 Found 402 hits with Last Name = 'méthot' and Initial = 'n'
Found 402 hits with Last Name = 'méthot' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cruzipain
(Trypanosoma cruzi) | BDBM50331776
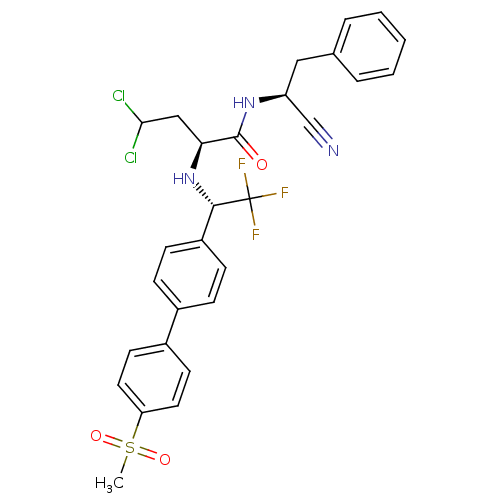
((S)-4,4-dichloro-N-((S)-1-cyano-2-phenylethyl)-2-(...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(Cl)Cl)C(=O)N[C@@H](Cc1ccccc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C28H26Cl2F3N3O3S/c1-40(38,39)23-13-11-20(12-14-23)19-7-9-21(10-8-19)26(28(31,32)33)36-24(16-25(29)30)27(37)35-22(17-34)15-18-5-3-2-4-6-18/h2-14,22,24-26,36H,15-16H2,1H3,(H,35,37)/t22-,24-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50372597
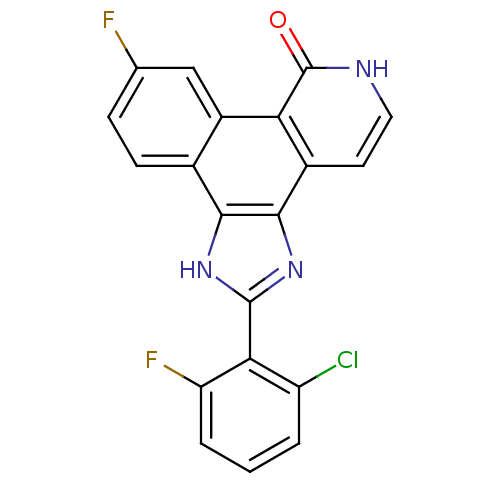
(CHEMBL272424)Show SMILES Fc1ccc2c3[nH]c(nc3c3cc[nH]c(=O)c3c2c1)-c1c(F)cccc1Cl Show InChI InChI=1S/C20H10ClF2N3O/c21-13-2-1-3-14(23)16(13)19-25-17-10-5-4-9(22)8-12(10)15-11(18(17)26-19)6-7-24-20(15)27/h1-8H,(H,24,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 |
Bioorg Med Chem Lett 17: 6816-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.033
BindingDB Entry DOI: 10.7270/Q25X29RT |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331767
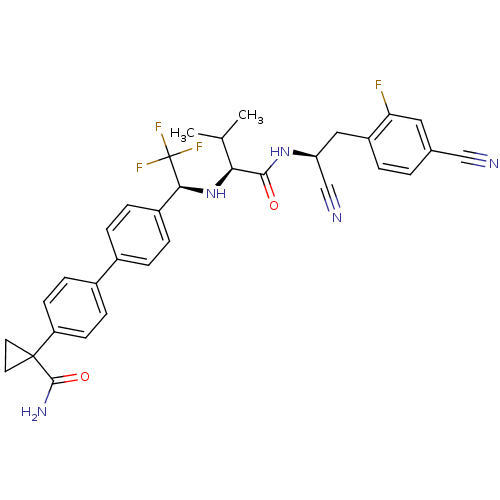
(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-(4-cyano-2-fluo...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C33H31F4N5O2/c1-19(2)28(30(43)41-26(18-39)16-24-4-3-20(17-38)15-27(24)34)42-29(33(35,36)37)23-7-5-21(6-8-23)22-9-11-25(12-10-22)32(13-14-32)31(40)44/h3-12,15,19,26,28-29,42H,13-14,16H2,1-2H3,(H2,40,44)(H,41,43)/t26-,28-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331769
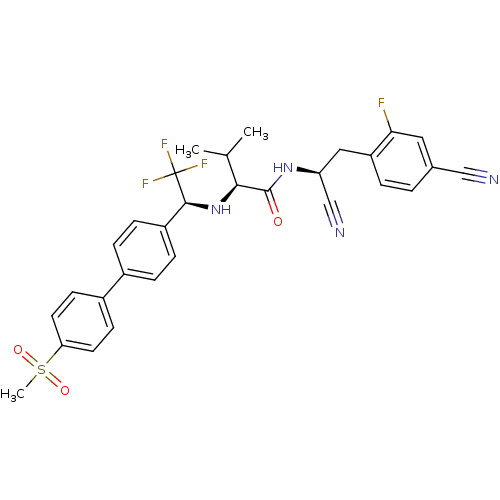
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C30H28F4N4O3S/c1-18(2)27(29(39)37-24(17-36)15-23-5-4-19(16-35)14-26(23)31)38-28(30(32,33)34)22-8-6-20(7-9-22)21-10-12-25(13-11-21)42(3,40)41/h4-14,18,24,27-28,38H,15H2,1-3H3,(H,37,39)/t24-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50214542
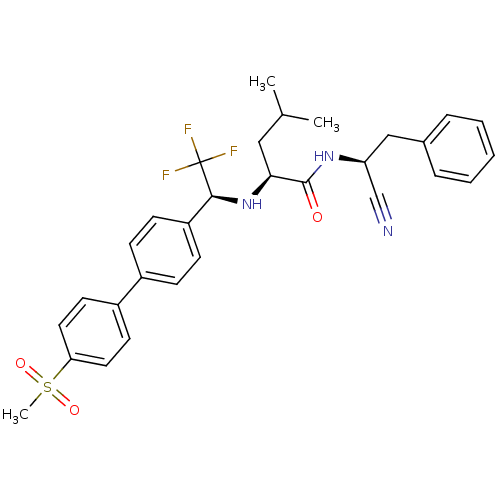
((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin F
(Homo sapiens (Human)) | BDBM50214542

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N Show InChI InChI=1S/C30H32F3N3O3S/c1-20(2)17-27(29(37)35-25(19-34)18-21-7-5-4-6-8-21)36-28(30(31,32)33)24-11-9-22(10-12-24)23-13-15-26(16-14-23)40(3,38)39/h4-16,20,25,27-28,36H,17-18H2,1-3H3,(H,35,37)/t25-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat F expressed in rabbit HIG82 cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302429
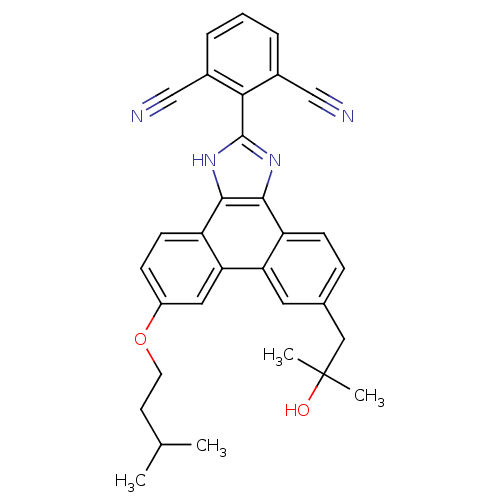
(2-(9-(2-hydroxy-2-methylpropyl)-6-(isopentyloxy)-1...)Show SMILES CC(C)CCOc1ccc2c3[nH]c(nc3c3ccc(CC(C)(C)O)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C32H30N4O2/c1-19(2)12-13-38-23-9-11-25-27(15-23)26-14-20(16-32(3,4)37)8-10-24(26)29-30(25)36-31(35-29)28-21(17-33)6-5-7-22(28)18-34/h5-11,14-15,19,37H,12-13,16H2,1-4H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331768
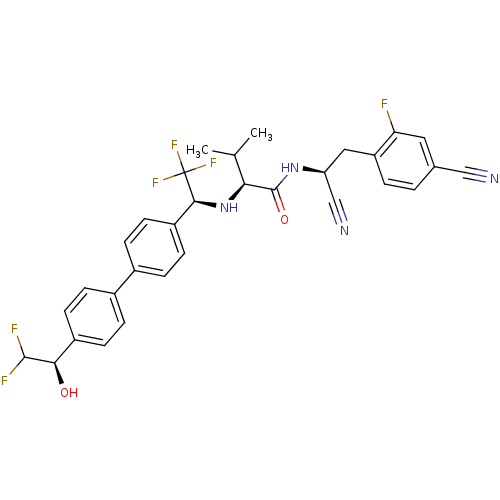
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H28F6N4O2/c1-17(2)26(30(43)40-24(16-39)14-23-4-3-18(15-38)13-25(23)32)41-28(31(35,36)37)22-11-7-20(8-12-22)19-5-9-21(10-6-19)27(42)29(33)34/h3-13,17,24,26-29,41-42H,14H2,1-2H3,(H,40,43)/t24-,26-,27+,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50372597

(CHEMBL272424)Show SMILES Fc1ccc2c3[nH]c(nc3c3cc[nH]c(=O)c3c2c1)-c1c(F)cccc1Cl Show InChI InChI=1S/C20H10ClF2N3O/c21-13-2-1-3-14(23)16(13)19-25-17-10-5-4-9(22)8-12(10)15-11(18(17)26-19)6-7-24-20(15)27/h1-8H,(H,24,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 |
Bioorg Med Chem Lett 17: 6816-20 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.033
BindingDB Entry DOI: 10.7270/Q25X29RT |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331769

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C30H28F4N4O3S/c1-18(2)27(29(39)37-24(17-36)15-23-5-4-19(16-35)14-26(23)31)38-28(30(32,33)34)22-8-6-20(7-9-22)21-10-12-25(13-11-21)42(3,40)41/h4-14,18,24,27-28,38H,15H2,1-3H3,(H,37,39)/t24-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50270592
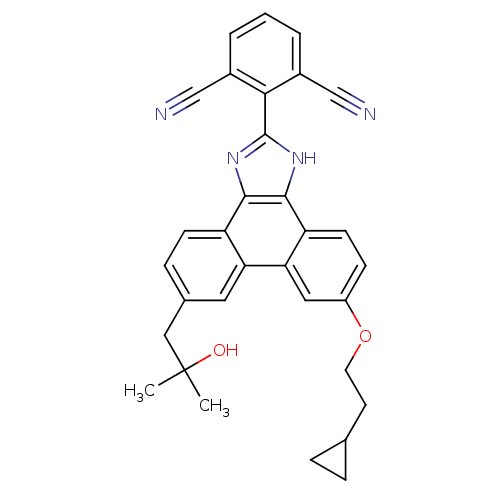
(2-(6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpr...)Show SMILES CC(C)(O)Cc1ccc2c3nc([nH]c3c3ccc(OCCC4CC4)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C32H28N4O2/c1-32(2,37)16-20-8-10-24-26(14-20)27-15-23(38-13-12-19-6-7-19)9-11-25(27)30-29(24)35-31(36-30)28-21(17-33)4-3-5-22(28)18-34/h3-5,8-11,14-15,19,37H,6-7,12-13,16H2,1-2H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50270029
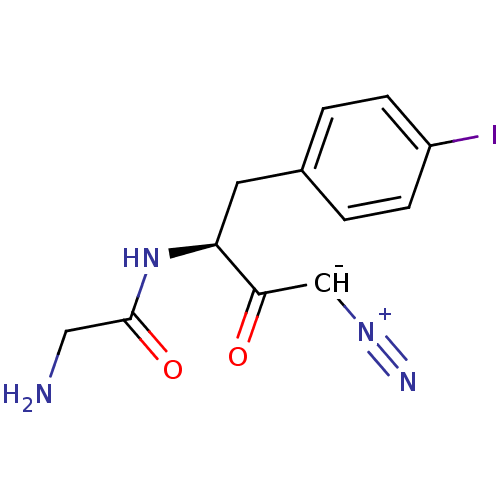
(2-Amino-N-[(S)-3-diazo-1-(4-iodo-benzyl)-2-oxo-pro...)Show SMILES NCC(=O)N[C@@H](Cc1ccc(I)cc1)C(=O)[CH-][N+]#N |r| Show InChI InChI=1S/C12H13IN4O2/c13-9-3-1-8(2-4-9)5-10(11(18)7-16-15)17-12(19)6-14/h1-4,7,10H,5-6,14H2,(H,17,19)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin C after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50270591
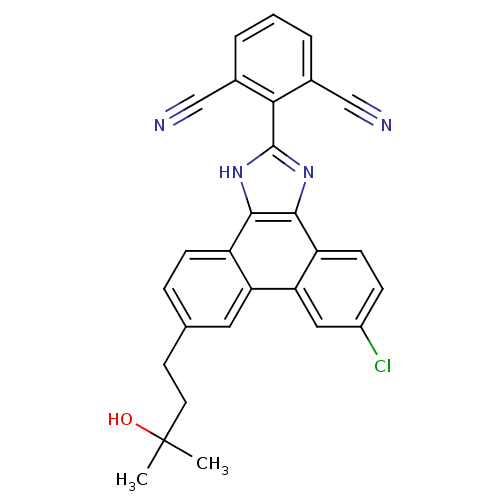
(2-(6-chloro-9-(3-hydroxy-3-methylbutyl)-1H-phenant...)Show SMILES CC(C)(O)CCc1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C28H21ClN4O/c1-28(2,34)11-10-16-6-8-20-22(12-16)23-13-19(29)7-9-21(23)26-25(20)32-27(33-26)24-17(14-30)4-3-5-18(24)15-31/h3-9,12-13,34H,10-11H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302428

(2-(9-(2-hydroxy-2-methylpropyl)-6-(4,4,4-trifluoro...)Show SMILES CC(C)(O)Cc1ccc2c3nc([nH]c3c3ccc(OCCCC(F)(F)F)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C31H25F3N4O2/c1-30(2,39)15-18-7-9-22-24(13-18)25-14-21(40-12-4-11-31(32,33)34)8-10-23(25)28-27(22)37-29(38-28)26-19(16-35)5-3-6-20(26)17-36/h3,5-10,13-14,39H,4,11-12,15H2,1-2H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302421
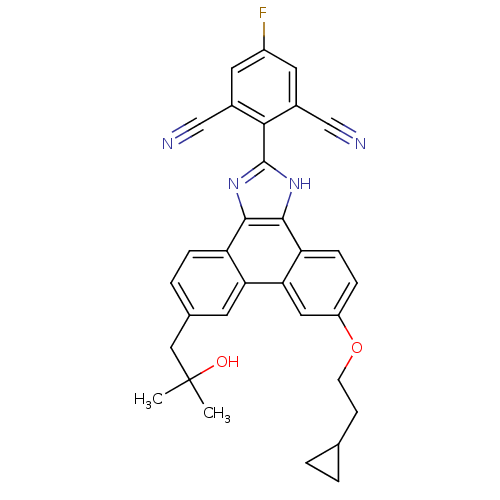
(2-(6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpr...)Show SMILES CC(C)(O)Cc1ccc2c3nc([nH]c3c3ccc(OCCC4CC4)cc3c2c1)-c1c(cc(F)cc1C#N)C#N Show InChI InChI=1S/C32H27FN4O2/c1-32(2,38)15-19-5-7-24-26(11-19)27-14-23(39-10-9-18-3-4-18)6-8-25(27)30-29(24)36-31(37-30)28-20(16-34)12-22(33)13-21(28)17-35/h5-8,11-14,18,38H,3-4,9-10,15H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19516

((2S)-N-[(3S,5E)-6-(benzenesulfonyl)-4-oxo-1-phenyl...)Show SMILES CC(C)C[C@@H](NC(=O)N1CCOCC1)C(=O)N[C@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:27.29| Show InChI InChI=1S/C28H37N3O5S/c1-22(2)21-26(30-28(33)31-16-18-36-19-17-31)27(32)29-24(14-13-23-9-5-3-6-10-23)15-20-37(34,35)25-11-7-4-8-12-25/h3-12,15,20,22,24,26H,13-14,16-19,21H2,1-2H3,(H,29,32)(H,30,33)/t24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S after 10 mins |
J Biol Chem 282: 20836-46 (2007)
Article DOI: 10.1074/jbc.M702615200
BindingDB Entry DOI: 10.7270/Q2125TJM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cruzipain
(Trypanosoma cruzi) | BDBM50331787
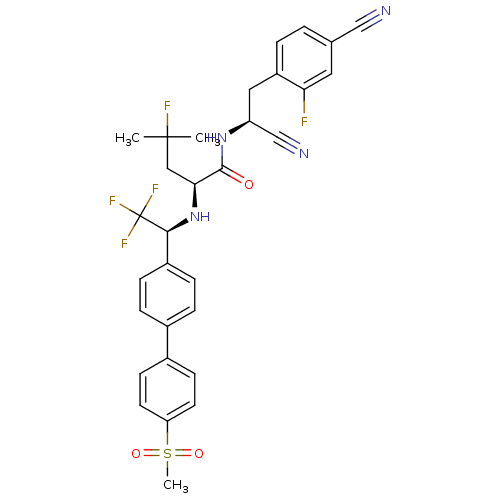
((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H29F5N4O3S/c1-30(2,33)16-27(29(41)39-24(18-38)15-23-5-4-19(17-37)14-26(23)32)40-28(31(34,35)36)22-8-6-20(7-9-22)21-10-12-25(13-11-21)44(3,42)43/h4-14,24,27-28,40H,15-16H2,1-3H3,(H,39,41)/t24-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337659
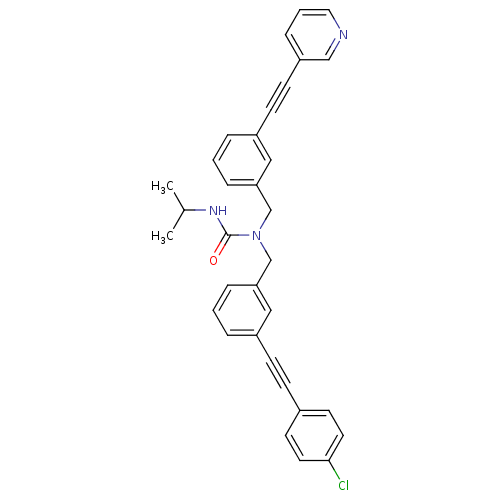
(1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccc(Cl)cc1)Cc1cccc(c1)C#Cc1cccnc1 Show InChI InChI=1S/C33H28ClN3O/c1-25(2)36-33(38)37(24-31-9-4-7-28(21-31)13-14-29-10-5-19-35-22-29)23-30-8-3-6-27(20-30)12-11-26-15-17-32(34)18-16-26/h3-10,15-22,25H,23-24H2,1-2H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631
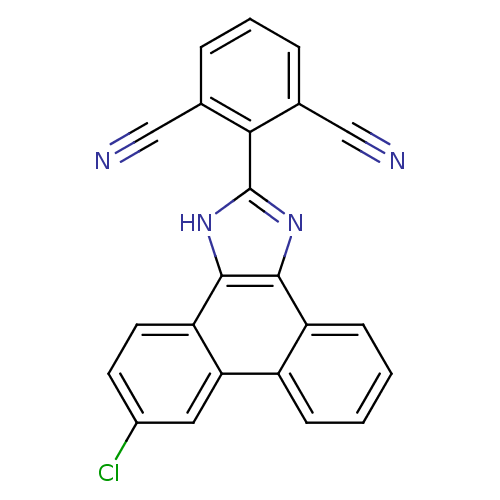
(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337658
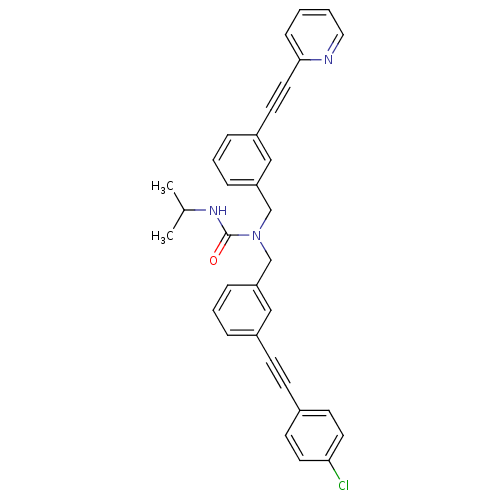
(1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccc(Cl)cc1)Cc1cccc(c1)C#Cc1ccccn1 Show InChI InChI=1S/C33H28ClN3O/c1-25(2)36-33(38)37(24-30-10-6-8-28(22-30)16-19-32-11-3-4-20-35-32)23-29-9-5-7-27(21-29)13-12-26-14-17-31(34)18-15-26/h3-11,14-15,17-18,20-22,25H,23-24H2,1-2H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337654
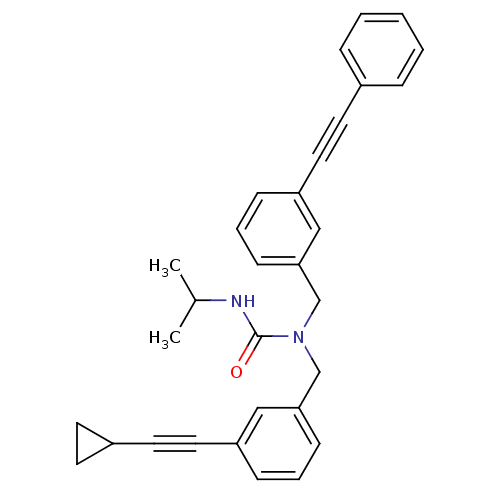
(1-(3-(cyclopropylethynyl)benzyl)-3-isopropyl-1-(3-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#CC1CC1)Cc1cccc(c1)C#Cc1ccccc1 Show InChI InChI=1S/C31H30N2O/c1-24(2)32-31(34)33(23-30-13-7-11-28(21-30)19-17-26-14-15-26)22-29-12-6-10-27(20-29)18-16-25-8-4-3-5-9-25/h3-13,20-21,24,26H,14-15,22-23H2,1-2H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302407
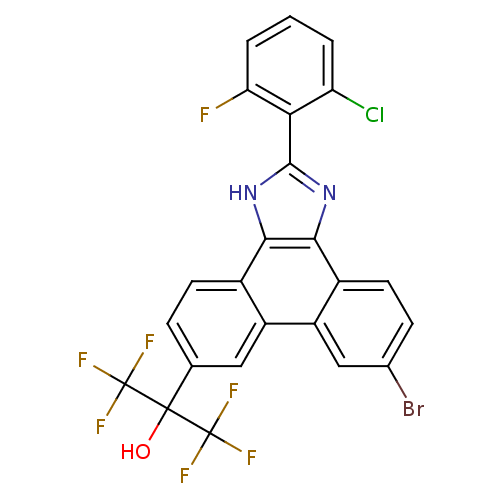
(2-(6-bromo-2-(2-chloro-6-fluorophenyl)-1H-phenanth...)Show SMILES OC(c1ccc2c3[nH]c(nc3c3ccc(Br)cc3c2c1)-c1c(F)cccc1Cl)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H11BrClF7N2O/c25-11-5-7-13-15(9-11)14-8-10(22(36,23(28,29)30)24(31,32)33)4-6-12(14)19-20(13)35-21(34-19)18-16(26)2-1-3-17(18)27/h1-9,36H,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631

(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302414

(2-(6-chloro-9-(3-cyanopropoxy)-1H-phenanthro[9,10-...)Show SMILES Clc1ccc2c3nc([nH]c3c3ccc(OCCCC#N)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C27H16ClN5O/c28-18-6-8-20-22(12-18)23-13-19(34-11-2-1-10-29)7-9-21(23)26-25(20)32-27(33-26)24-16(14-30)4-3-5-17(24)15-31/h3-9,12-13H,1-2,11H2,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302415
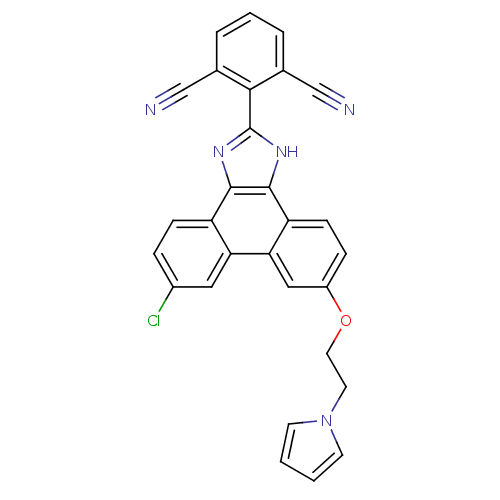
(2-(9-(2-(1H-pyrrol-1-yl)ethoxy)-6-chloro-1H-phenan...)Show SMILES Clc1ccc2c3nc([nH]c3c3ccc(OCCn4cccc4)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C29H18ClN5O/c30-20-6-8-22-24(14-20)25-15-21(36-13-12-35-10-1-2-11-35)7-9-23(25)28-27(22)33-29(34-28)26-18(16-31)4-3-5-19(26)17-32/h1-11,14-15H,12-13H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302418
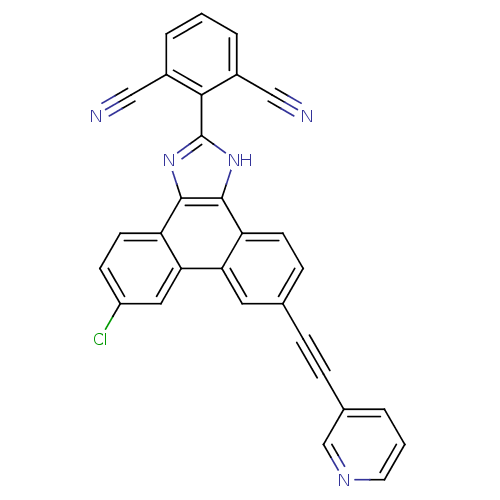
(2-(6-chloro-9-(pyridin-3-ylethynyl)-1H-phenanthro[...)Show SMILES Clc1ccc2c3nc([nH]c3c3ccc(cc3c2c1)C#Cc1cccnc1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C30H14ClN5/c31-22-9-11-24-26(14-22)25-13-18(6-7-19-3-2-12-34-17-19)8-10-23(25)28-29(24)36-30(35-28)27-20(15-32)4-1-5-21(27)16-33/h1-5,8-14,17H,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302419
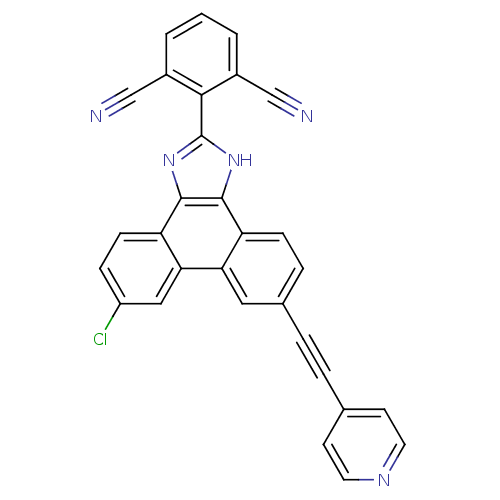
(2-(6-chloro-9-(pyridin-4-ylethynyl)-1H-phenanthro[...)Show SMILES Clc1ccc2c3nc([nH]c3c3ccc(cc3c2c1)C#Cc1ccncc1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C30H14ClN5/c31-22-7-9-24-26(15-22)25-14-19(5-4-18-10-12-34-13-11-18)6-8-23(25)28-29(24)36-30(35-28)27-20(16-32)2-1-3-21(27)17-33/h1-3,6-15H,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50271108

(2-(6-chloro-9-(3-hydroxy-3-methylbut-1-ynyl)-1H-ph...)Show SMILES CC(C)(O)C#Cc1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C28H17ClN4O/c1-28(2,34)11-10-16-6-8-20-22(12-16)23-13-19(29)7-9-21(23)26-25(20)32-27(33-26)24-17(14-30)4-3-5-18(24)15-31/h3-9,12-13,34H,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302420

(2-(6-chloro-9-((1-hydroxycyclopentyl)ethynyl)-1H-p...)Show SMILES OC1(CCCC1)C#Cc1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C30H19ClN4O/c31-21-7-9-23-25(15-21)24-14-18(10-13-30(36)11-1-2-12-30)6-8-22(24)27-28(23)35-29(34-27)26-19(16-32)4-3-5-20(26)17-33/h3-9,14-15,36H,1-2,11-12H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302424
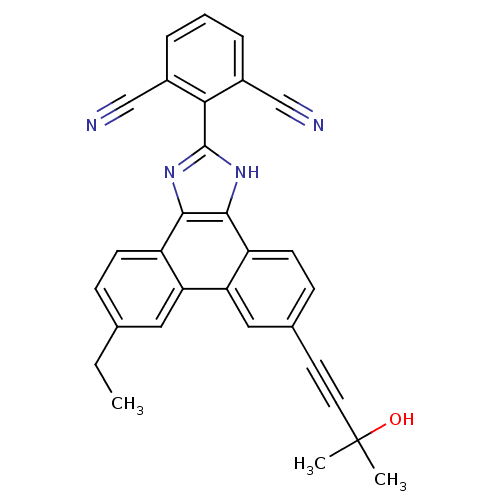
(2-(6-ethyl-9-(3-hydroxy-3-methylbut-1-ynyl)-1H-phe...)Show SMILES CCc1ccc2c3nc([nH]c3c3ccc(cc3c2c1)C#CC(C)(C)O)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C30H22N4O/c1-4-18-8-10-22-24(14-18)25-15-19(12-13-30(2,3)35)9-11-23(25)28-27(22)33-29(34-28)26-20(16-31)6-5-7-21(26)17-32/h5-11,14-15,35H,4H2,1-3H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302425

(2-(6-(cyclopropylethynyl)-9-(3-hydroxy-3-methylbut...)Show SMILES CC(C)(O)C#Cc1ccc2c3[nH]c(nc3c3ccc(cc3c2c1)C#CC1CC1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C33H22N4O/c1-33(2,38)15-14-22-11-13-26-28(17-22)27-16-21(9-8-20-6-7-20)10-12-25(27)30-31(26)37-32(36-30)29-23(18-34)4-3-5-24(29)19-35/h3-5,10-13,16-17,20,38H,6-7H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM19518

((2S)-N-(cyanomethyl)-4-methyl-2-{[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C26H32F3N5O/c1-18(2)17-23(25(35)32-12-11-30)33-24(26(27,28)29)21-5-3-19(4-6-21)20-7-9-22(10-8-20)34-15-13-31-14-16-34/h3-10,18,23-24,31,33H,12-17H2,1-2H3,(H,32,35)/t23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331768

((S)-N-((S)-1-cyano-2-(4-cyano-2-fluorophenyl)ethyl...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)[C@@H](O)C(F)F)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C31H28F6N4O2/c1-17(2)26(30(43)40-24(16-39)14-23-4-3-18(15-38)13-25(23)32)41-28(31(35,36)37)22-11-7-20(8-12-22)19-5-9-21(10-6-19)27(42)29(33)34/h3-13,17,24,26-29,41-42H,14H2,1-2H3,(H,40,43)/t24-,26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331778
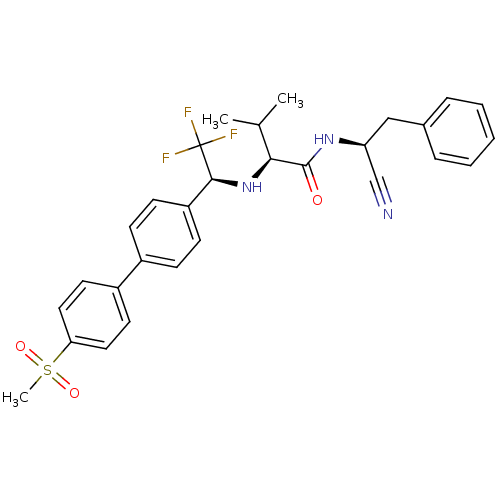
((S)-N-((S)-1-cyano-2-phenylethyl)-3-methyl-2-((S)-...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C29H30F3N3O3S/c1-19(2)26(28(36)34-24(18-33)17-20-7-5-4-6-8-20)35-27(29(30,31)32)23-11-9-21(10-12-23)22-13-15-25(16-14-22)39(3,37)38/h4-16,19,24,26-27,35H,17H2,1-3H3,(H,34,36)/t24-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337657
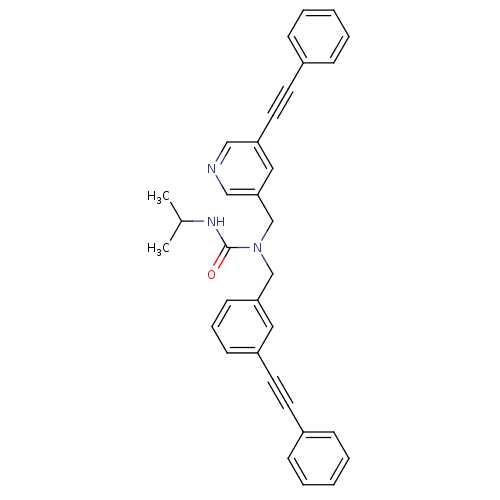
(3-isopropyl-1-(3-(phenylethynyl)benzyl)-1-((5-(phe...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccccc1)Cc1cncc(c1)C#Cc1ccccc1 Show InChI InChI=1S/C33H29N3O/c1-26(2)35-33(37)36(24-31-15-9-14-29(20-31)18-16-27-10-5-3-6-11-27)25-32-21-30(22-34-23-32)19-17-28-12-7-4-8-13-28/h3-15,20-23,26H,24-25H2,1-2H3,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302426
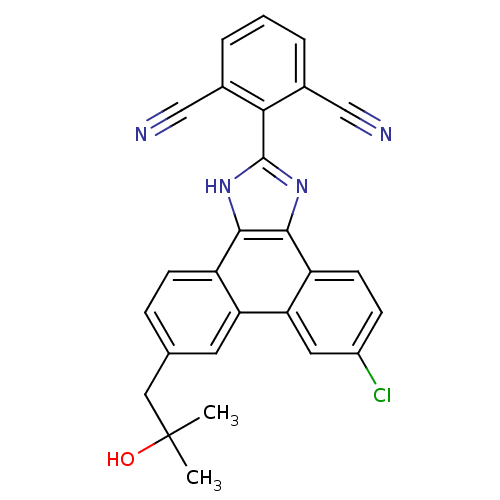
(2-(6-chloro-9-(2-hydroxy-2-methylpropyl)-1H-phenan...)Show SMILES CC(C)(O)Cc1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C27H19ClN4O/c1-27(2,33)12-15-6-8-19-21(10-15)22-11-18(28)7-9-20(22)25-24(19)31-26(32-25)23-16(13-29)4-3-5-17(23)14-30/h3-11,33H,12H2,1-2H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302399
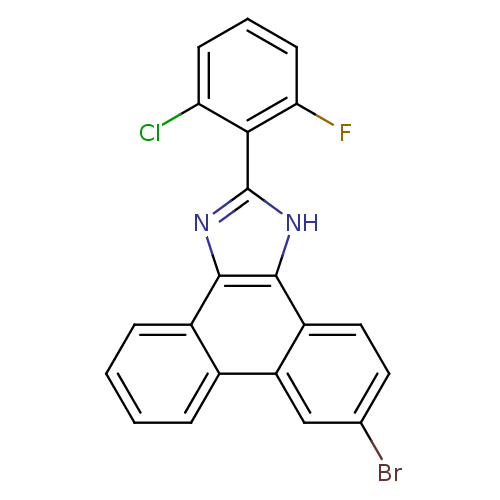
(6-bromo-2-(2-chloro-6-fluorophenyl)-1H-phenanthro[...)Show SMILES Fc1cccc(Cl)c1-c1nc2c([nH]1)c1ccc(Br)cc1c1ccccc21 Show InChI InChI=1S/C21H11BrClFN2/c22-11-8-9-14-15(10-11)12-4-1-2-5-13(12)19-20(14)26-21(25-19)18-16(23)6-3-7-17(18)24/h1-10H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302416

(2-(6-chloro-9-(4-(methylsulfonyl)phenyl)-1H-phenan...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C30H17ClN4O2S/c1-38(36,37)22-9-5-17(6-10-22)18-7-11-23-25(13-18)26-14-21(31)8-12-24(26)29-28(23)34-30(35-29)27-19(15-32)3-2-4-20(27)16-33/h2-14H,1H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302417
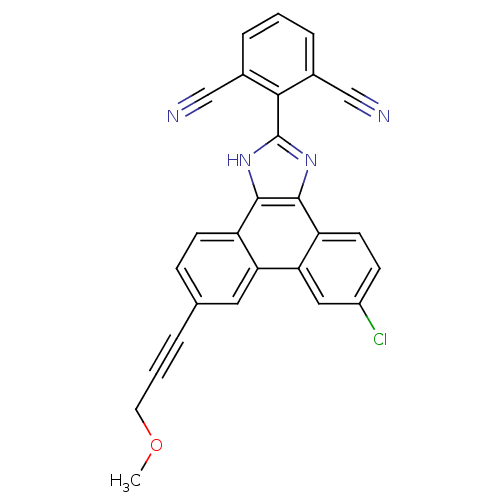
(2-(6-chloro-9-(3-methoxyprop-1-ynyl)-1H-phenanthro...)Show SMILES COCC#Cc1ccc2c3[nH]c(nc3c3ccc(Cl)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C27H15ClN4O/c1-33-11-3-4-16-7-9-20-22(12-16)23-13-19(28)8-10-21(23)26-25(20)31-27(32-26)24-17(14-29)5-2-6-18(24)15-30/h2,5-10,12-13H,11H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337661
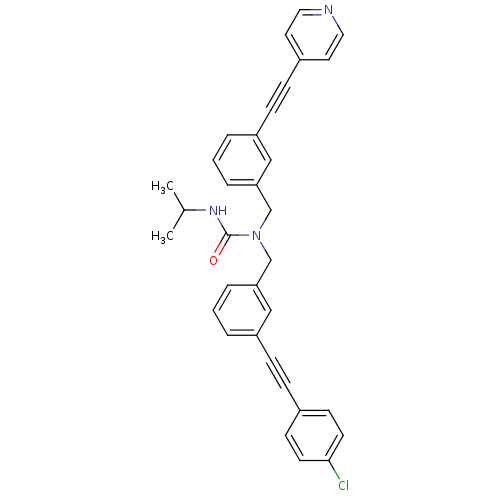
(1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccncc1)Cc1cccc(c1)C#Cc1ccc(Cl)cc1 Show InChI InChI=1S/C33H28ClN3O/c1-25(2)36-33(38)37(24-31-8-4-6-29(22-31)12-10-27-17-19-35-20-18-27)23-30-7-3-5-28(21-30)11-9-26-13-15-32(34)16-14-26/h3-8,13-22,25H,23-24H2,1-2H3,(H,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302427

(2-(6-(cyclopropylmethoxy)-9-(2-hydroxy-2-methylpro...)Show SMILES CC(C)(O)Cc1ccc2c3nc([nH]c3c3ccc(OCC4CC4)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C31H26N4O2/c1-31(2,36)14-19-8-10-23-25(12-19)26-13-22(37-17-18-6-7-18)9-11-24(26)29-28(23)34-30(35-29)27-20(15-32)4-3-5-21(27)16-33/h3-5,8-13,18,36H,6-7,14,17H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302430
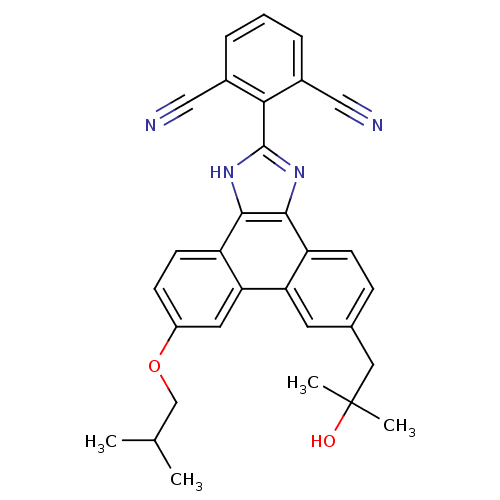
(2-(9-(2-hydroxy-2-methylpropyl)-6-isobutoxy-1H-phe...)Show SMILES CC(C)COc1ccc2c3[nH]c(nc3c3ccc(CC(C)(C)O)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C31H28N4O2/c1-18(2)17-37-22-9-11-24-26(13-22)25-12-19(14-31(3,4)36)8-10-23(25)28-29(24)35-30(34-28)27-20(15-32)6-5-7-21(27)16-33/h5-13,18,36H,14,17H2,1-4H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50331767

(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-(4-cyano-2-fluo...)Show SMILES CC(C)[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccc(cc1F)C#N)C#N |r| Show InChI InChI=1S/C33H31F4N5O2/c1-19(2)28(30(43)41-26(18-39)16-24-4-3-20(17-38)15-27(24)34)42-29(33(35,36)37)23-7-5-21(6-8-23)22-9-11-25(12-10-22)32(13-14-32)31(40)44/h3-12,15,19,26,28-29,42H,13-14,16H2,1-2H3,(H2,40,44)(H,41,43)/t26-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cat K expressed in Ramos cells |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331770
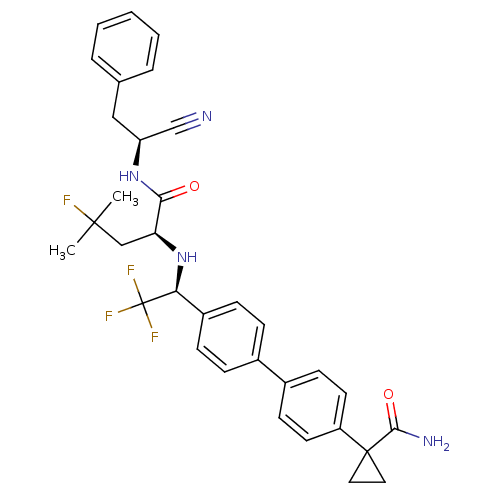
(1-(4'-((S)-1-((S)-1-((S)-1-cyano-2-phenylethylamin...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C33H34F4N4O2/c1-31(2,34)19-27(29(42)40-26(20-38)18-21-6-4-3-5-7-21)41-28(33(35,36)37)24-10-8-22(9-11-24)23-12-14-25(15-13-23)32(16-17-32)30(39)43/h3-15,26-28,41H,16-19H2,1-2H3,(H2,39,43)(H,40,42)/t26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302403
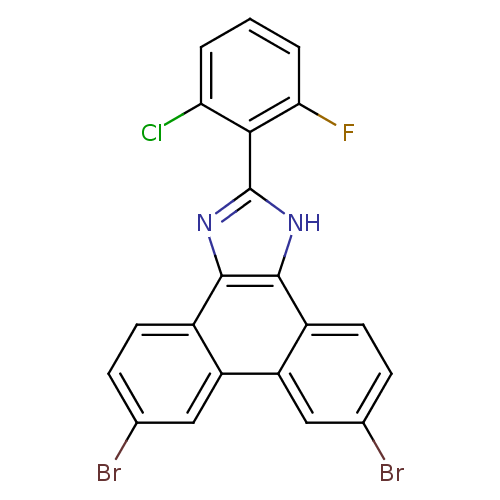
(6,9-dibromo-2-(2-chloro-6-fluorophenyl)-1H-phenant...)Show SMILES Fc1cccc(Cl)c1-c1nc2c([nH]1)c1ccc(Br)cc1c1cc(Br)ccc21 Show InChI InChI=1S/C21H10Br2ClFN2/c22-10-4-6-12-14(8-10)15-9-11(23)5-7-13(15)20-19(12)26-21(27-20)18-16(24)2-1-3-17(18)25/h1-9H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGH2 from human recombinant PGES1 expressed in CHOK1 cells |
Bioorg Med Chem Lett 19: 5837-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.085
BindingDB Entry DOI: 10.7270/Q2MG7PKK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337660
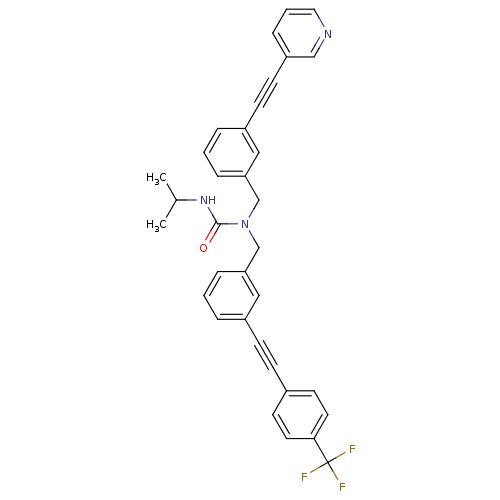
(3-isopropyl-1-(3-(pyridin-3-ylethynyl)benzyl)-1-(3...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccc(cc1)C(F)(F)F)Cc1cccc(c1)C#Cc1cccnc1 Show InChI InChI=1S/C34H28F3N3O/c1-25(2)39-33(41)40(24-31-9-4-7-28(21-31)13-14-29-10-5-19-38-22-29)23-30-8-3-6-27(20-30)12-11-26-15-17-32(18-16-26)34(35,36)37/h3-10,15-22,25H,23-24H2,1-2H3,(H,39,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337656
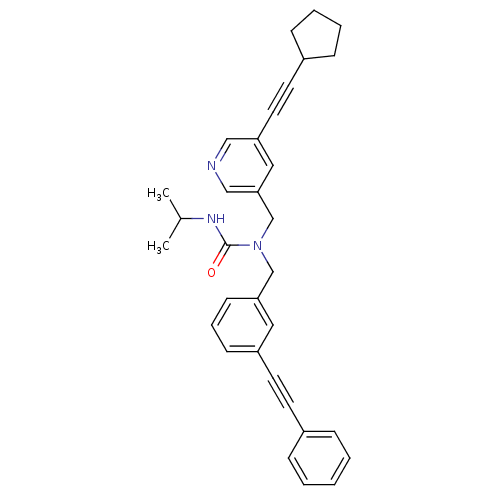
(1-((5-(cyclopentylethynyl)pyridin-3-yl)methyl)-3-i...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccccc1)Cc1cncc(c1)C#CC1CCCC1 Show InChI InChI=1S/C32H33N3O/c1-25(2)34-32(36)35(24-31-20-29(21-33-22-31)18-16-27-11-6-7-12-27)23-30-14-8-13-28(19-30)17-15-26-9-4-3-5-10-26/h3-5,8-10,13-14,19-22,25,27H,6-7,11-12,23-24H2,1-2H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337653

(1-(biphenyl-3-ylmethyl)-3-isopropyl-1-(3-(phenylet...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccccc1)Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C32H30N2O/c1-25(2)33-32(35)34(24-29-15-10-18-31(22-29)30-16-7-4-8-17-30)23-28-14-9-13-27(21-28)20-19-26-11-5-3-6-12-26/h3-18,21-22,25H,23-24H2,1-2H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 |
Bioorg Med Chem Lett 21: 1488-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.006
BindingDB Entry DOI: 10.7270/Q2XS5VNP |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM50168766
(3-(1-(4-chlorobenzyl)-5-(2-fluoro-2'-methylbipheny...)Show SMILES Cc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(cc12)-c1ccc(c(F)c1)-c1ccccc1C Show InChI InChI=1S/C34H31ClFNO2/c1-21-7-5-6-8-27(21)28-15-11-25(18-30(28)36)24-12-16-31-29(17-24)22(2)32(19-34(3,4)33(38)39)37(31)20-23-9-13-26(35)14-10-23/h5-18H,19-20H2,1-4H3,(H,38,39) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human prostaglandin E2 synthase (mPGES-1) |
Bioorg Med Chem Lett 15: 3352-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.027
BindingDB Entry DOI: 10.7270/Q21R6Q1X |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50331771
((S)-N-((S)-1-cyano-2-phenylethyl)-4-fluoro-4-methy...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(=O)N1CCOCC1)C(F)(F)F)C(=O)N[C@@H](Cc1ccccc1)C#N |r| Show InChI InChI=1S/C37H40F4N4O3/c1-35(2,38)23-31(33(46)43-30(24-42)22-25-6-4-3-5-7-25)44-32(37(39,40)41)28-10-8-26(9-11-28)27-12-14-29(15-13-27)36(16-17-36)34(47)45-18-20-48-21-19-45/h3-15,30-32,44H,16-23H2,1-2H3,(H,43,46)/t30-,31-,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of trypanosoma cruzi Cruzipain |
Bioorg Med Chem Lett 20: 7444-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.015
BindingDB Entry DOI: 10.7270/Q29S1R8D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data