

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50219206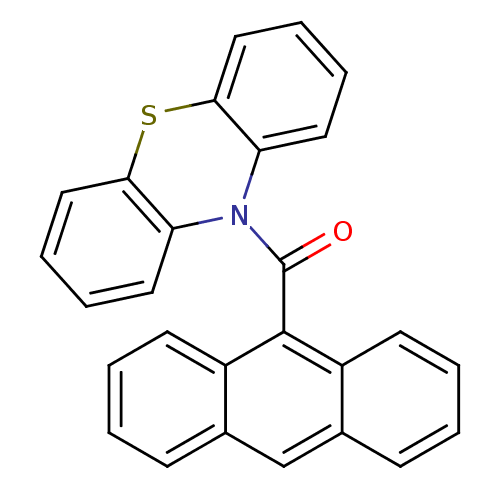 (Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50308418 (CHEMBL605824 | N-[2-(N',N'-diisopropylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50292603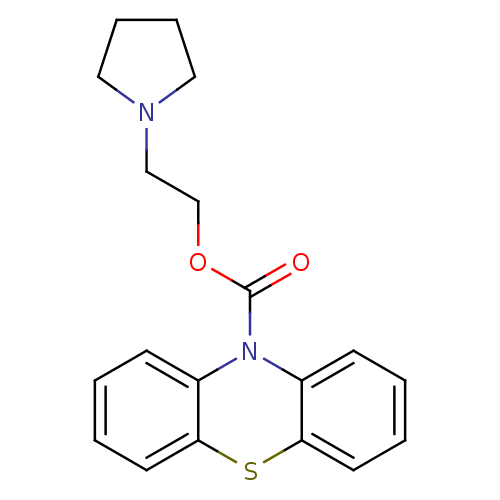 (2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50292604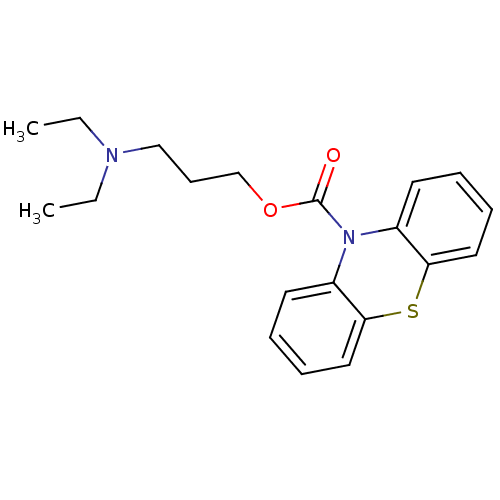 (3-(diethylamino)propyl 10H-phenothiazine-10-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at histamine H1 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50292603 (2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM120262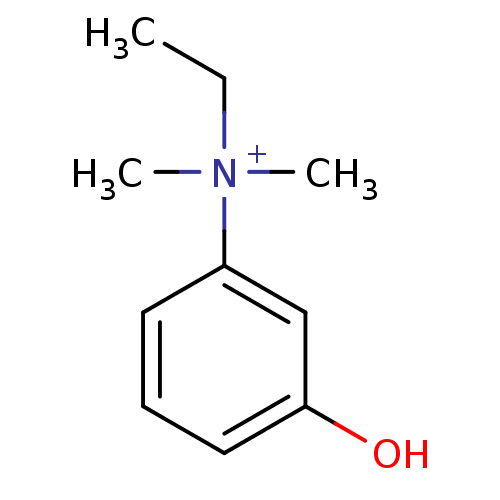 (EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50292604 (3-(diethylamino)propyl 10H-phenothiazine-10-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308418 (CHEMBL605824 | N-[2-(N',N'-diisopropylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50292603 (2-(pyrrolidin-1-yl)ethyl 10H-phenothiazine-10-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at serotonin 5HT3 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31904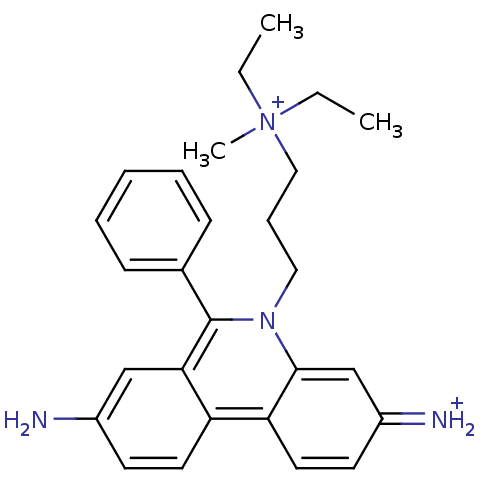 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50100134 (2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50292604 (3-(diethylamino)propyl 10H-phenothiazine-10-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at serotonin 5HT3 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50100134 (2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50219223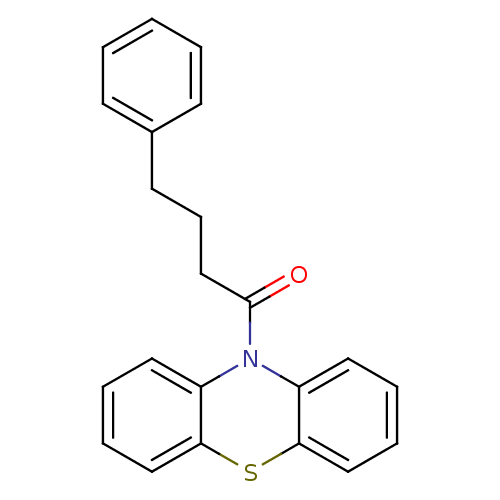 (1-(10H-phenothiazin-10-yl)-4-phenylbutan-1-one | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University Curated by ChEMBL | Assay Description Antagonist activity at histamine H2 receptor (unknown origin) by PDSP assay | Bioorg Med Chem Lett 23: 3822-5 (2013) Article DOI: 10.1016/j.bmcl.2013.04.082 BindingDB Entry DOI: 10.7270/Q2H133DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31904 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM120262 (EDROPHONIUM BROMIDE | EDROPHONIUM CHLORIDE | Edrop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University | Assay Description Inhibition constant using AChE or BuChE. | Biochemistry 51: 7046-53 (2012) Article DOI: 10.1021/bi300955k BindingDB Entry DOI: 10.7270/Q2J101R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283145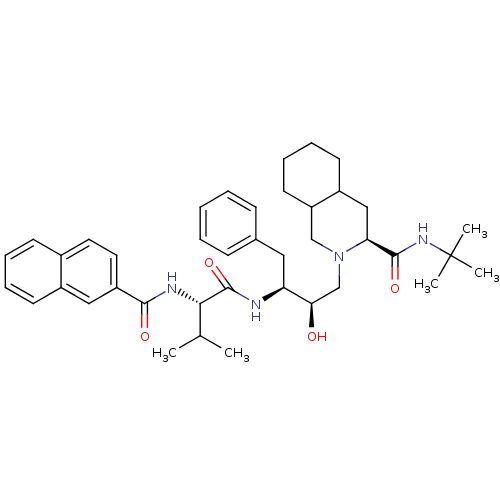 ((4S,4aS,5S)-2-((2R,3S)-2-Hydroxy-3-{3-methyl-2-[(n...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308469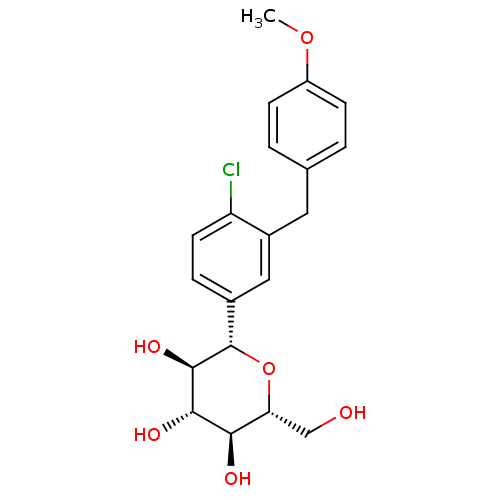 ((2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-methoxybenzyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283146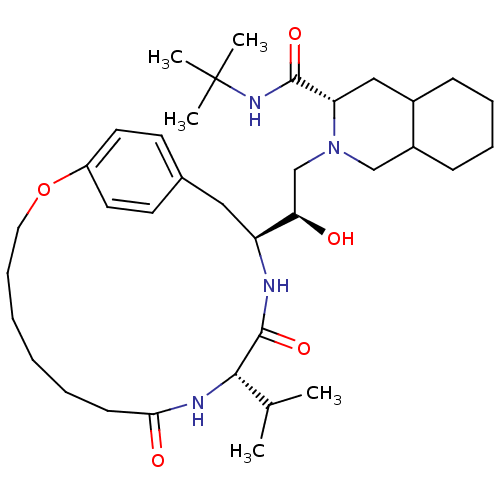 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-4-((S)-4-hydroxy-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005701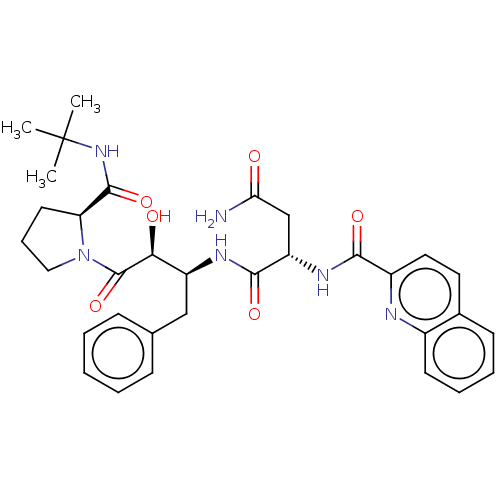 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 435-438 (1996) Article DOI: 10.1016/0960-894X(96)00034-0 BindingDB Entry DOI: 10.7270/Q2W095X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM20880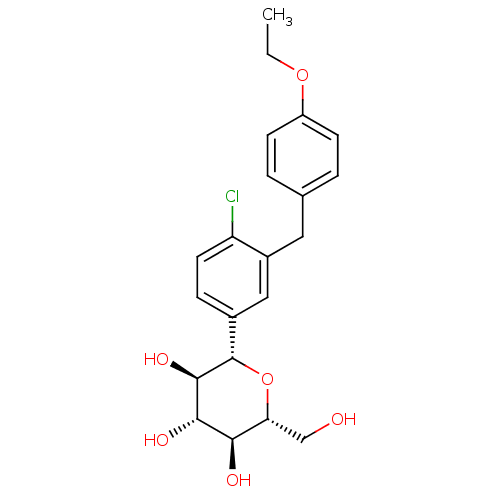 ((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313364 ((2S,3R,4R,5S,6R)-2-(3-(4-ethylbenzyl)-4-methylphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283142 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,10S)-10-isopro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313371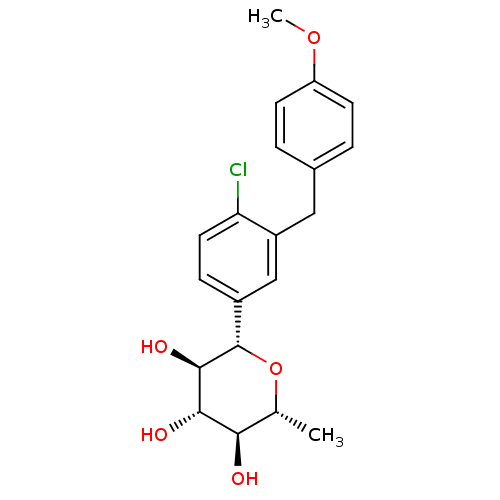 ((2S,3R,4S,5S,6R)-2-(4-chloro-3-(4-methoxybenzyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283143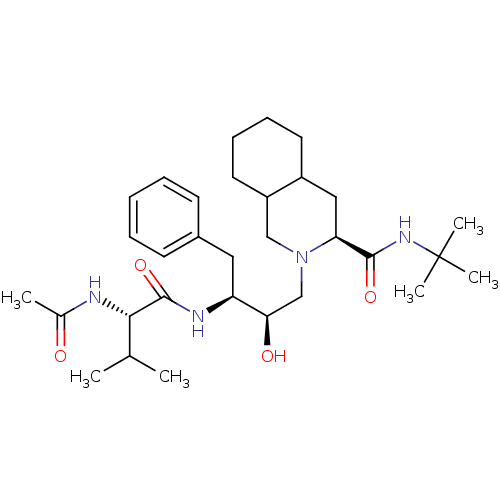 ((4S,4aS,5S)-2-{(2R,3S)-3-[2-((S)-Acetylamino)-3-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313374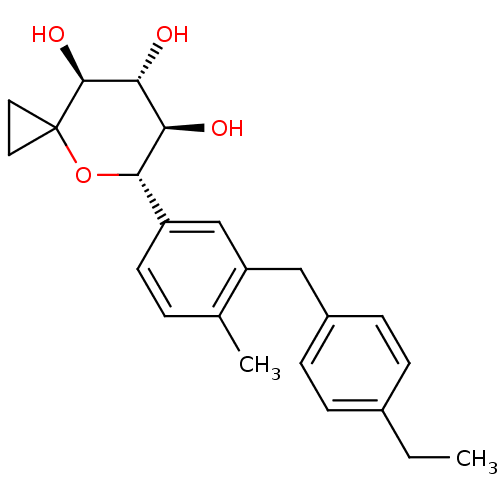 ((5S,6R,7R,8S)-5-(3-(4-ethylbenzyl)-4-methylphenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283147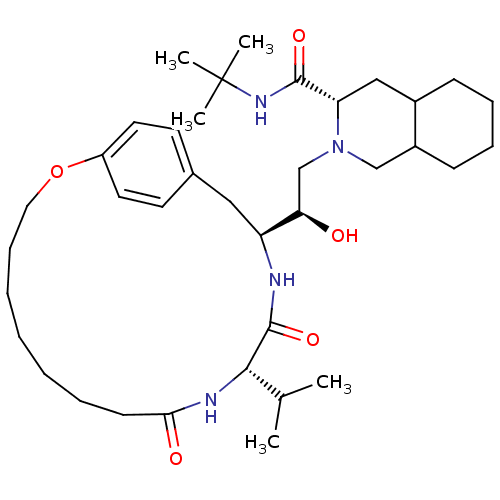 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,12S)-12-isopro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313367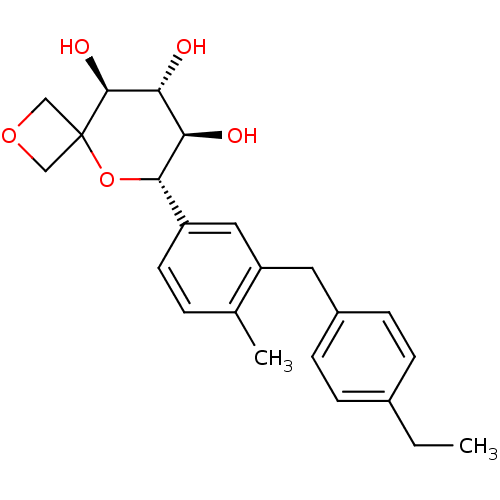 ((6S,7R,8R,9S)-6-(3-(4-ethylbenzyl)-4-methylphenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313384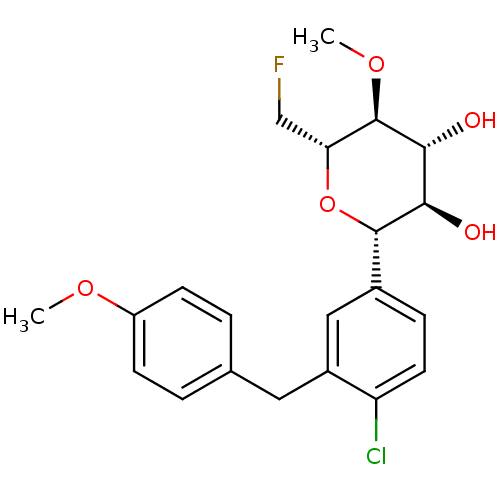 ((2S,3R,4R,5S,6S)-2-(4-chloro-3-(4-methoxybenzyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283154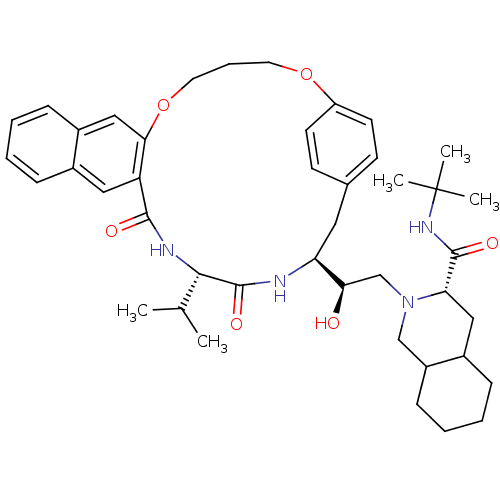 (3N-(tert-butyl)-2-{2-hydroxy-2-[19-isopropyl-17,20...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283155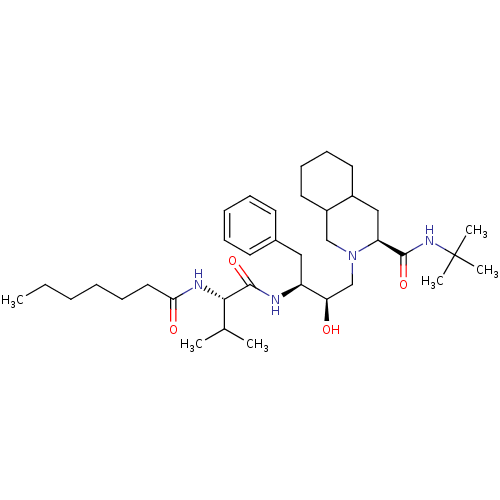 ((4S,4aS,5S)-2-{(2R,3S)-3-[2-((S)-Heptanoylamino)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313368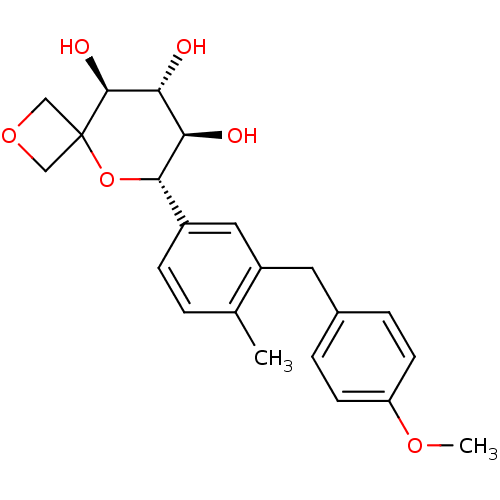 ((6S,7R,8R,9S)-6-(3-(4-methoxybenzyl)-4-methylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283156 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((Z)-(1S,10S)-10-is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313375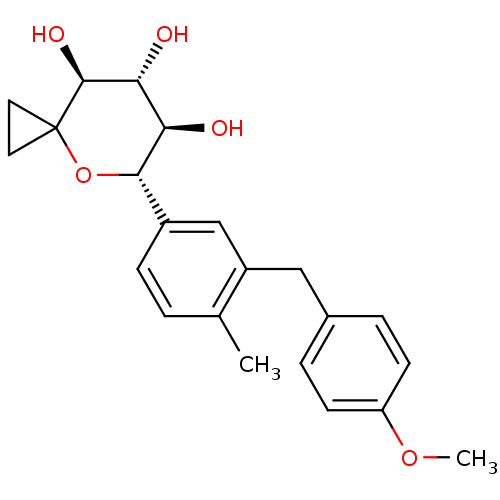 ((5S,6R,7R,8S)-5-(3-(4-methoxybenzyl)-4-methylpheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283158 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,15S)-15-isopro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283148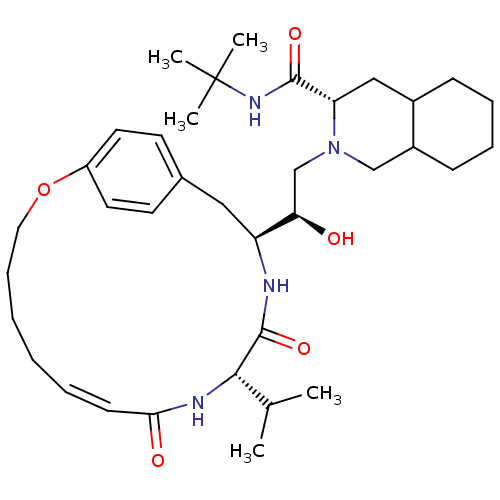 ((6S,7S,8aS)-2-{(R)-2-Hydroxy-4-((S)-4-hydroxy-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity of compound against HIV protease from BRU (IIIB) strain of HIV- 1 virus was determined | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283151 (2-[22-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283157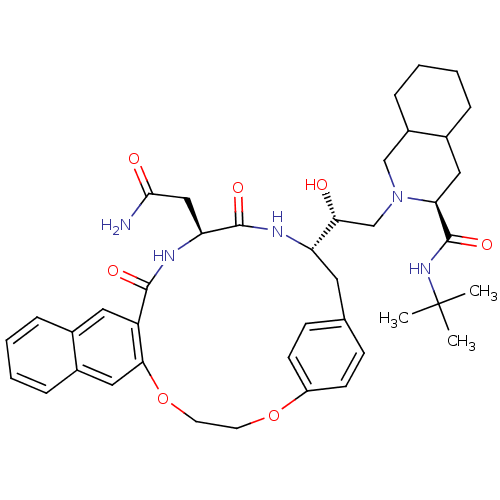 (2-[21-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313377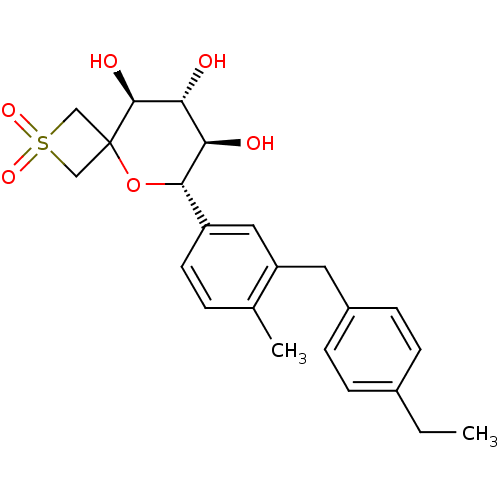 ((6S,7R,8R,9S)-6-[3-(4-Ethyl-benzyl)-4-methyl-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288787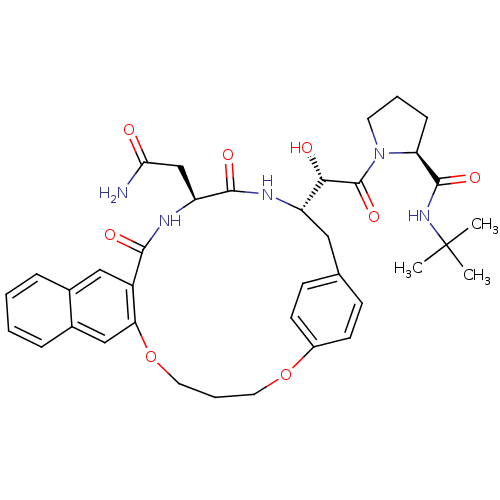 (CHEMBL137282 | Macrocyclic Norstatine derivative) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 435-438 (1996) Article DOI: 10.1016/0960-894X(96)00034-0 BindingDB Entry DOI: 10.7270/Q2W095X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313381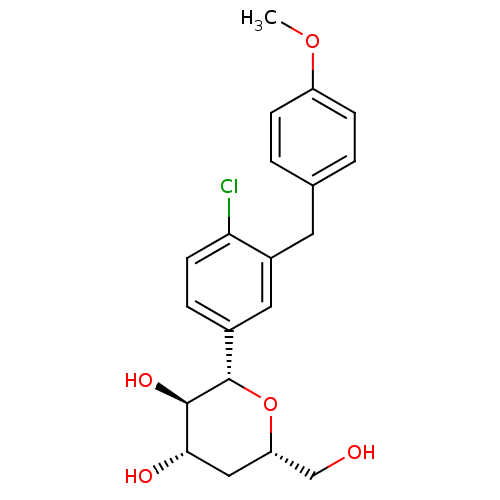 ((2S,3R,4S,6S)-2-(4-chloro-3-(4-methoxybenzyl)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288786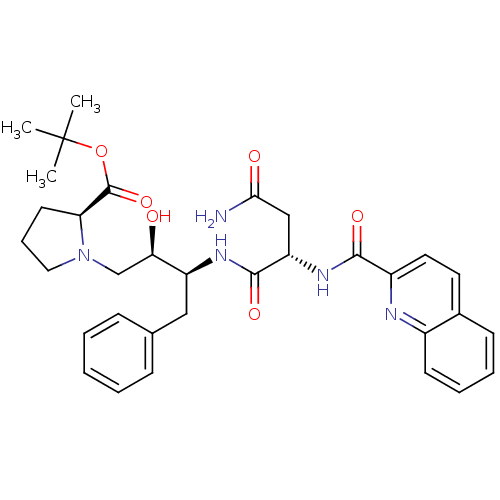 ((S)-1-((2R,3S)-3-{(S)-3-Carbamoyl-2-[(quinoline-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of HIV protease | Bioorg Med Chem Lett 6: 435-438 (1996) Article DOI: 10.1016/0960-894X(96)00034-0 BindingDB Entry DOI: 10.7270/Q2W095X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313369 ((6S,7R,8R,9S)-6-(4-chloro-3-(4-methoxybenzyl)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283144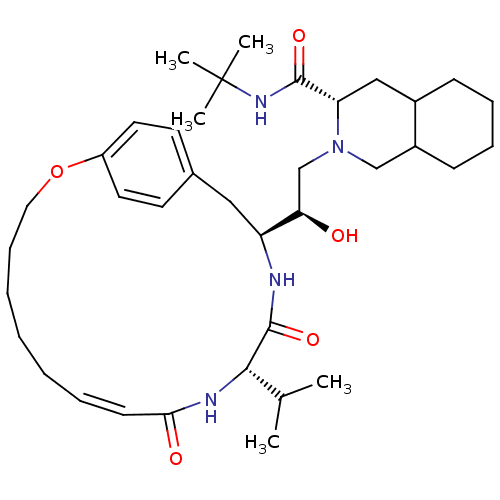 ((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((Z)-(1S,12S)-12-is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313370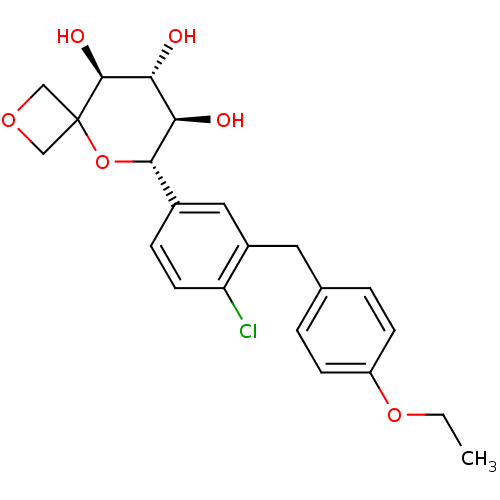 ((6S,7R,8R,9S)-6-(4-chloro-3-(4-ethoxybenzyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313373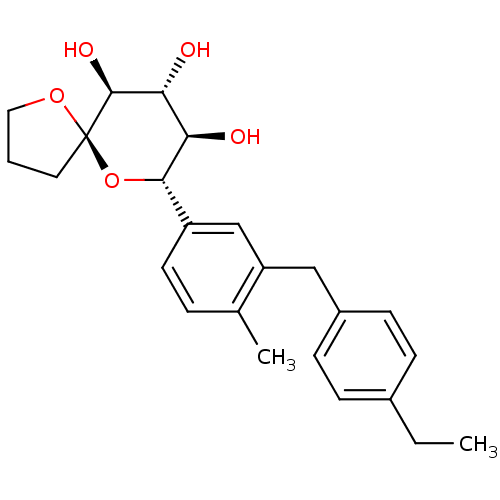 ((5R,7S,8R,9R,10S)-7-(3-(4-ethylbenzyl)-4-methylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50313372 ((3S,4R,5R,6S)-6-(4-chloro-3-(4-methoxybenzyl)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50313371 ((2S,3R,4S,5S,6R)-2-(4-chloro-3-(4-methoxybenzyl)ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of sodium-dependent methyl-alpha-D-[U-14C]glucopyranoside uptake after 2 hrs | Bioorg Med Chem Lett 20: 1569-72 (2010) Article DOI: 10.1016/j.bmcl.2010.01.075 BindingDB Entry DOI: 10.7270/Q2DF6RBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283153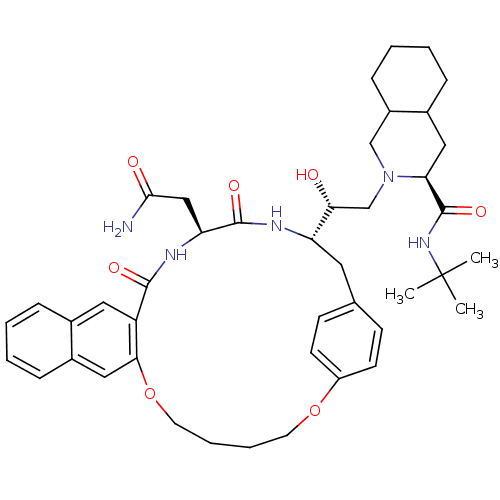 (2-[23-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition HIV-1 IIIB protease. | Bioorg Med Chem Lett 4: 2217-2222 (1994) Article DOI: 10.1016/S0960-894X(00)80074-8 BindingDB Entry DOI: 10.7270/Q2PG1RP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |