

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112660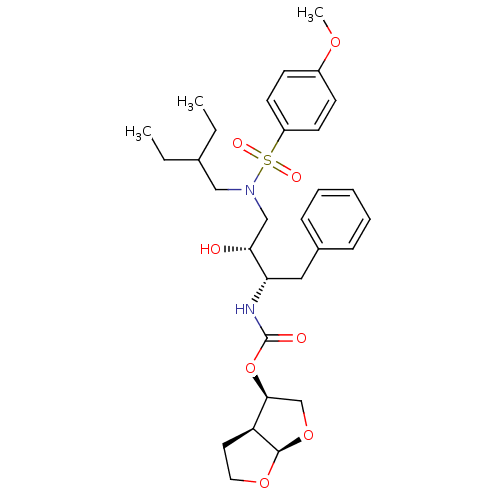 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000200 | -72.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112661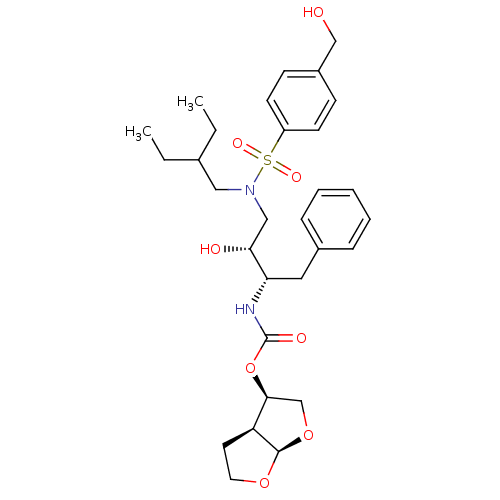 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493155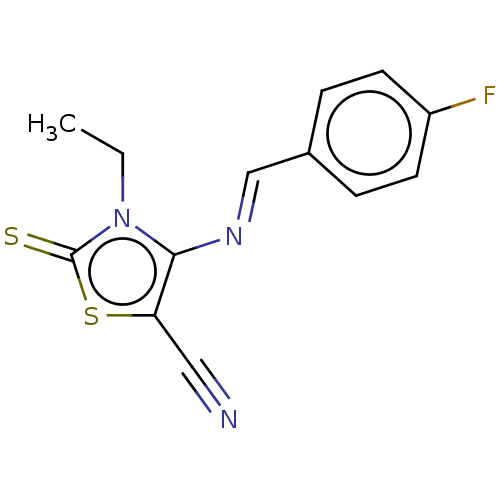 (CHEMBL2419148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493155 (CHEMBL2419148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493144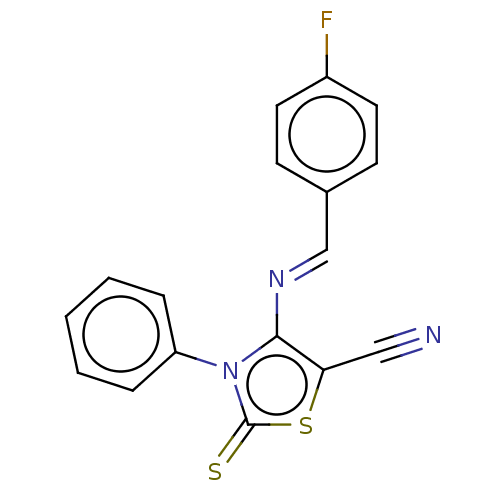 (CHEMBL2419144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493144 (CHEMBL2419144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493151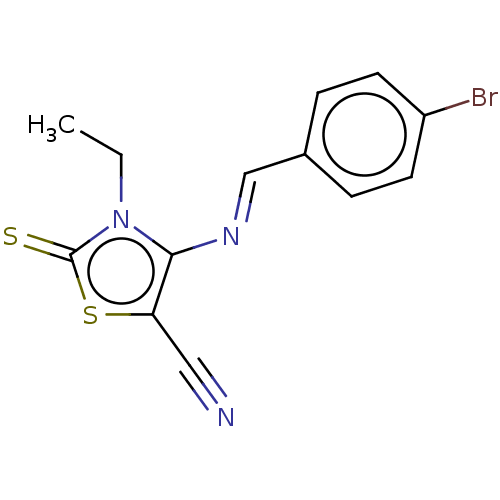 (CHEMBL2419139) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50493151 (CHEMBL2419139) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | -69.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000900 | -68.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00150 | -67.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| neuronal acetylcholine receptor subunit alpha-2 (Xenopus) | BDBM86041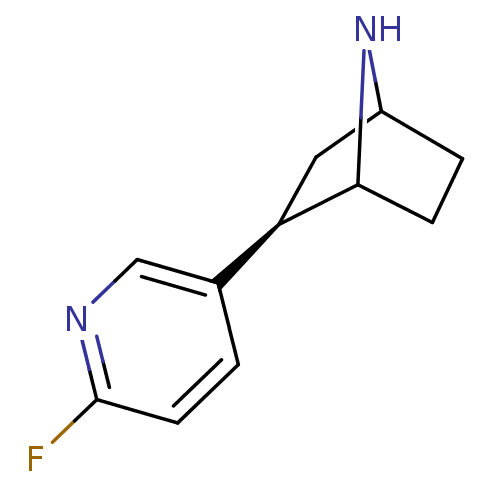 (NFEP) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 1246-52 (2002) Article DOI: 10.1124/jpet.102.035899 BindingDB Entry DOI: 10.7270/Q2HQ3XG3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414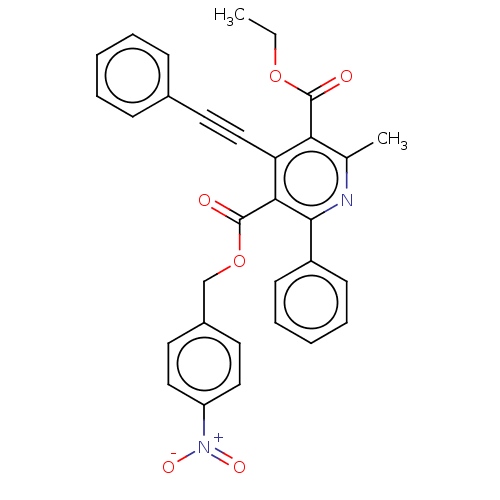 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1 (Xenopus) | BDBM86042 (NEP) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 1246-52 (2002) Article DOI: 10.1124/jpet.102.035899 BindingDB Entry DOI: 10.7270/Q2HQ3XG3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043393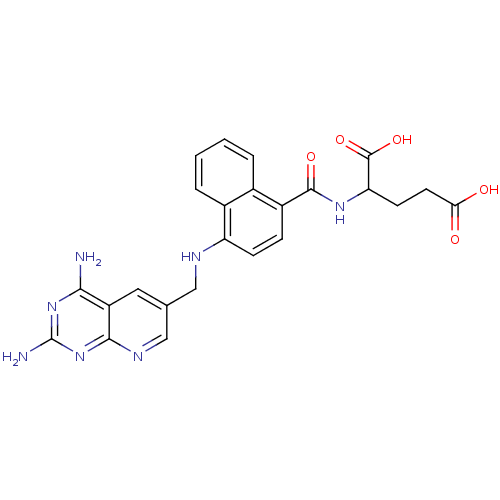 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1 (Xenopus) | BDBM86041 (NFEP) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 1246-52 (2002) Article DOI: 10.1124/jpet.102.035899 BindingDB Entry DOI: 10.7270/Q2HQ3XG3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | -65.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1 (Xenopus) | BDBM86040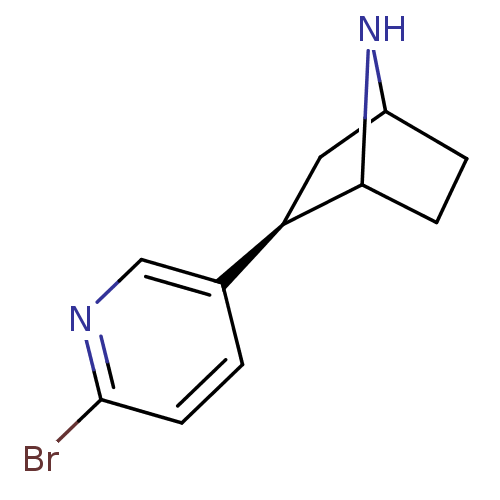 (NBEP) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami Curated by PDSP Ki Database | J Pharmacol Exp Ther 302: 1246-52 (2002) Article DOI: 10.1124/jpet.102.035899 BindingDB Entry DOI: 10.7270/Q2HQ3XG3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50493145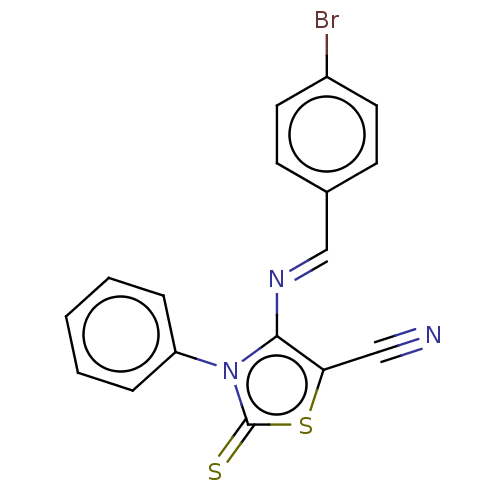 (CHEMBL2419149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50493145 (CHEMBL2419149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043396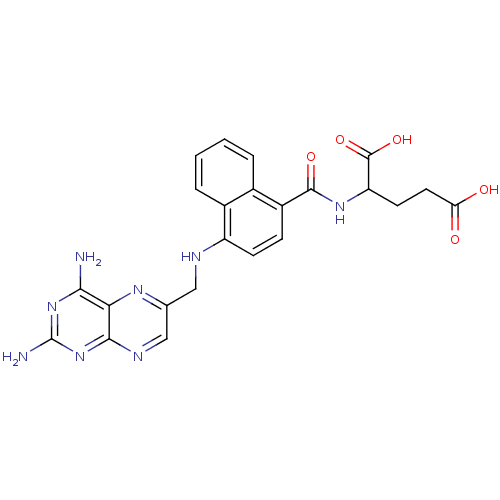 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043399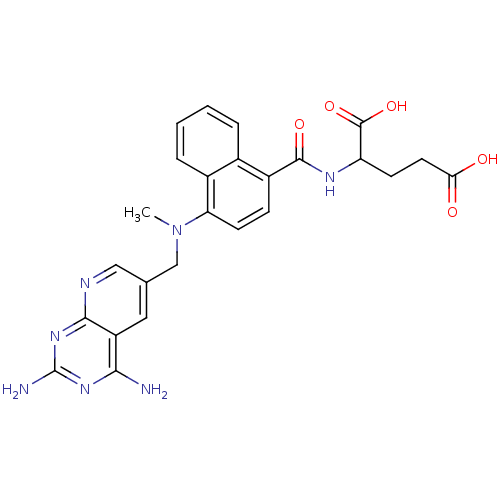 (2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50493144 (CHEMBL2419144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50493144 (CHEMBL2419144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting | Bioorg Med Chem 21: 6077-83 (2013) Article DOI: 10.1016/j.bmc.2013.07.005 BindingDB Entry DOI: 10.7270/Q2CC13NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043395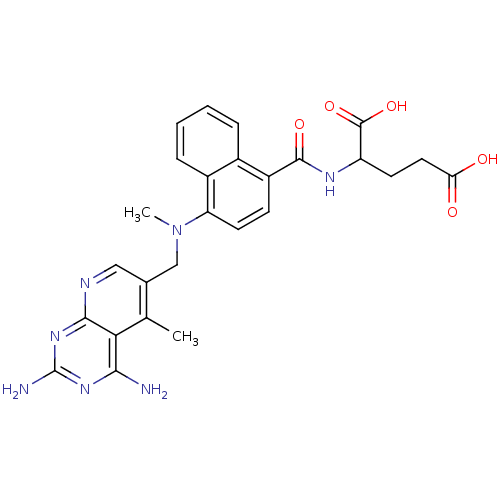 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50430060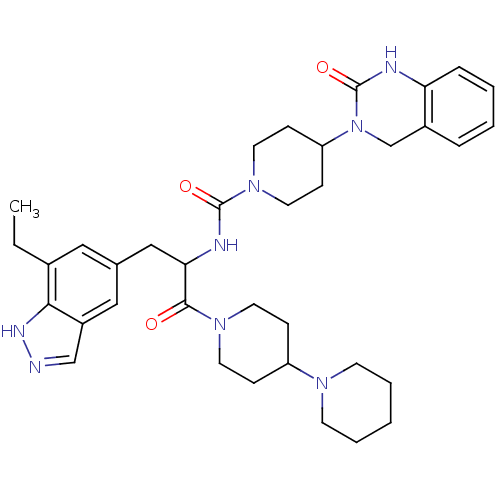 (CHEMBL2336421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | <0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | <0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50552678 (CHEMBL4759036) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00518 BindingDB Entry DOI: 10.7270/Q26H4NG9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112659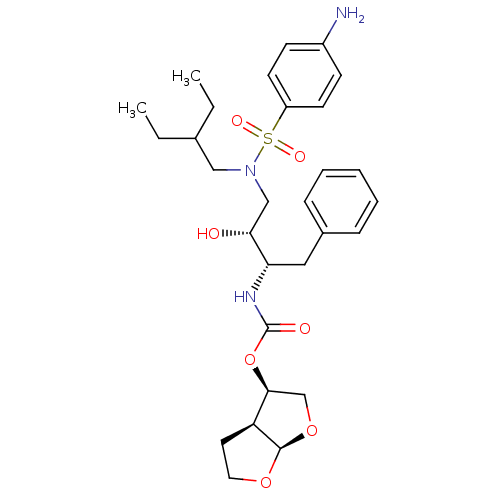 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043400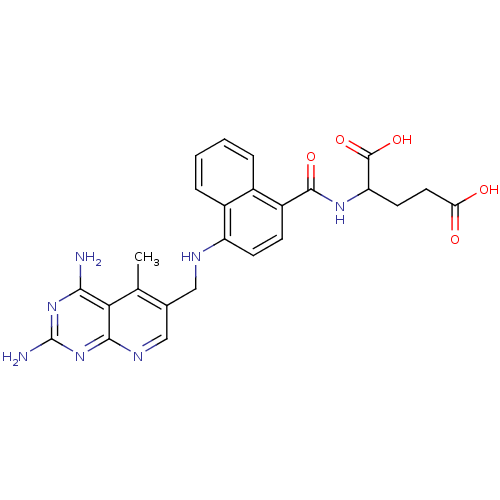 (2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043398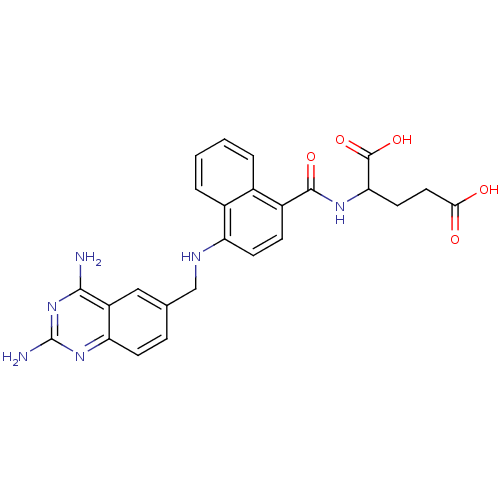 (2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50043394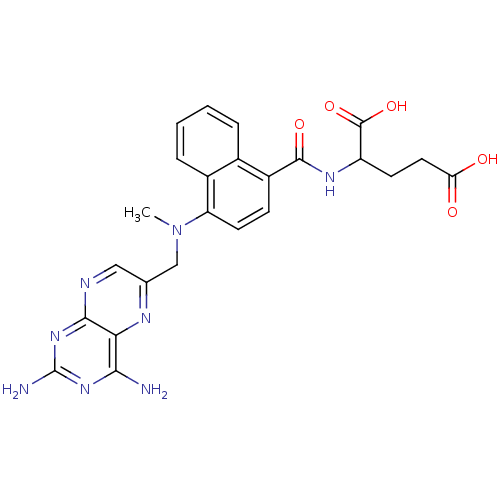 (2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase in L1210 cells | J Med Chem 36: 4161-71 (1994) BindingDB Entry DOI: 10.7270/Q2BC3XNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00528 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against Dihydrofolate reductase derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008293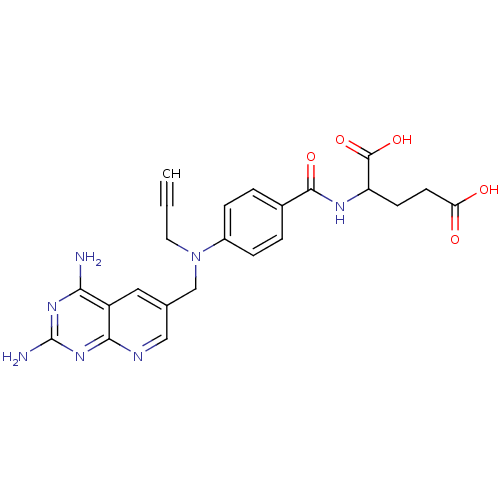 (2-{4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against dihydrofolate reductase(DHFR) derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112654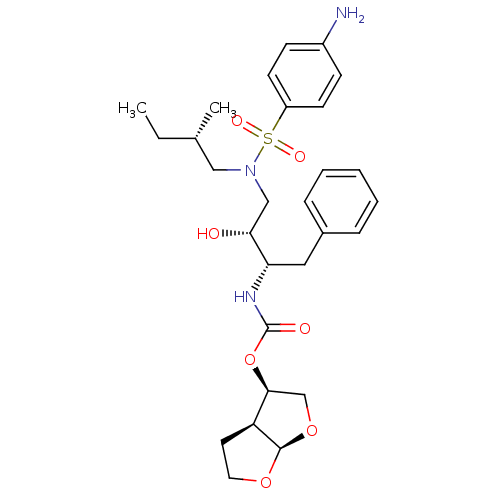 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50601724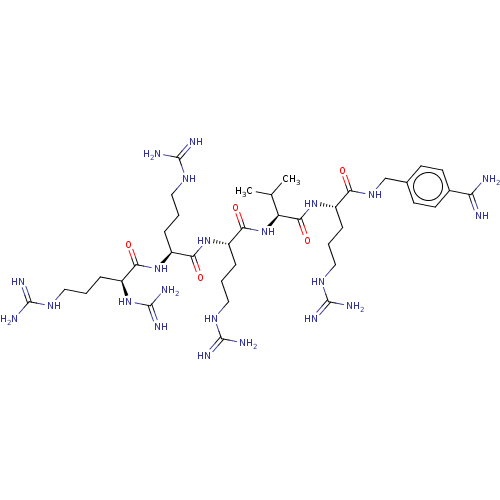 (CHEMBL5190391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00518 BindingDB Entry DOI: 10.7270/Q26H4NG9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM602107 (US11643417, Ex. No. 120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49VQ0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008292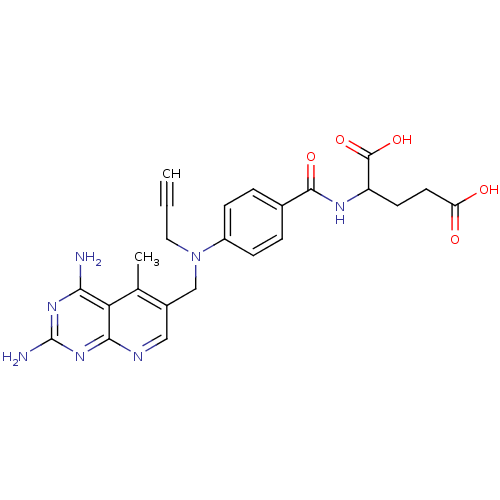 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description The compound was tested for its inhibitory activity against dihydrofolate reductase(DHFR) derived from L1210 cells. | J Med Chem 35: 332-7 (1992) BindingDB Entry DOI: 10.7270/Q2N58KBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50273292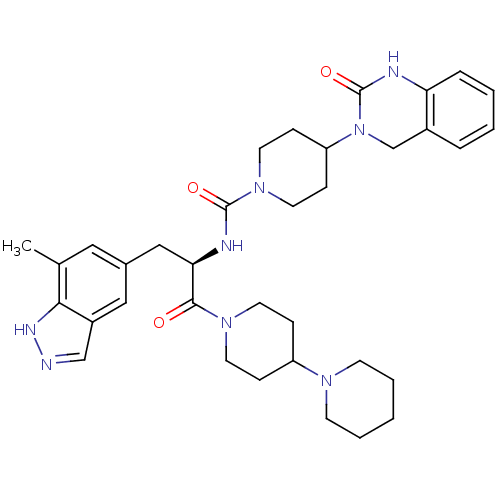 ((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Binding affinity to CGRP receptor (unknown origin) | Bioorg Med Chem Lett 23: 1870-3 (2013) Article DOI: 10.1016/j.bmcl.2013.01.011 BindingDB Entry DOI: 10.7270/Q2WD41XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156455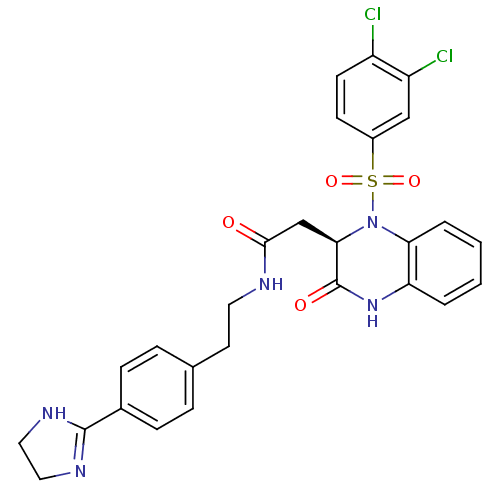 ((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human BK1 receptor E273 mutant | Bioorg Med Chem Lett 16: 2791-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.112 BindingDB Entry DOI: 10.7270/Q28915GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Furin (Homo sapiens (Human)) | BDBM50601696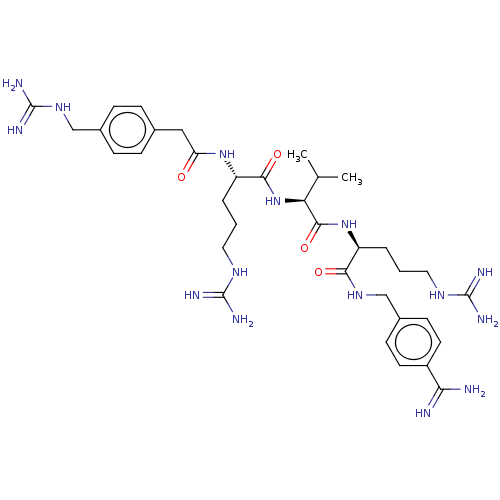 (CHEMBL5189844) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00518 BindingDB Entry DOI: 10.7270/Q26H4NG9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79923 total ) | Next | Last >> |