compound 52a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453329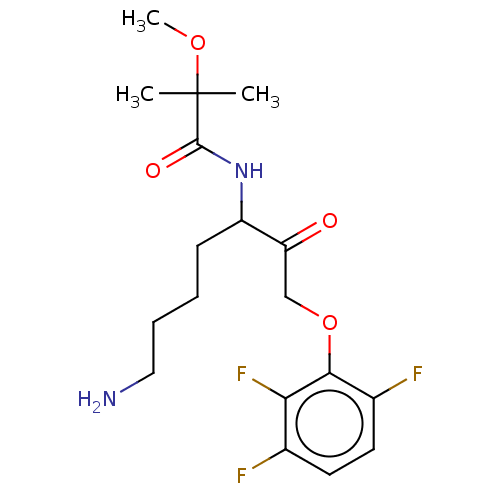 (BDBM553832 | US10730826, Compound 47a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453352 (BDBM553856 | US10730826, Compound 42a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453359 (BDBM553862 | US10730826, Compound 52a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453329 (BDBM553832 | US10730826, Compound 47a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453333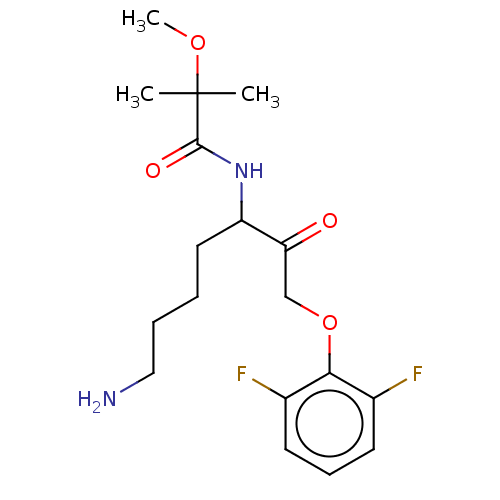 (BDBM553836 | US10730826, Compound 48a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453333 (BDBM553836 | US10730826, Compound 48a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453293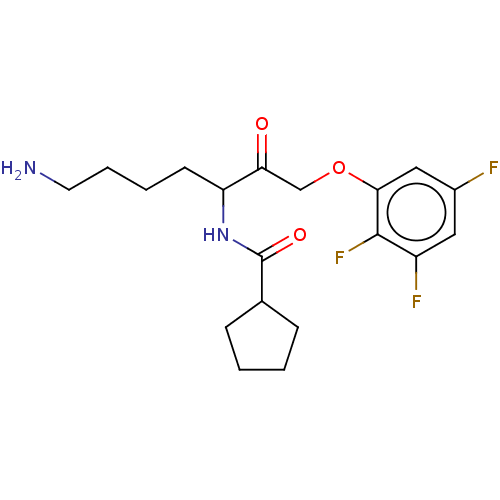 (US10730826, Compound 4a | US11325884, Compound 4a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453293 (US10730826, Compound 4a | US11325884, Compound 4a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453309 (US10730826, Compound 10a | US10730826, Compound 9a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453312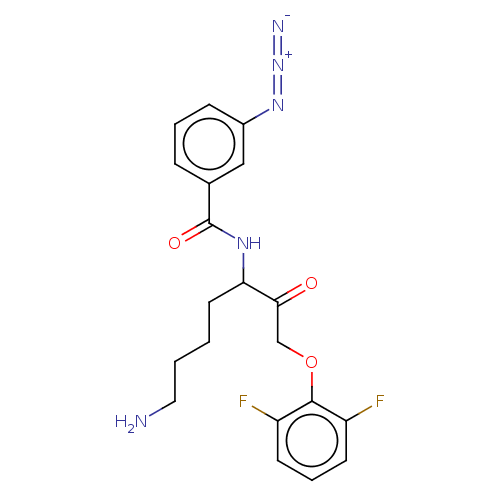 (BDBM453343 | BDBM553818 | US10730826, Compound 12a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453342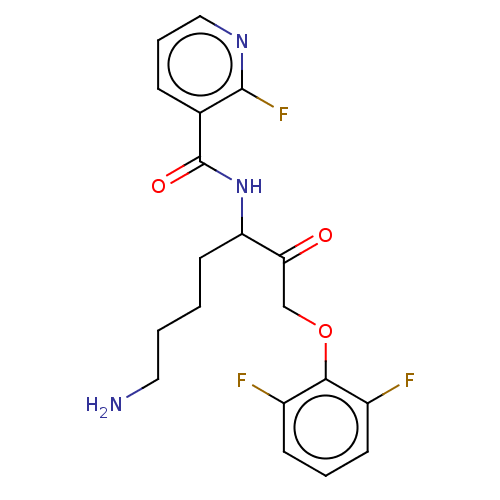 (BDBM553845 | US10730826, Compound 32a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453312 (BDBM453343 | BDBM553818 | US10730826, Compound 12a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453309 (US10730826, Compound 10a | US10730826, Compound 9a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453312 (BDBM453343 | BDBM553818 | US10730826, Compound 12a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453342 (BDBM553845 | US10730826, Compound 32a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453312 (BDBM453343 | BDBM553818 | US10730826, Compound 12a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453353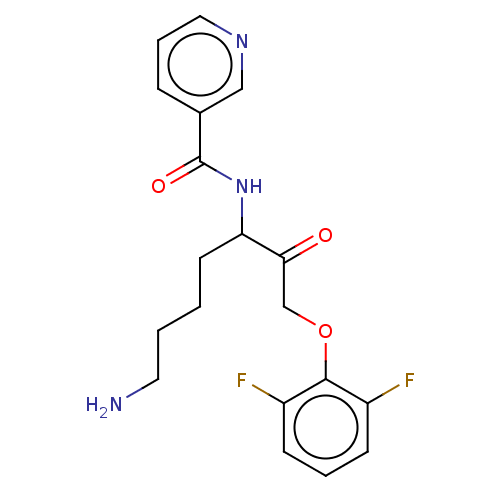 (BDBM553857 | US10730826, Compound 43a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain W83 (Porphyromonas gingivalis) | BDBM453369 (BDBM553871 | US10730826, Compound 63a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
CORTEXYME, INC. US Patent | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos... | US Patent US10730826 (2020) BindingDB Entry DOI: 10.7270/Q2QR5158 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453330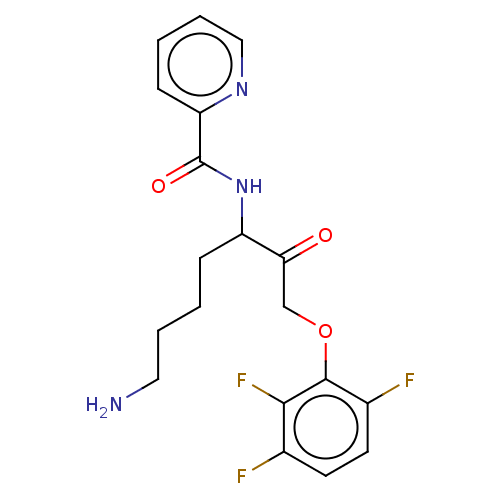 (BDBM553833 | US10730826, Compound 50a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453335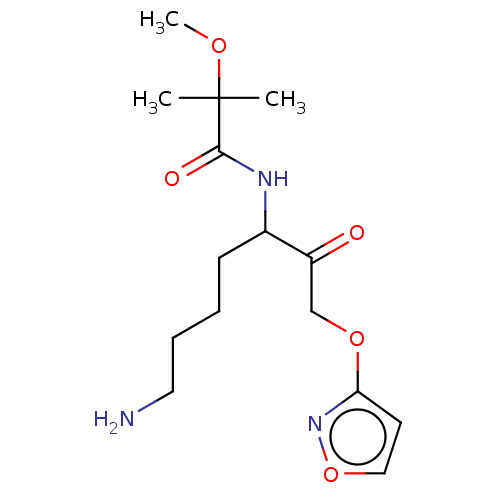 (BDBM553838 | US10730826, Compound 57a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lys-gingipain (Porphyromonas gingivalis) | BDBM453336 (BDBM553839 | US10730826, Compound 26a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GF0XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1039 total ) | Next | Last >> |