 Found 553 hits with Last Name = 'machius' and Initial = 'm'
Found 553 hits with Last Name = 'machius' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384584
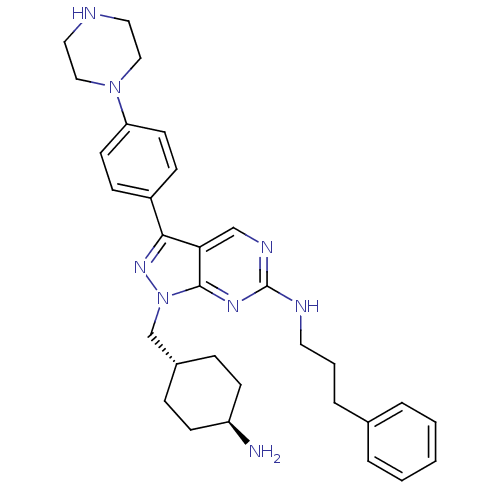
(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384583
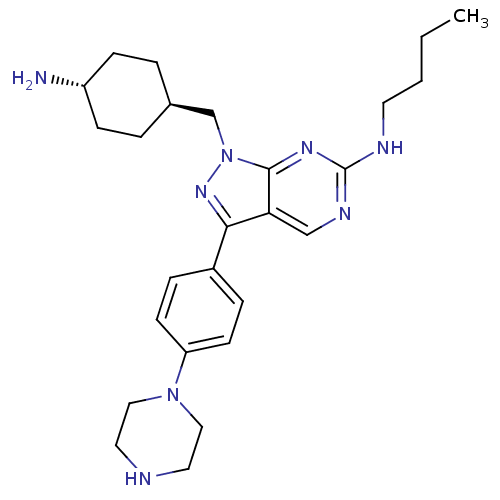
(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384582
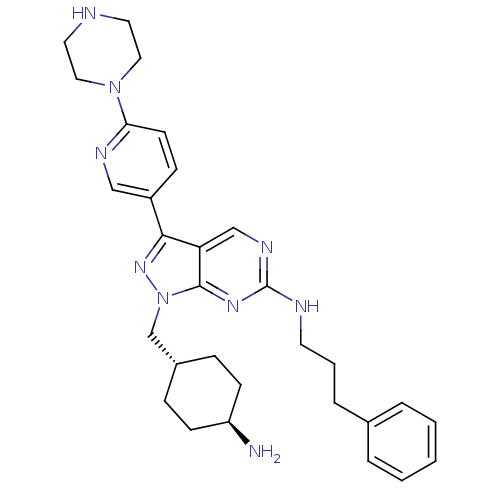
(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384585
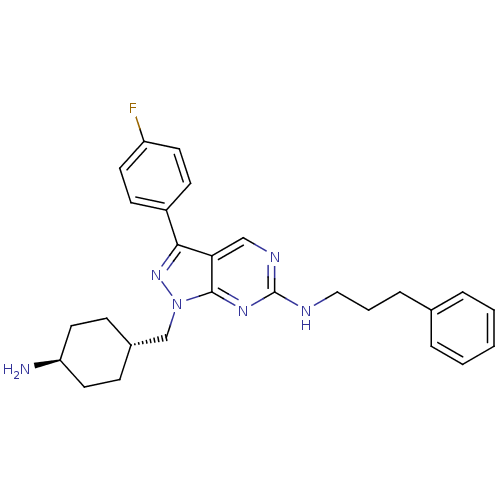
(CHEMBL2036809)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(F)cc3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(-.82,-49.25,;.22,-48.11,;1.72,-48.43,;2.76,-47.29,;2.28,-45.83,;3.31,-44.69,;2.84,-43.22,;3.75,-41.97,;2.84,-40.71,;3.31,-39.25,;4.82,-38.93,;5.3,-37.47,;4.27,-36.32,;4.74,-34.86,;2.75,-36.65,;2.28,-38.11,;1.36,-41.19,;.03,-40.43,;-1.3,-41.2,;-1.3,-42.74,;-2.64,-43.51,;-3.97,-42.74,;-3.97,-41.2,;-5.3,-40.43,;-5.3,-38.89,;-3.96,-38.13,;-3.96,-36.59,;-5.29,-35.82,;-6.63,-36.59,;-6.63,-38.13,;.03,-43.51,;1.36,-42.74,;.78,-45.5,;-.25,-46.64,)| Show InChI InChI=1S/C27H31FN6/c28-22-12-10-21(11-13-22)25-24-17-31-27(30-16-4-7-19-5-2-1-3-6-19)32-26(24)34(33-25)18-20-8-14-23(29)15-9-20/h1-3,5-6,10-13,17,20,23H,4,7-9,14-16,18,29H2,(H,30,31,32)/t20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384581
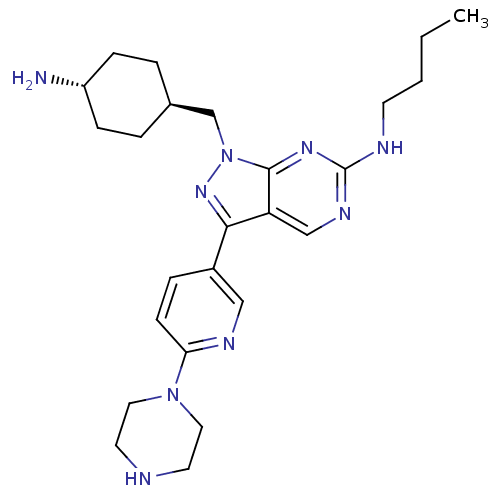
(CHEMBL2036804)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(nc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(-8.83,-18.78,;-8.83,-20.32,;-7.5,-21.09,;-7.5,-22.63,;-6.17,-23.41,;-4.83,-22.64,;-4.83,-21.09,;-3.5,-20.32,;-2.17,-21.08,;-.69,-20.61,;.22,-21.86,;-.69,-23.12,;-.22,-24.58,;-1.25,-25.72,;-.77,-27.18,;-1.81,-28.32,;-3.31,-28,;-4.35,-29.14,;-3.78,-26.53,;-2.76,-25.39,;-2.17,-22.64,;-3.5,-23.41,;-.22,-19.14,;-1.25,-18.01,;-.78,-16.54,;.73,-16.22,;1.77,-17.36,;1.29,-18.82,;1.2,-14.75,;2.71,-14.44,;3.19,-12.98,;2.16,-11.83,;.65,-12.15,;.17,-13.62,)| Show InChI InChI=1S/C25H37N9/c1-2-3-10-28-25-30-16-21-23(19-6-9-22(29-15-19)33-13-11-27-12-14-33)32-34(24(21)31-25)17-18-4-7-20(26)8-5-18/h6,9,15-16,18,20,27H,2-5,7-8,10-14,17,26H2,1H3,(H,28,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384584

(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444070
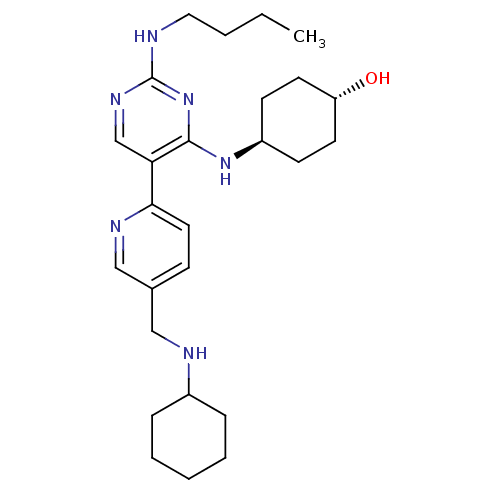
(CHEMBL3092795)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CNC2CCCCC2)cn1 |r,wU:11.10,wD:14.14,(-.85,-56.03,;.49,-55.27,;1.83,-56.04,;3.16,-55.27,;4.49,-56.04,;5.83,-55.27,;7.16,-56.04,;8.5,-55.27,;8.49,-53.72,;7.16,-52.95,;7.15,-51.42,;5.82,-50.65,;4.49,-51.42,;3.16,-50.65,;3.15,-49.1,;1.82,-48.33,;4.49,-48.34,;5.82,-49.1,;5.83,-53.73,;9.83,-52.94,;11.16,-53.71,;12.49,-52.94,;12.48,-51.4,;13.81,-50.62,;15.15,-51.39,;16.48,-50.61,;17.81,-51.38,;19.14,-50.61,;19.14,-49.07,;17.8,-48.3,;16.46,-49.08,;11.14,-50.63,;9.81,-51.41,)| Show InChI InChI=1S/C26H40N6O/c1-2-3-15-27-26-30-18-23(25(32-26)31-21-10-12-22(33)13-11-21)24-14-9-19(17-29-24)16-28-20-7-5-4-6-8-20/h9,14,17-18,20-22,28,33H,2-8,10-13,15-16H2,1H3,(H2,27,30,31,32)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444072
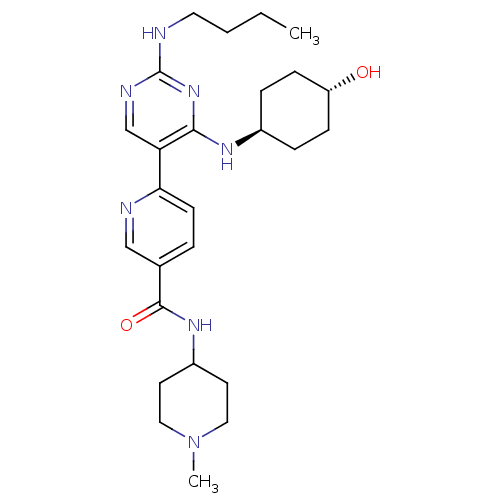
(CHEMBL3092793)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(cn1)C(=O)NC1CCN(C)CC1 |r,wU:11.10,wD:14.14,(42.31,-47.3,;43.64,-46.53,;44.98,-47.3,;46.31,-46.53,;47.64,-47.3,;48.98,-46.53,;50.31,-47.3,;51.65,-46.53,;51.65,-44.98,;50.31,-44.22,;50.31,-42.68,;48.97,-41.91,;47.64,-42.68,;46.31,-41.91,;46.31,-40.36,;44.97,-39.59,;47.64,-39.6,;48.97,-40.36,;48.98,-44.99,;52.98,-44.2,;54.31,-44.98,;55.64,-44.2,;55.63,-42.66,;54.29,-41.9,;52.96,-42.67,;56.96,-41.88,;56.95,-40.34,;58.3,-42.65,;59.63,-41.87,;60.96,-42.64,;62.29,-41.87,;62.29,-40.33,;63.62,-39.56,;60.95,-39.56,;59.61,-40.34,)| Show InChI InChI=1S/C26H39N7O2/c1-3-4-13-27-26-29-17-22(24(32-26)30-19-6-8-21(34)9-7-19)23-10-5-18(16-28-23)25(35)31-20-11-14-33(2)15-12-20/h5,10,16-17,19-21,34H,3-4,6-9,11-15H2,1-2H3,(H,31,35)(H2,27,29,30,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444073
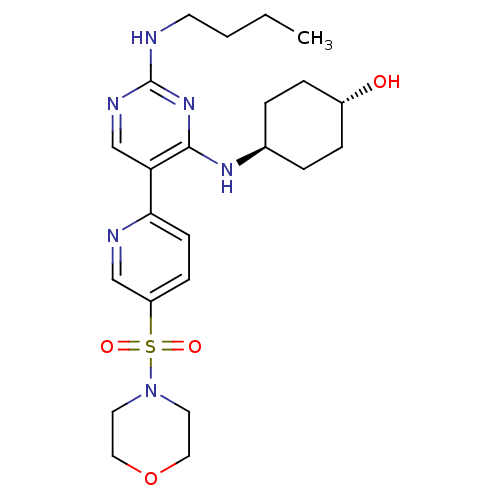
(CHEMBL3092792)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(cn1)S(=O)(=O)N1CCOCC1 |r,wU:11.10,wD:14.14,(17.06,-43.23,;18.4,-42.47,;19.73,-43.24,;21.06,-42.47,;22.4,-43.24,;23.73,-42.47,;25.07,-43.24,;26.4,-42.47,;26.4,-40.92,;25.06,-40.15,;25.06,-38.61,;23.72,-37.85,;22.4,-38.62,;21.06,-37.85,;21.06,-36.3,;19.73,-35.53,;22.39,-35.54,;23.72,-36.3,;23.73,-40.93,;27.73,-40.14,;29.06,-40.91,;30.39,-40.14,;30.39,-38.6,;29.04,-37.83,;27.72,-38.61,;31.72,-37.82,;32.48,-36.48,;30.94,-36.49,;33.05,-38.59,;33.05,-40.13,;34.38,-40.89,;35.71,-40.12,;35.71,-38.58,;34.37,-37.81,)| Show InChI InChI=1S/C23H34N6O4S/c1-2-3-10-24-23-26-16-20(22(28-23)27-17-4-6-18(30)7-5-17)21-9-8-19(15-25-21)34(31,32)29-11-13-33-14-12-29/h8-9,15-18,30H,2-7,10-14H2,1H3,(H2,24,26,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384582

(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201078
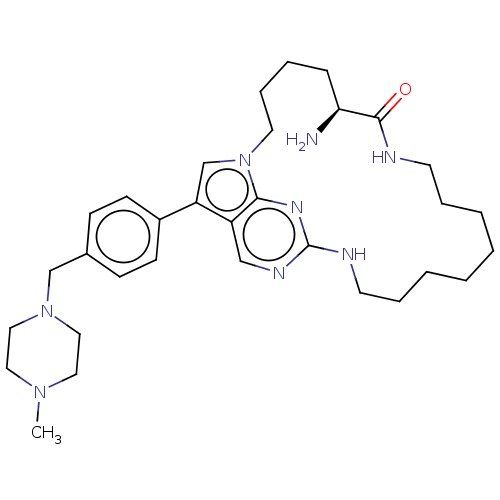
(CHEMBL3964573)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC[C@H](N)C(=O)NCCCCCCCCNc4ncc2c3n4)CC1 |r| Show InChI InChI=1S/C32H48N8O/c1-38-18-20-39(21-19-38)23-25-11-13-26(14-12-25)28-24-40-17-9-6-10-29(33)31(41)34-15-7-4-2-3-5-8-16-35-32-36-22-27(28)30(40)37-32/h11-14,22,24,29H,2-10,15-21,23,33H2,1H3,(H,34,41)(H,35,36,37)/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444068
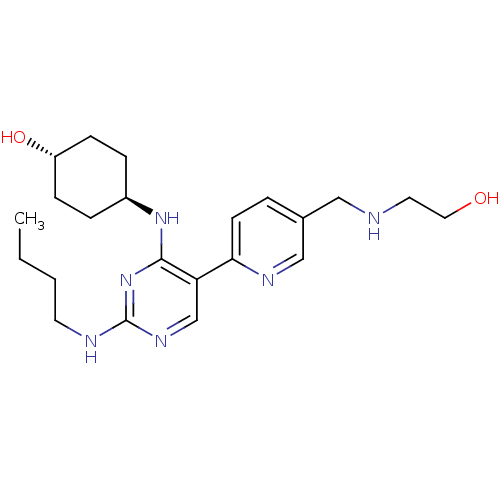
(CHEMBL3092797)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CNCCO)cn1 |r,wU:11.10,wD:14.14,(43.3,-58.78,;44.63,-58.01,;45.97,-58.78,;47.3,-58.01,;48.63,-58.78,;49.97,-58.01,;51.3,-58.78,;52.64,-58.01,;52.63,-56.46,;51.3,-55.7,;51.29,-54.16,;49.96,-53.39,;48.63,-54.16,;47.29,-53.39,;47.29,-51.84,;45.96,-51.07,;48.63,-51.08,;49.96,-51.84,;49.97,-56.47,;53.96,-55.68,;55.3,-56.46,;56.63,-55.68,;56.62,-54.14,;57.95,-53.36,;59.29,-54.13,;60.62,-53.35,;61.96,-54.11,;63.28,-53.34,;55.27,-53.38,;53.95,-54.15,)| Show InChI InChI=1S/C22H34N6O2/c1-2-3-10-24-22-26-15-19(20-9-4-16(14-25-20)13-23-11-12-29)21(28-22)27-17-5-7-18(30)8-6-17/h4,9,14-15,17-18,23,29-30H,2-3,5-8,10-13H2,1H3,(H2,24,26,27,28)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444075
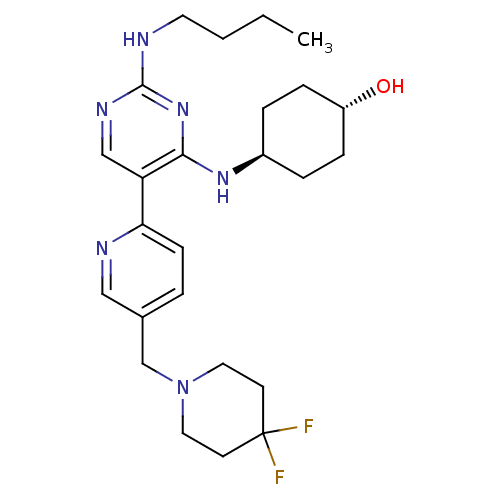
(CHEMBL3092790)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CN2CCC(F)(F)CC2)cn1 |r,wU:11.10,wD:14.14,(52.92,-33.3,;54.26,-32.53,;55.59,-33.3,;56.93,-32.54,;58.26,-33.31,;59.59,-32.54,;60.93,-33.31,;62.26,-32.54,;62.26,-30.99,;60.92,-30.22,;60.92,-28.68,;59.58,-27.92,;58.26,-28.69,;56.92,-27.91,;56.92,-26.37,;55.59,-25.6,;58.26,-25.6,;59.58,-26.37,;59.6,-30.99,;63.59,-30.21,;64.92,-30.98,;66.25,-30.21,;66.25,-28.67,;67.58,-27.89,;68.91,-28.65,;68.91,-30.19,;70.24,-30.96,;71.58,-30.19,;72.35,-31.52,;73.12,-30.18,;71.57,-28.64,;70.23,-27.87,;64.9,-27.9,;63.58,-28.68,)| Show InChI InChI=1S/C25H36F2N6O/c1-2-3-12-28-24-30-16-21(23(32-24)31-19-5-7-20(34)8-6-19)22-9-4-18(15-29-22)17-33-13-10-25(26,27)11-14-33/h4,9,15-16,19-20,34H,2-3,5-8,10-14,17H2,1H3,(H2,28,30,31,32)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444069
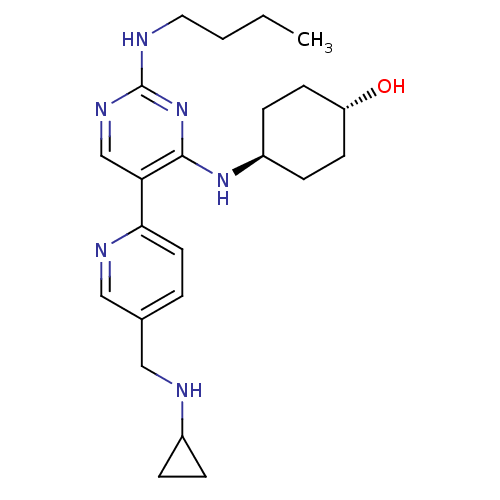
(CHEMBL3092796)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CNC2CC2)cn1 |r,wU:11.10,wD:14.14,(20.96,-58.36,;22.3,-57.6,;23.63,-58.37,;24.96,-57.6,;26.3,-58.37,;27.63,-57.6,;28.96,-58.37,;30.3,-57.6,;30.3,-56.05,;28.96,-55.28,;28.96,-53.74,;27.62,-52.98,;26.29,-53.75,;24.96,-52.98,;24.96,-51.43,;23.62,-50.66,;26.29,-50.67,;27.62,-51.43,;27.63,-56.06,;31.63,-55.27,;32.96,-56.04,;34.29,-55.27,;34.28,-53.73,;35.61,-52.95,;36.95,-53.72,;38.28,-52.94,;39.81,-52.93,;39.04,-51.6,;32.94,-52.96,;31.61,-53.74,)| Show InChI InChI=1S/C23H34N6O/c1-2-3-12-24-23-27-15-20(22(29-23)28-18-7-9-19(30)10-8-18)21-11-4-16(14-26-21)13-25-17-5-6-17/h4,11,14-15,17-19,25,30H,2-3,5-10,12-13H2,1H3,(H2,24,27,28,29)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384584

(CHEMBL2036807 | US9744172, Compound UNC607A)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(cc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(41.81,-28.1,;42.85,-26.96,;44.35,-27.28,;45.39,-26.14,;44.91,-24.68,;45.94,-23.54,;45.47,-22.07,;46.38,-20.82,;45.47,-19.56,;45.94,-18.1,;47.45,-17.78,;47.93,-16.32,;46.9,-15.17,;45.38,-15.5,;44.91,-16.96,;47.36,-13.71,;48.87,-13.39,;49.35,-11.94,;48.32,-10.79,;46.81,-11.11,;46.33,-12.57,;43.99,-20.04,;42.66,-19.28,;41.33,-20.05,;41.33,-21.6,;39.99,-22.36,;38.66,-21.59,;38.66,-20.05,;37.33,-19.28,;37.33,-17.74,;38.67,-16.98,;38.67,-15.44,;37.34,-14.67,;36,-15.45,;36,-16.98,;42.66,-22.37,;43.99,-21.6,;43.41,-24.35,;42.38,-25.49,)| Show InChI InChI=1S/C31H40N8/c32-26-12-8-24(9-13-26)22-39-30-28(21-35-31(36-30)34-16-4-7-23-5-2-1-3-6-23)29(37-39)25-10-14-27(15-11-25)38-19-17-33-18-20-38/h1-3,5-6,10-11,14-15,21,24,26,33H,4,7-9,12-13,16-20,22,32H2,(H,34,35,36)/t24-,26- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444074
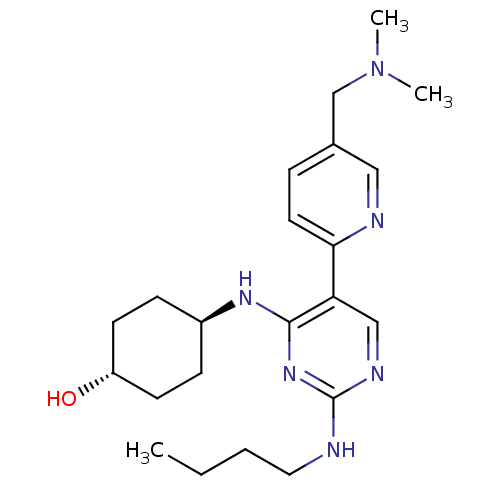
(CHEMBL3092791)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CN(C)C)cn1 |r,wU:11.10,wD:14.14,(-4.06,-43.37,;-2.72,-42.6,;-1.39,-43.37,;-.05,-42.6,;1.29,-43.37,;2.62,-42.6,;3.96,-43.37,;5.29,-42.6,;5.29,-41.05,;3.95,-40.29,;3.95,-38.75,;2.61,-37.98,;1.29,-38.75,;-.06,-37.98,;-.06,-36.44,;-1.39,-35.67,;1.29,-35.67,;2.61,-36.44,;2.63,-41.06,;6.62,-40.28,;7.95,-41.05,;9.28,-40.27,;9.27,-38.73,;10.6,-37.96,;11.94,-38.72,;13.26,-37.94,;11.95,-40.26,;7.93,-37.97,;6.6,-38.75,)| Show InChI InChI=1S/C22H34N6O/c1-4-5-12-23-22-25-14-19(20-11-6-16(13-24-20)15-28(2)3)21(27-22)26-17-7-9-18(29)10-8-17/h6,11,13-14,17-18,29H,4-5,7-10,12,15H2,1-3H3,(H2,23,25,26,27)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444042
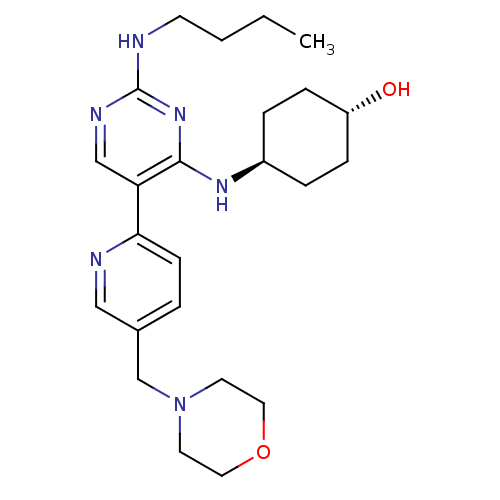
(CHEMBL3092807)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CN2CCOCC2)cn1 |r,wU:11.10,wD:14.14,(58.28,-22.87,;59.62,-22.1,;60.95,-22.87,;62.28,-22.11,;63.62,-22.88,;64.95,-22.11,;66.29,-22.88,;67.62,-22.11,;67.62,-20.56,;66.28,-19.79,;66.28,-18.25,;64.94,-17.49,;63.62,-18.26,;62.28,-17.48,;62.28,-15.94,;60.94,-15.17,;63.61,-15.17,;64.94,-15.94,;64.95,-20.56,;68.95,-19.78,;70.28,-20.55,;71.61,-19.78,;71.6,-18.24,;72.93,-17.46,;74.27,-18.22,;74.27,-19.76,;75.6,-20.53,;76.93,-19.76,;76.93,-18.21,;75.59,-17.44,;70.26,-17.47,;68.93,-18.25,)| Show InChI InChI=1S/C24H36N6O2/c1-2-3-10-25-24-27-16-21(23(29-24)28-19-5-7-20(31)8-6-19)22-9-4-18(15-26-22)17-30-11-13-32-14-12-30/h4,9,15-16,19-20,31H,2-3,5-8,10-14,17H2,1H3,(H2,25,27,28,29)/t19-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384581

(CHEMBL2036804)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(nc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(-8.83,-18.78,;-8.83,-20.32,;-7.5,-21.09,;-7.5,-22.63,;-6.17,-23.41,;-4.83,-22.64,;-4.83,-21.09,;-3.5,-20.32,;-2.17,-21.08,;-.69,-20.61,;.22,-21.86,;-.69,-23.12,;-.22,-24.58,;-1.25,-25.72,;-.77,-27.18,;-1.81,-28.32,;-3.31,-28,;-4.35,-29.14,;-3.78,-26.53,;-2.76,-25.39,;-2.17,-22.64,;-3.5,-23.41,;-.22,-19.14,;-1.25,-18.01,;-.78,-16.54,;.73,-16.22,;1.77,-17.36,;1.29,-18.82,;1.2,-14.75,;2.71,-14.44,;3.19,-12.98,;2.16,-11.83,;.65,-12.15,;.17,-13.62,)| Show InChI InChI=1S/C25H37N9/c1-2-3-10-28-25-30-16-21-23(19-6-9-22(29-15-19)33-13-11-27-12-14-33)32-34(24(21)31-25)17-18-4-7-20(26)8-5-18/h6,9,15-16,18,20,27H,2-5,7-8,10-14,17,26H2,1H3,(H,28,30,31)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444066

(CHEMBL3092799)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](N)CC2)n1)-c1ccccn1 |r,wU:11.10,wD:14.14,(15.98,-7.45,;17.32,-6.68,;18.65,-7.45,;19.98,-6.68,;21.32,-7.45,;22.65,-6.69,;23.98,-7.46,;25.32,-6.68,;25.32,-5.13,;23.98,-4.37,;23.98,-2.83,;22.64,-2.06,;21.31,-2.84,;19.98,-2.06,;19.98,-.52,;18.64,.26,;21.31,.25,;22.64,-.52,;22.65,-5.14,;26.65,-4.36,;27.98,-5.13,;29.31,-4.36,;29.3,-2.81,;27.96,-2.05,;26.64,-2.82,)| Show InChI InChI=1S/C19H28N6/c1-2-3-11-22-19-23-13-16(17-6-4-5-12-21-17)18(25-19)24-15-9-7-14(20)8-10-15/h4-6,12-15H,2-3,7-11,20H2,1H3,(H2,22,23,24,25)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201039
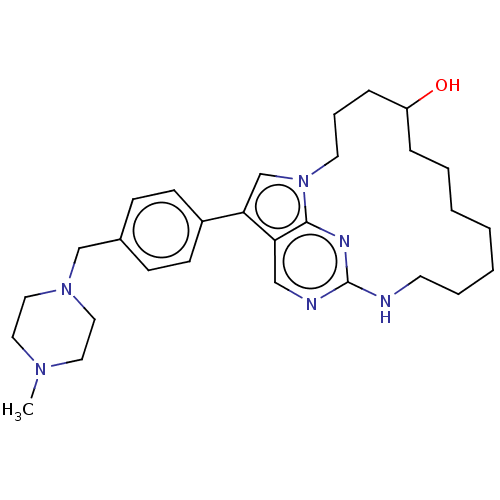
(CHEMBL3910520)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC(O)CCCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C29H42N6O/c1-33-16-18-34(19-17-33)21-23-10-12-24(13-11-23)27-22-35-15-7-9-25(36)8-5-3-2-4-6-14-30-29-31-20-26(27)28(35)32-29/h10-13,20,22,25,36H,2-9,14-19,21H2,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444240
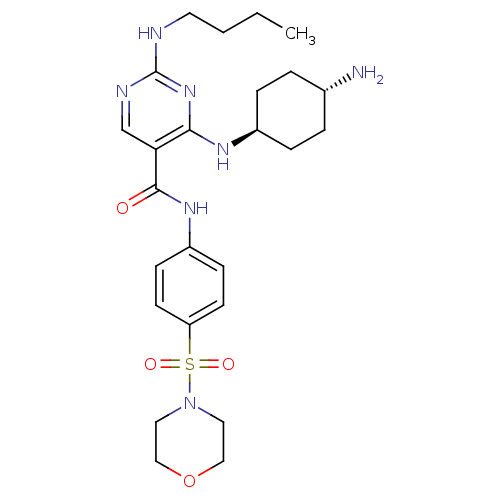
(CHEMBL3093635)Show SMILES CCCCNc1ncc(C(=O)Nc2ccc(cc2)S(=O)(=O)N2CCOCC2)c(N[C@H]2CC[C@H](N)CC2)n1 |r,wU:29.30,wD:32.34,(.04,-21.39,;1.37,-20.63,;2.71,-21.4,;4.04,-20.63,;5.37,-21.4,;6.71,-20.63,;8.04,-21.4,;9.38,-20.63,;9.37,-19.08,;10.7,-18.3,;10.7,-16.76,;12.04,-19.07,;13.37,-18.29,;14.71,-19.07,;16.03,-18.29,;16.03,-16.75,;14.68,-15.99,;13.36,-16.76,;17.36,-15.97,;16.58,-14.63,;18.13,-14.62,;18.7,-16.74,;18.69,-18.27,;20.02,-19.03,;21.36,-18.26,;21.35,-16.72,;20.01,-15.95,;8.04,-18.32,;8.03,-16.78,;6.7,-16.01,;5.36,-16.79,;4.03,-16.01,;4.03,-14.47,;2.69,-13.7,;5.36,-13.71,;6.69,-14.47,;6.71,-19.09,)| Show InChI InChI=1S/C25H37N7O4S/c1-2-3-12-27-25-28-17-22(23(31-25)29-19-6-4-18(26)5-7-19)24(33)30-20-8-10-21(11-9-20)37(34,35)32-13-15-36-16-14-32/h8-11,17-19H,2-7,12-16,26H2,1H3,(H,30,33)(H2,27,28,29,31)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9693-700 (2014)
Article DOI: 10.1021/jm4013888
BindingDB Entry DOI: 10.7270/Q21G0NQ1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201153
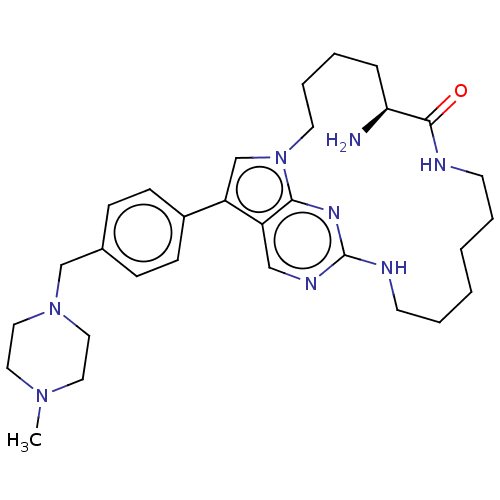
(CHEMBL3892515)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC[C@H](N)C(=O)NCCCCCCNc4ncc2c3n4)CC1 |r| Show InChI InChI=1S/C30H44N8O/c1-36-16-18-37(19-17-36)21-23-9-11-24(12-10-23)26-22-38-15-7-4-8-27(31)29(39)32-13-5-2-3-6-14-33-30-34-20-25(26)28(38)35-30/h9-12,20,22,27H,2-8,13-19,21,31H2,1H3,(H,32,39)(H,33,34,35)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50201078

(CHEMBL3964573)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC[C@H](N)C(=O)NCCCCCCCCNc4ncc2c3n4)CC1 |r| Show InChI InChI=1S/C32H48N8O/c1-38-18-20-39(21-19-38)23-25-11-13-26(14-12-25)28-24-40-17-9-6-10-29(33)31(41)34-15-7-4-2-3-5-8-16-35-32-36-22-27(28)30(40)37-32/h11-14,22,24,29H,2-10,15-21,23,33H2,1H3,(H,34,41)(H,35,36,37)/t29-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201077
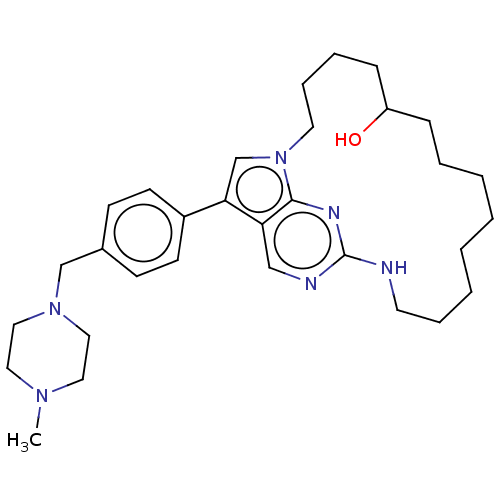
(CHEMBL3967759)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCC(O)CCCCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C31H46N6O/c1-35-18-20-36(21-19-35)23-25-12-14-26(15-13-25)29-24-37-17-9-7-11-27(38)10-6-4-2-3-5-8-16-32-31-33-22-28(29)30(37)34-31/h12-15,22,24,27,38H,2-11,16-21,23H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444080

(CHEMBL3092805)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(cn1)N1CCN(C)CC1 |r,wU:11.10,wD:14.14,(13.34,-23.32,;14.67,-22.55,;16,-23.32,;17.34,-22.56,;18.67,-23.33,;20.01,-22.56,;21.34,-23.33,;22.68,-22.56,;22.67,-21.01,;21.34,-20.24,;21.33,-18.7,;20,-17.94,;18.67,-18.71,;17.33,-17.93,;17.33,-16.39,;16,-15.62,;18.67,-15.62,;20,-16.39,;20.01,-21.01,;24,-20.23,;25.33,-21,;26.66,-20.23,;26.66,-18.69,;25.31,-17.92,;23.99,-18.7,;27.99,-17.91,;29.31,-18.68,;30.64,-17.91,;30.64,-16.37,;31.97,-15.6,;29.3,-15.6,;27.97,-16.37,)| Show InChI InChI=1S/C24H37N7O/c1-3-4-11-25-24-27-17-21(23(29-24)28-18-5-8-20(32)9-6-18)22-10-7-19(16-26-22)31-14-12-30(2)13-15-31/h7,10,16-18,20,32H,3-6,8-9,11-15H2,1-2H3,(H2,25,27,28,29)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Tyro3 using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384576

(CHEMBL2036808)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(F)cc1 |r,wU:13.12,wD:16.16,(54.06,-16.98,;54.06,-18.52,;55.39,-19.29,;55.39,-20.83,;56.72,-21.6,;58.06,-20.83,;58.06,-19.29,;59.39,-18.52,;60.72,-19.28,;62.2,-18.8,;63.11,-20.06,;62.2,-21.31,;62.67,-22.78,;61.64,-23.92,;62.12,-25.38,;61.08,-26.52,;59.58,-26.2,;58.54,-27.34,;59.11,-24.73,;60.14,-23.59,;60.72,-20.83,;59.39,-21.6,;62.67,-17.34,;64.18,-17.02,;64.66,-15.56,;63.63,-14.41,;64.1,-12.95,;62.11,-14.74,;61.64,-16.2,)| Show InChI InChI=1S/C22H29FN6/c1-2-3-12-25-22-26-13-19-20(16-6-8-17(23)9-7-16)28-29(21(19)27-22)14-15-4-10-18(24)11-5-15/h6-9,13,15,18H,2-5,10-12,14,24H2,1H3,(H,25,26,27)/t15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384585

(CHEMBL2036809)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(F)cc3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(-.82,-49.25,;.22,-48.11,;1.72,-48.43,;2.76,-47.29,;2.28,-45.83,;3.31,-44.69,;2.84,-43.22,;3.75,-41.97,;2.84,-40.71,;3.31,-39.25,;4.82,-38.93,;5.3,-37.47,;4.27,-36.32,;4.74,-34.86,;2.75,-36.65,;2.28,-38.11,;1.36,-41.19,;.03,-40.43,;-1.3,-41.2,;-1.3,-42.74,;-2.64,-43.51,;-3.97,-42.74,;-3.97,-41.2,;-5.3,-40.43,;-5.3,-38.89,;-3.96,-38.13,;-3.96,-36.59,;-5.29,-35.82,;-6.63,-36.59,;-6.63,-38.13,;.03,-43.51,;1.36,-42.74,;.78,-45.5,;-.25,-46.64,)| Show InChI InChI=1S/C27H31FN6/c28-22-12-10-21(11-13-22)25-24-17-31-27(30-16-4-7-19-5-2-1-3-6-19)32-26(24)34(33-25)18-20-8-14-23(29)15-9-20/h1-3,5-6,10-13,17,20,23H,4,7-9,14-16,18,29H2,(H,30,31,32)/t20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201165

(CHEMBL3932614)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCC(O)CCCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C30H44N6O/c1-34-17-19-35(20-18-34)22-24-11-13-25(14-12-24)28-23-36-16-8-6-10-26(37)9-5-3-2-4-7-15-31-30-32-21-27(28)29(36)33-30/h11-14,21,23,26,37H,2-10,15-20,22H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201148
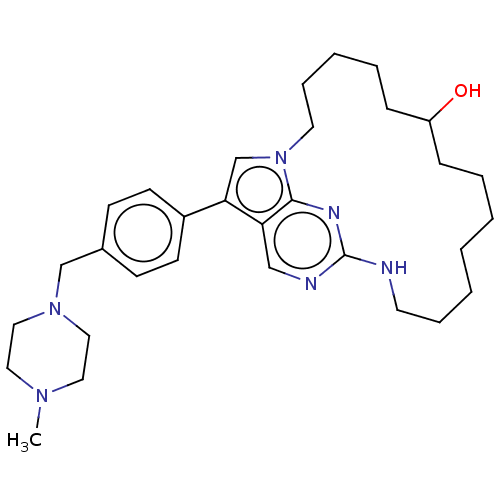
(CHEMBL3946971)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCCC(O)CCCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C31H46N6O/c1-35-18-20-36(21-19-35)23-25-12-14-26(15-13-25)29-24-37-17-9-5-7-11-27(38)10-6-3-2-4-8-16-32-31-33-22-28(29)30(37)34-31/h12-15,22,24,27,38H,2-11,16-21,23H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444071
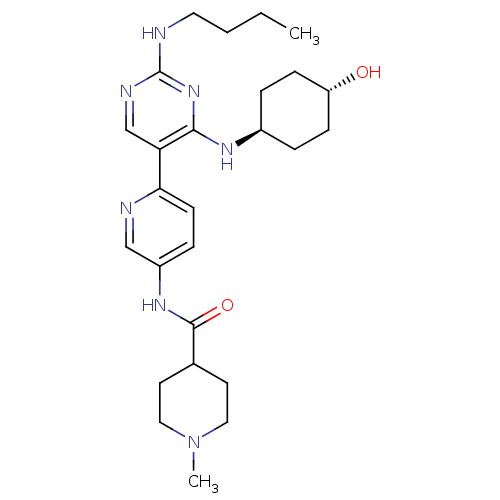
(CHEMBL3092794)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(NC(=O)C2CCN(C)CC2)cn1 |r,wU:11.10,wD:14.14,(41.44,-46.25,;42.77,-45.48,;44.11,-46.25,;45.44,-45.48,;46.77,-46.25,;48.11,-45.48,;49.44,-46.25,;50.78,-45.48,;50.78,-43.93,;49.44,-43.17,;49.44,-41.63,;48.1,-40.86,;46.77,-41.63,;45.44,-40.86,;45.44,-39.32,;44.1,-38.54,;46.77,-38.55,;48.1,-39.31,;48.11,-43.94,;52.11,-43.16,;53.44,-43.93,;54.77,-43.15,;54.76,-41.61,;56.09,-40.83,;57.43,-41.6,;57.44,-43.14,;58.76,-40.82,;60.09,-41.59,;61.42,-40.82,;61.42,-39.28,;62.75,-38.51,;60.08,-38.51,;58.74,-39.29,;53.42,-40.85,;52.09,-41.62,)| Show InChI InChI=1S/C26H39N7O2/c1-3-4-13-27-26-29-17-22(24(32-26)30-19-5-8-21(34)9-6-19)23-10-7-20(16-28-23)31-25(35)18-11-14-33(2)15-12-18/h7,10,16-19,21,34H,3-6,8-9,11-15H2,1-2H3,(H,31,35)(H2,27,29,30,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384582

(CHEMBL2036805)Show SMILES N[C@H]1CC[C@H](Cn2nc(-c3ccc(nc3)N3CCNCC3)c3cnc(NCCCc4ccccc4)nc23)CC1 |r,wU:4.4,wD:1.0,(11.21,-28.56,;12.24,-27.41,;13.75,-27.74,;14.79,-26.59,;14.31,-25.14,;15.34,-24,;14.86,-22.53,;15.77,-21.28,;14.86,-20.02,;15.34,-18.56,;14.31,-17.42,;14.78,-15.96,;16.29,-15.63,;17.32,-16.78,;16.84,-18.24,;16.76,-14.17,;18.27,-13.85,;18.74,-12.39,;17.71,-11.25,;16.21,-11.56,;15.72,-13.03,;13.39,-20.5,;12.05,-19.74,;10.72,-20.51,;10.72,-22.05,;9.39,-22.82,;8.05,-22.05,;8.05,-20.51,;6.72,-19.74,;6.72,-18.2,;8.06,-17.44,;8.06,-15.9,;6.73,-15.13,;5.39,-15.9,;5.4,-17.44,;12.05,-22.82,;13.39,-22.05,;12.8,-24.81,;11.77,-25.95,)| Show InChI InChI=1S/C30H39N9/c31-25-11-8-23(9-12-25)21-39-29-26(20-35-30(36-29)33-14-4-7-22-5-2-1-3-6-22)28(37-39)24-10-13-27(34-19-24)38-17-15-32-16-18-38/h1-3,5-6,10,13,19-20,23,25,32H,4,7-9,11-12,14-18,21,31H2,(H,33,35,36)/t23-,25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201167

(CHEMBL3973561)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCC[C@H](N)C(=O)NCCCCNc4ncc2c3n4)CC1 |r| Show InChI InChI=1S/C27H38N8O/c1-33-13-15-34(16-14-33)18-20-6-8-21(9-7-20)23-19-35-12-4-5-24(28)26(36)29-10-2-3-11-30-27-31-17-22(23)25(35)32-27/h6-9,17,19,24H,2-5,10-16,18,28H2,1H3,(H,29,36)(H,30,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444079
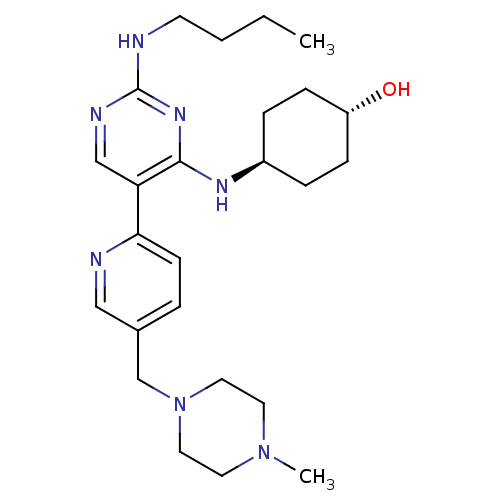
(CHEMBL3092806)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(CN2CCN(C)CC2)cn1 |r,wU:11.10,wD:14.14,(37.6,-21.62,;38.94,-20.85,;40.27,-21.62,;41.6,-20.85,;42.94,-21.62,;44.27,-20.86,;45.61,-21.63,;46.94,-20.85,;46.94,-19.3,;45.6,-18.54,;45.6,-17,;44.26,-16.23,;42.94,-17.01,;41.6,-16.23,;41.6,-14.69,;40.26,-13.92,;42.93,-13.92,;44.26,-14.69,;44.27,-19.31,;48.27,-18.53,;49.6,-19.3,;50.93,-18.53,;50.92,-16.98,;52.25,-16.21,;53.59,-16.97,;53.59,-18.51,;54.92,-19.27,;56.25,-18.5,;57.59,-19.27,;56.25,-16.96,;54.91,-16.19,;49.58,-16.22,;48.25,-17,)| Show InChI InChI=1S/C25H39N7O/c1-3-4-11-26-25-28-17-22(24(30-25)29-20-6-8-21(33)9-7-20)23-10-5-19(16-27-23)18-32-14-12-31(2)13-15-32/h5,10,16-17,20-21,33H,3-4,6-9,11-15,18H2,1-2H3,(H2,26,28,29,30)/t20-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50384583

(CHEMBL2036806)Show SMILES CCCCNc1ncc2c(nn(C[C@H]3CC[C@H](N)CC3)c2n1)-c1ccc(cc1)N1CCNCC1 |r,wU:13.12,wD:16.16,(19.9,-18.03,;19.9,-19.57,;21.23,-20.34,;21.23,-21.88,;22.56,-22.65,;23.9,-21.88,;23.9,-20.34,;25.23,-19.57,;26.56,-20.33,;28.04,-19.85,;28.95,-21.11,;28.04,-22.36,;28.51,-23.83,;27.48,-24.97,;27.96,-26.43,;26.92,-27.57,;25.42,-27.25,;24.38,-28.39,;24.95,-25.78,;25.98,-24.64,;26.56,-21.88,;25.23,-22.65,;28.51,-18.39,;30.02,-18.07,;30.5,-16.61,;29.47,-15.46,;27.95,-15.79,;27.48,-17.25,;29.93,-14,;31.44,-13.68,;31.92,-12.22,;30.89,-11.08,;29.38,-11.39,;28.9,-12.86,)| Show InChI InChI=1S/C26H38N8/c1-2-3-12-29-26-30-17-23-24(20-6-10-22(11-7-20)33-15-13-28-14-16-33)32-34(25(23)31-26)18-19-4-8-21(27)9-5-19/h6-7,10-11,17,19,21,28H,2-5,8-9,12-16,18,27H2,1H3,(H,29,30,31)/t19-,21- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Axl using KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluid capillary electrophoresis assay |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201075
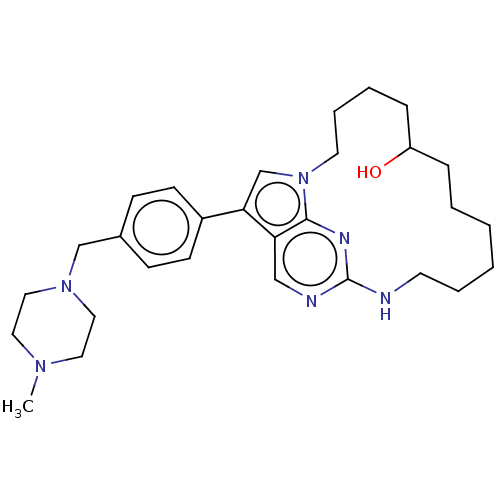
(CHEMBL3956694)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCC(O)CCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C29H42N6O/c1-33-16-18-34(19-17-33)21-23-10-12-24(13-11-23)27-22-35-15-7-5-9-25(36)8-4-2-3-6-14-30-29-31-20-26(27)28(35)32-29/h10-13,20,22,25,36H,2-9,14-19,21H2,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444253

(CHEMBL3093648)Show SMILES CCCCNc1ncc(C(=O)NC2CCN(CC2)c2ncccn2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:26.27,wD:29.31,(19.76,-36.09,;21.1,-35.32,;22.43,-36.09,;23.76,-35.32,;25.1,-36.09,;26.43,-35.32,;27.76,-36.09,;29.1,-35.32,;29.1,-33.77,;30.43,-33,;30.42,-31.46,;31.76,-33.76,;33.09,-32.99,;34.43,-33.76,;35.76,-32.99,;35.76,-31.45,;34.42,-30.68,;33.08,-31.45,;37.09,-30.68,;38.42,-31.45,;39.75,-30.68,;39.75,-29.14,;38.41,-28.37,;37.08,-29.14,;27.76,-33.01,;27.76,-31.47,;26.42,-30.7,;25.09,-31.48,;23.75,-30.71,;23.75,-29.16,;22.42,-28.39,;25.08,-28.4,;26.41,-29.16,;26.43,-33.78,)| Show InChI InChI=1S/C24H36N8O2/c1-2-3-11-25-23-28-16-20(21(31-23)29-17-5-7-19(33)8-6-17)22(34)30-18-9-14-32(15-10-18)24-26-12-4-13-27-24/h4,12-13,16-19,33H,2-3,5-11,14-15H2,1H3,(H,30,34)(H2,25,28,29,31)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9693-700 (2014)
Article DOI: 10.1021/jm4013888
BindingDB Entry DOI: 10.7270/Q21G0NQ1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201152
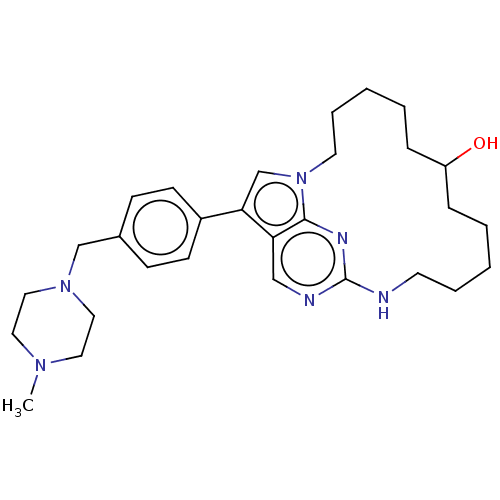
(CHEMBL3938180)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCCC(O)CCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C29H42N6O/c1-33-16-18-34(19-17-33)21-23-10-12-24(13-11-23)27-22-35-15-7-3-5-9-25(36)8-4-2-6-14-30-29-31-20-26(27)28(35)32-29/h10-13,20,22,25,36H,2-9,14-19,21H2,1H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201076
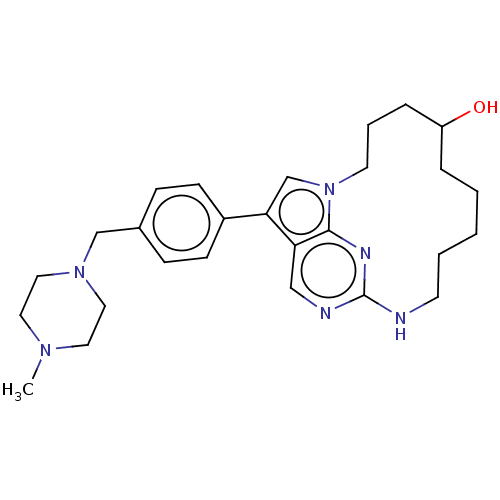
(CHEMBL3951096)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC(O)CCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C27H38N6O/c1-31-14-16-32(17-15-31)19-21-8-10-22(11-9-21)25-20-33-13-5-7-23(34)6-3-2-4-12-28-27-29-18-24(25)26(33)30-27/h8-11,18,20,23,34H,2-7,12-17,19H2,1H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50201078

(CHEMBL3964573)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC[C@H](N)C(=O)NCCCCCCCCNc4ncc2c3n4)CC1 |r| Show InChI InChI=1S/C32H48N8O/c1-38-18-20-39(21-19-38)23-25-11-13-26(14-12-25)28-24-40-17-9-6-10-29(33)31(41)34-15-7-4-2-3-5-8-16-35-32-36-22-27(28)30(40)37-32/h11-14,22,24,29H,2-10,15-21,23,33H2,1H3,(H,34,41)(H,35,36,37)/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Axl (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444241
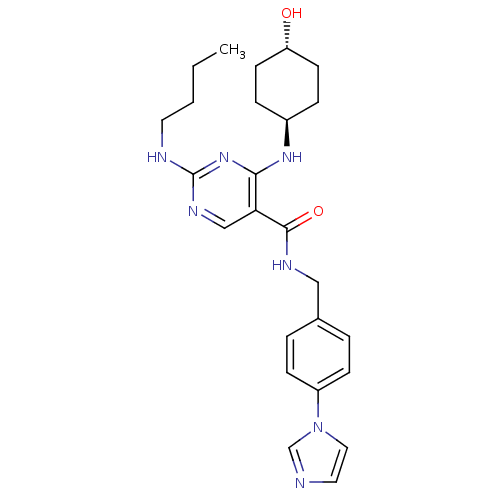
(CHEMBL3093756 | US9649309, Compound UNC2881A)Show SMILES CCCCNc1ncc(C(=O)NCc2ccc(cc2)-n2ccnc2)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:26.27,wD:29.31,(48.43,-9.69,;49.77,-8.92,;51.1,-9.69,;52.43,-8.92,;53.77,-9.69,;55.1,-8.93,;56.43,-9.7,;57.77,-8.92,;57.77,-7.37,;59.1,-6.6,;59.09,-5.06,;60.43,-7.36,;61.76,-6.59,;63.1,-7.35,;63.1,-8.89,;64.44,-9.66,;65.77,-8.88,;65.76,-7.33,;64.42,-6.58,;67.11,-9.64,;68.5,-9.01,;69.54,-10.15,;68.78,-11.49,;67.27,-11.18,;56.43,-6.61,;56.43,-5.07,;55.09,-4.3,;53.76,-5.08,;52.42,-4.31,;52.42,-2.77,;51.09,-2,;53.75,-2,;55.08,-2.77,;55.1,-7.38,)| Show InChI InChI=1S/C25H33N7O2/c1-2-3-12-27-25-29-16-22(23(31-25)30-19-6-10-21(33)11-7-19)24(34)28-15-18-4-8-20(9-5-18)32-14-13-26-17-32/h4-5,8-9,13-14,16-17,19,21,33H,2-3,6-7,10-12,15H2,1H3,(H,28,34)(H2,27,29,30,31)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9693-700 (2014)
Article DOI: 10.1021/jm4013888
BindingDB Entry DOI: 10.7270/Q21G0NQ1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50444065
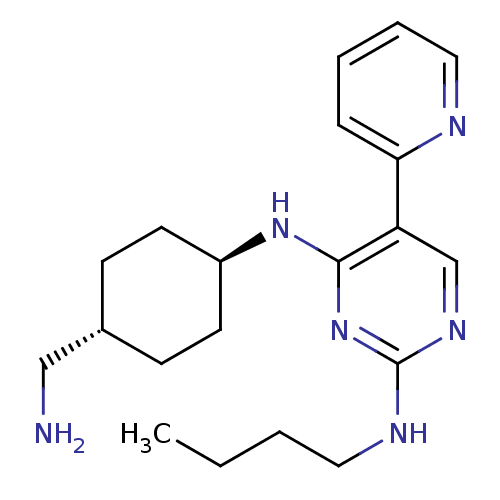
(CHEMBL3092800)Show SMILES CCCCNc1ncc(c(N[C@H]2CC[C@H](CN)CC2)n1)-c1ccccn1 |r,wU:11.10,wD:14.14,(31.68,-9.79,;33.01,-9.02,;34.35,-9.79,;35.68,-9.02,;37.01,-9.8,;38.35,-9.03,;39.68,-9.8,;41.02,-9.03,;41.01,-7.48,;39.68,-6.71,;39.67,-5.17,;38.34,-4.41,;37.01,-5.18,;35.67,-4.4,;35.67,-2.86,;34.34,-2.09,;34.34,-.55,;37.01,-2.09,;38.34,-2.86,;38.35,-7.48,;42.34,-6.7,;43.68,-7.47,;45,-6.7,;45,-5.16,;43.65,-4.39,;42.34,-5.16,)| Show InChI InChI=1S/C20H30N6/c1-2-3-11-23-20-24-14-17(18-6-4-5-12-22-18)19(26-20)25-16-9-7-15(13-21)8-10-16/h4-6,12,14-16H,2-3,7-11,13,21H2,1H3,(H2,23,24,25,26)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9683-92 (2014)
Article DOI: 10.1021/jm401387j
BindingDB Entry DOI: 10.7270/Q29W0GX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201154

(CHEMBL3984314)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCCC(O)CCCCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C32H48N6O/c1-36-19-21-37(22-20-36)24-26-13-15-27(16-14-26)30-25-38-18-10-6-8-12-28(39)11-7-4-2-3-5-9-17-33-32-34-23-29(30)31(38)35-32/h13-16,23,25,28,39H,2-12,17-22,24H2,1H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
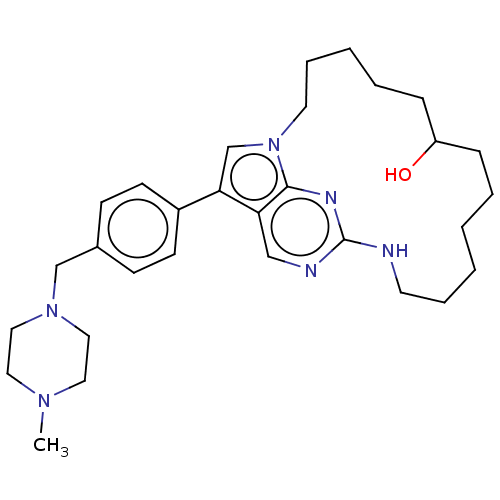
(Homo sapiens (Human)) | BDBM50201169
(CHEMBL3976261)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCCC(O)CCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C30H44N6O/c1-34-17-19-35(20-18-34)22-24-11-13-25(14-12-24)28-23-36-16-8-4-6-10-26(37)9-5-2-3-7-15-31-30-32-21-27(28)29(36)33-30/h11-14,21,23,26,37H,2-10,15-20,22H2,1H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
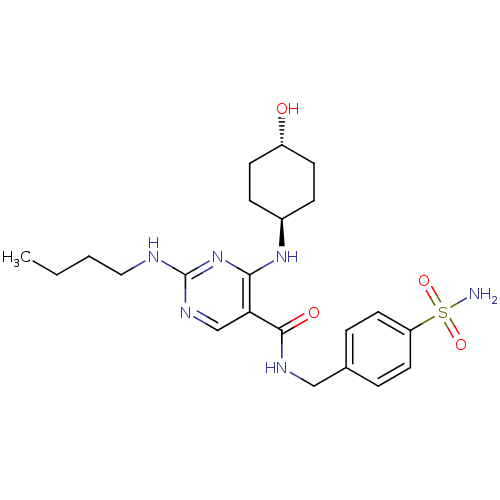
(Homo sapiens (Human)) | BDBM50444242
(CHEMBL3093755)Show SMILES CCCCNc1ncc(C(=O)NCc2ccc(cc2)S(N)(=O)=O)c(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:25.25,wD:28.29,(24.27,-9.22,;25.6,-8.45,;26.93,-9.22,;28.27,-8.45,;29.6,-9.22,;30.94,-8.45,;32.27,-9.22,;33.61,-8.45,;33.6,-6.9,;34.93,-6.12,;34.93,-4.58,;36.27,-6.89,;37.6,-6.11,;38.94,-6.88,;38.94,-8.42,;40.27,-9.18,;41.6,-8.41,;41.59,-6.86,;40.26,-6.1,;42.94,-9.17,;44.27,-8.39,;43.7,-10.5,;42.17,-10.5,;32.27,-6.14,;32.26,-4.6,;30.93,-3.83,;29.59,-4.61,;28.25,-3.84,;28.25,-2.29,;26.92,-1.52,;29.59,-1.53,;30.92,-2.29,;30.94,-6.91,)| Show InChI InChI=1S/C22H32N6O4S/c1-2-3-12-24-22-26-14-19(20(28-22)27-16-6-8-17(29)9-7-16)21(30)25-13-15-4-10-18(11-5-15)33(23,31)32/h4-5,10-11,14,16-17,29H,2-3,6-9,12-13H2,1H3,(H,25,30)(H2,23,31,32)(H2,24,26,27,28)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eshelman School of Pharmacy�Department of Pharmacology�Lineberger Compreh
Curated by ChEMBL
| Assay Description
Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay |
J Med Chem 56: 9693-700 (2014)
Article DOI: 10.1021/jm4013888
BindingDB Entry DOI: 10.7270/Q21G0NQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50201169

(CHEMBL3976261)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCCCC(O)CCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C30H44N6O/c1-34-17-19-35(20-18-34)22-24-11-13-25(14-12-24)28-23-36-16-8-4-6-10-26(37)9-5-2-3-7-15-31-30-32-21-27(28)29(36)33-30/h11-14,21,23,26,37H,2-10,15-20,22H2,1H3,(H,31,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50201173
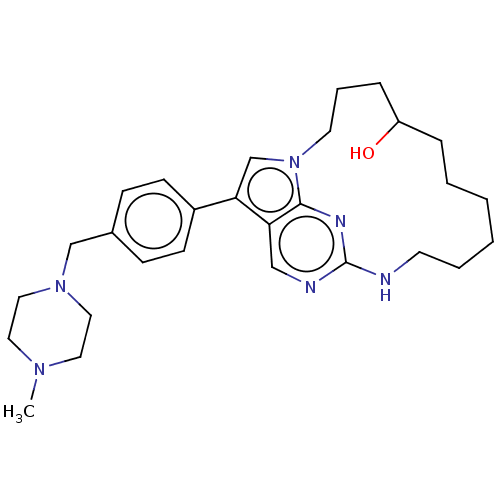
(CHEMBL3961597)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cn3CCCC(O)CCCCCCNc4ncc2c3n4)CC1 Show InChI InChI=1S/C28H40N6O/c1-32-15-17-33(18-16-32)20-22-9-11-23(12-10-22)26-21-34-14-6-8-24(35)7-4-2-3-5-13-29-28-30-19-25(26)27(34)31-28/h9-12,19,21,24,35H,2-8,13-18,20H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay |
ACS Med Chem Lett 7: 1044-1049 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00221
BindingDB Entry DOI: 10.7270/Q208679K |
More data for this
Ligand-Target Pair | |
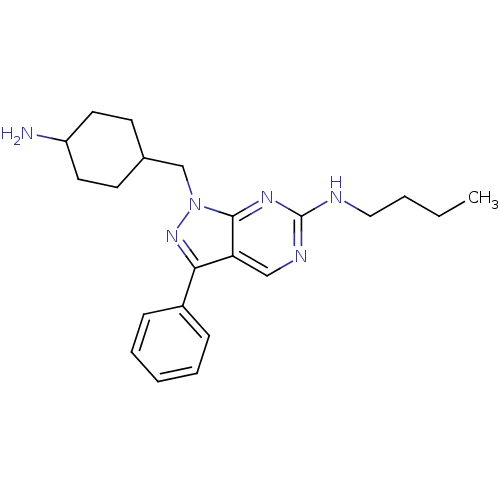
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50384602
(CHEMBL2036795 | US9744172, Compound UNC00000344A)Show SMILES CCCCNc1ncc2c(nn(CC3CCC(N)CC3)c2n1)-c1ccccc1 |(18.02,-41.5,;18.01,-43.04,;19.35,-43.81,;19.35,-45.35,;20.68,-46.13,;22.01,-45.36,;22.02,-43.81,;23.35,-43.04,;24.68,-43.81,;26.16,-43.33,;27.07,-44.58,;26.16,-45.84,;26.63,-47.3,;25.6,-48.44,;24.09,-48.11,;23.06,-49.25,;23.53,-50.72,;22.5,-51.86,;25.04,-51.04,;26.08,-49.9,;24.68,-45.36,;23.35,-46.13,;26.63,-41.86,;28.14,-41.54,;28.62,-40.08,;27.58,-38.94,;26.07,-39.26,;25.6,-40.73,)| Show InChI InChI=1S/C22H30N6/c1-2-3-13-24-22-25-14-19-20(17-7-5-4-6-8-17)27-28(21(19)26-22)15-16-9-11-18(23)12-10-16/h4-8,14,16,18H,2-3,9-13,15,23H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... |
ACS Med Chem Lett 3: 129-134 (2012)
Article DOI: 10.1021/ml200239k
BindingDB Entry DOI: 10.7270/Q2F76DMC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data