

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266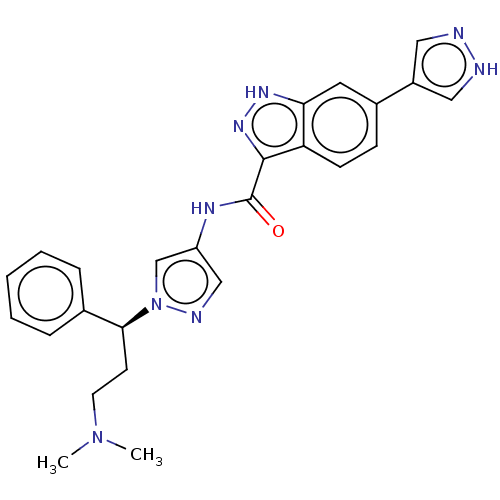 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175484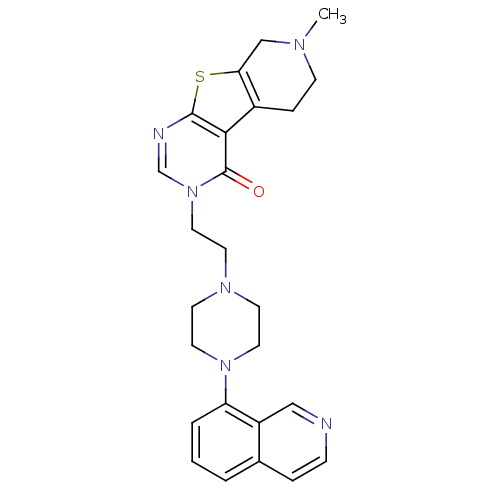 (3-[2-(4-isoquinolin-8-yl-piperazin-1-yl)-ethyl]-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037076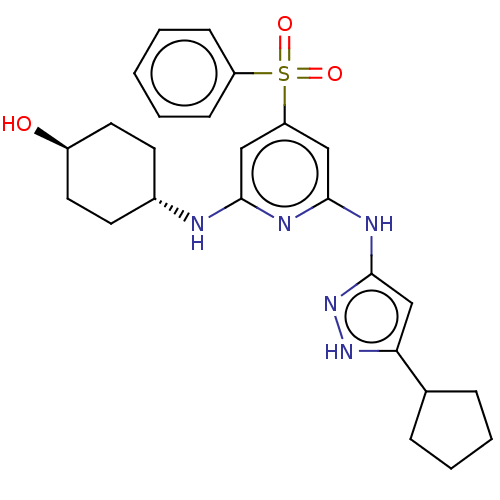 (CHEMBL3355737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175479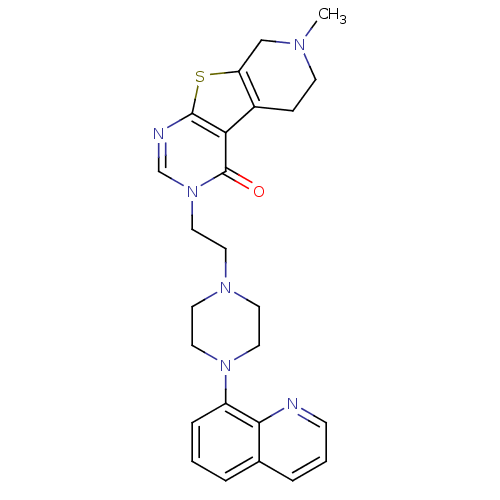 (7-methyl-3-[2-(4-quinolin-8-yl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022940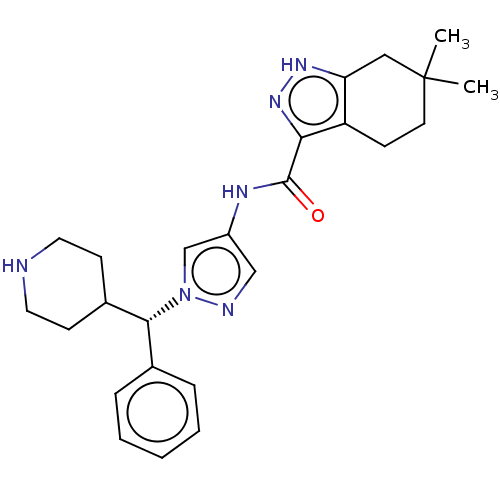 (CHEMBL3298373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037066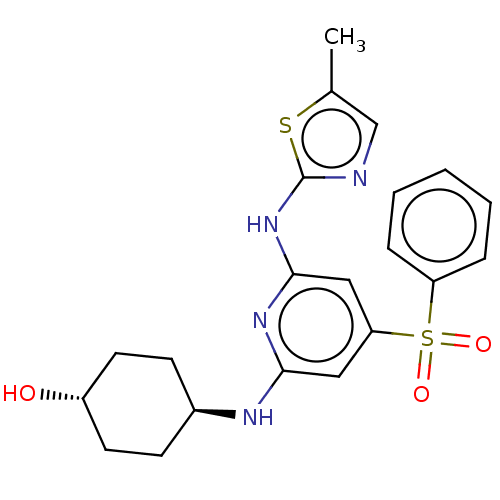 (CHEMBL3355728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175484 (3-[2-(4-isoquinolin-8-yl-piperazin-1-yl)-ethyl]-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175479 (7-methyl-3-[2-(4-quinolin-8-yl-piperazin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037077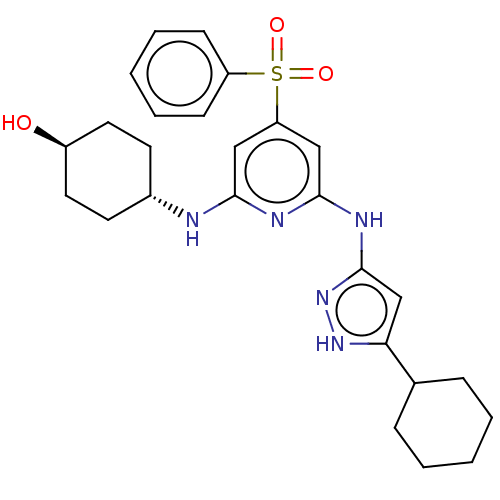 (CHEMBL3355738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185854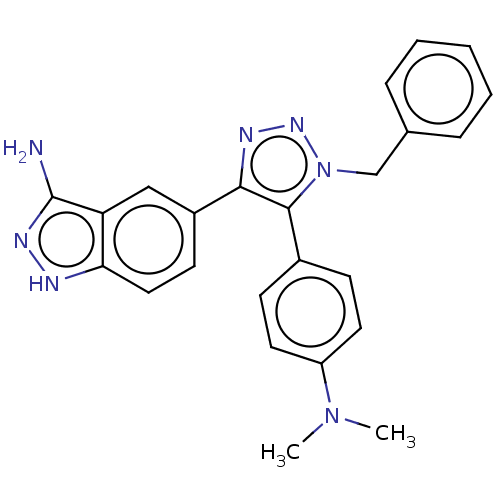 (US9163007, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50388878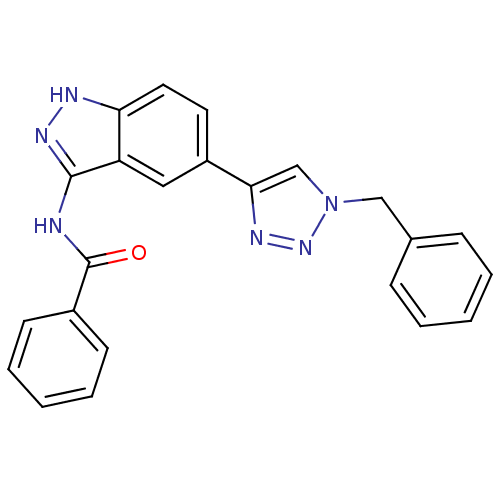 (CHEMBL1999931 | US9163007, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175485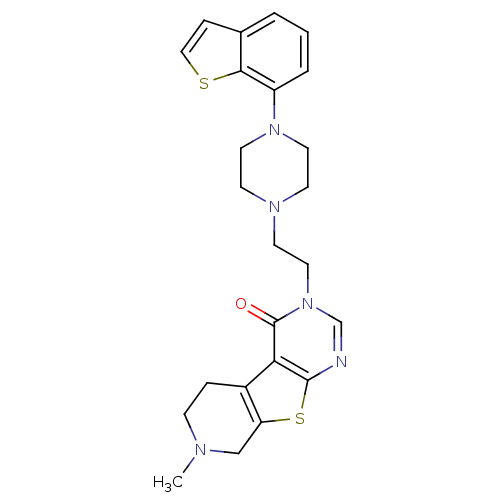 (3-[2-(4-benzo[b]thiophen-7-yl-piperazin-1-yl)-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175485 (3-[2-(4-benzo[b]thiophen-7-yl-piperazin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185901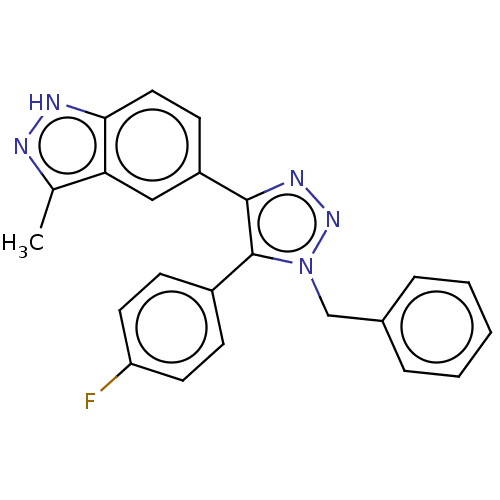 (US9163007, 408) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.428 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096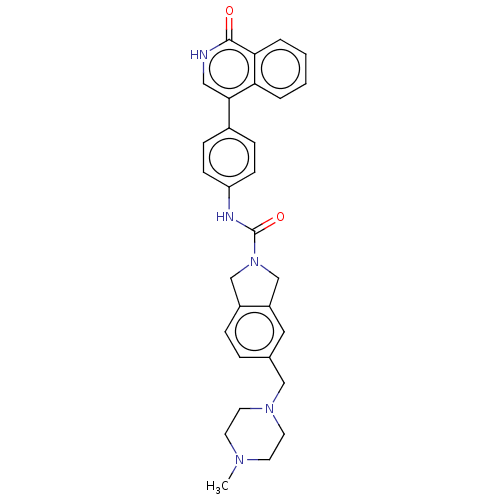 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443182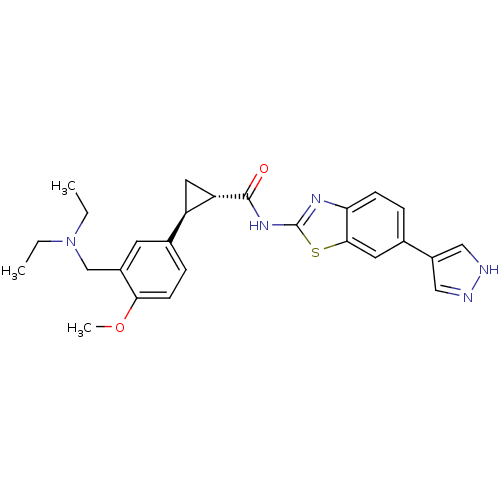 (CHEMBL3086538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175463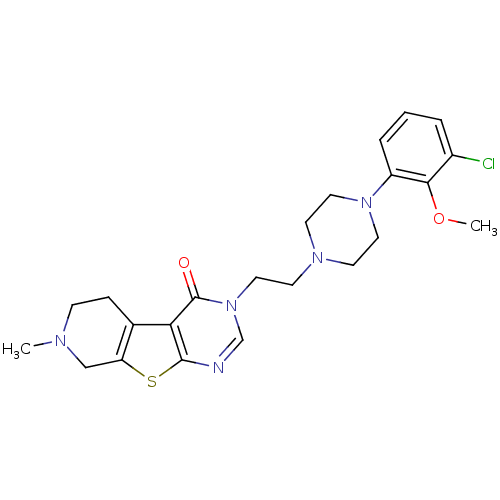 (3-{2-[4-(3-chloro-2-methoxy-phenyl)-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175467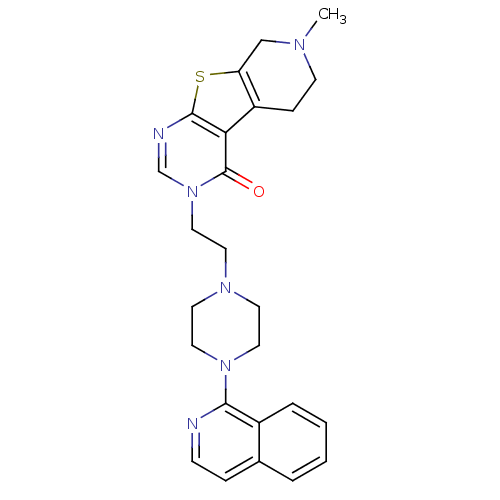 (3-[2-(4-isoquinolin-1-yl-piperazin-1-yl)-ethyl]-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185899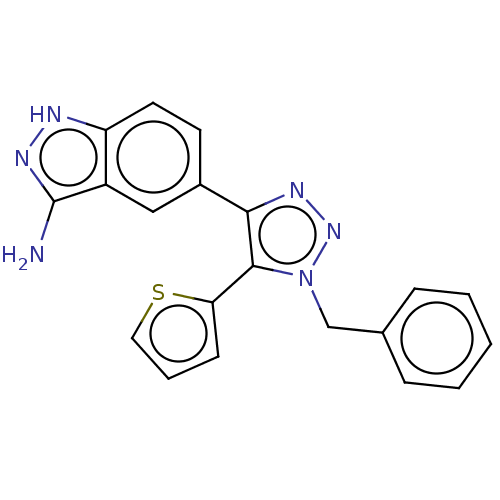 (US9163007, 406) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185888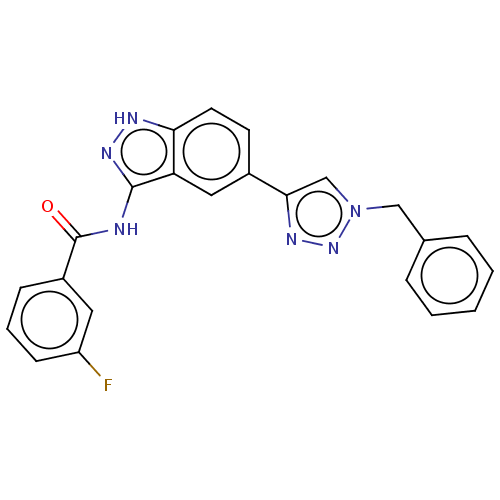 (US9163007, 395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175489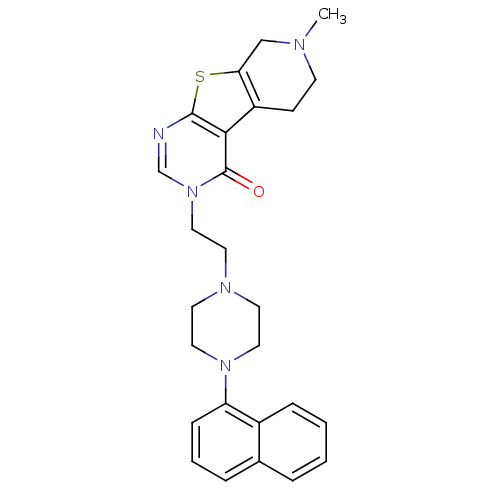 (7-methyl-3-[2-(4-naphthalen-1-yl-piperazin-1-yl)-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175477 (3-{2-[4-(2,3-dihydro-benzofuran-7-yl)-piperazin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175477 (3-{2-[4-(2,3-dihydro-benzofuran-7-yl)-piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50388878 (CHEMBL1999931 | US9163007, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443181 (CHEMBL3086535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185839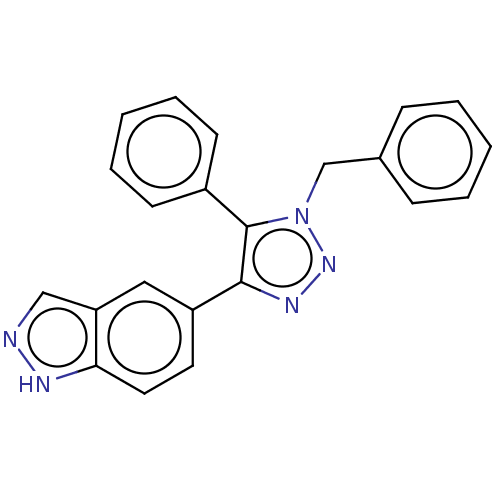 (US9163007, 87) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.601 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175486 (3-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175460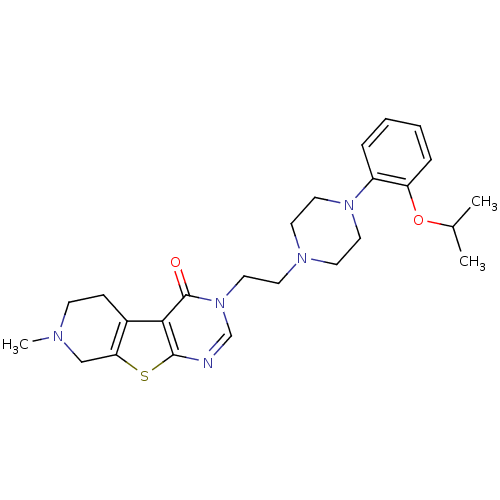 (3-{2-[4-(2-isopropoxy-phenyl)-piperazin-1-yl]-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022934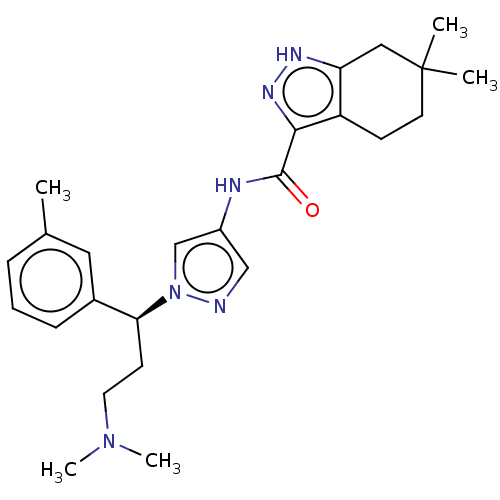 (CHEMBL3298375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022932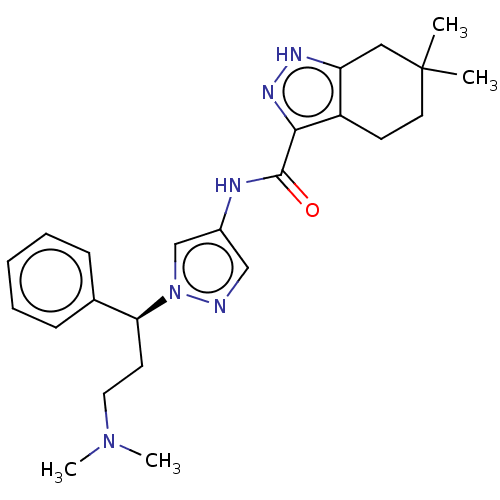 (CHEMBL3298371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175467 (3-[2-(4-isoquinolin-1-yl-piperazin-1-yl)-ethyl]-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175489 (7-methyl-3-[2-(4-naphthalen-1-yl-piperazin-1-yl)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443180 (CHEMBL3086536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185858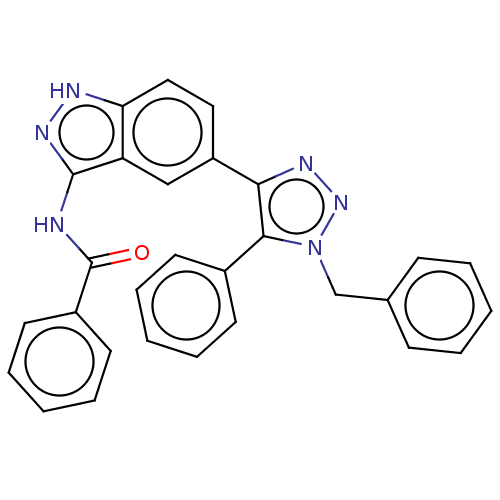 (US9163007, 198) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185846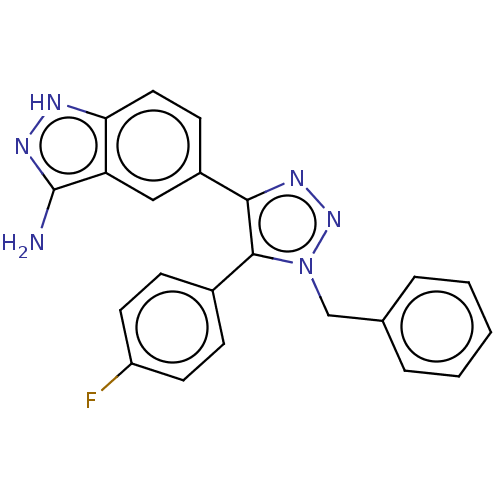 (US9163007, 151) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022927 (CHEMBL3298370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185858 (US9163007, 198) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175473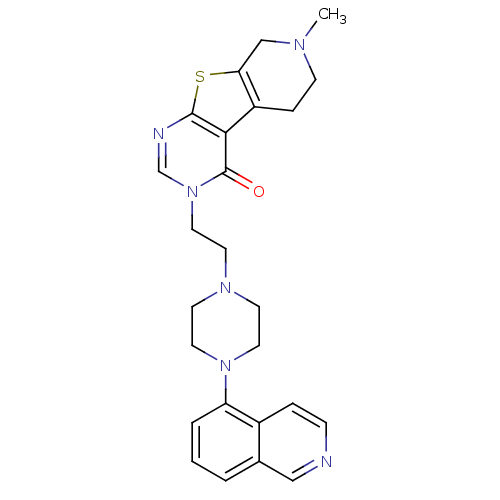 (3-[2-(4-isoquinolin-5-yl-piperazin-1-yl)-ethyl]-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50175470 (7-methyl-3-{2-[4-(2-phenoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [35S]GTPgammaS from human recombinant 5HT1A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185863 (US9163007, 205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175473 (3-[2-(4-isoquinolin-5-yl-piperazin-1-yl)-ethyl]-7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50175465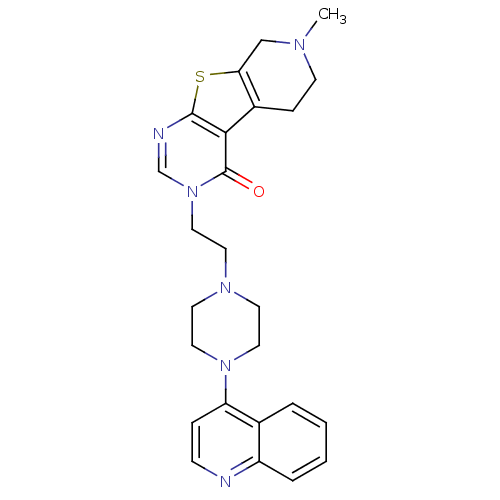 (7-methyl-3-[2-(4-quinolin-4-yl-piperazin-1-yl)-eth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against 5HT5A receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185888 (US9163007, 395) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185850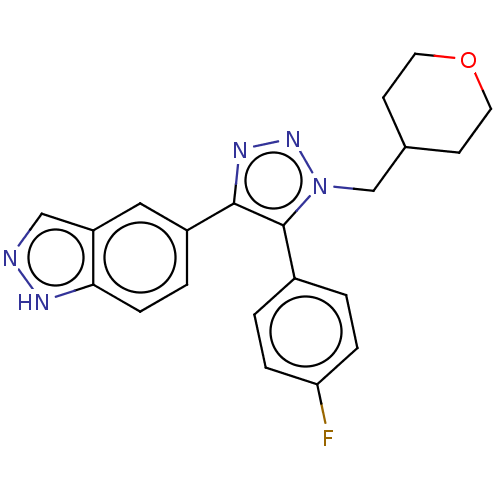 (US9163007, 167) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175470 (7-methyl-3-{2-[4-(2-phenoxy-phenyl)-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM185898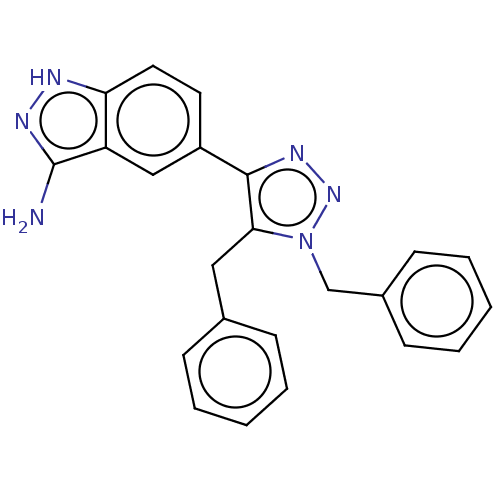 (US9163007, 405) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description 11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... | US Patent US9163007 (2015) BindingDB Entry DOI: 10.7270/Q2KW5DTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015271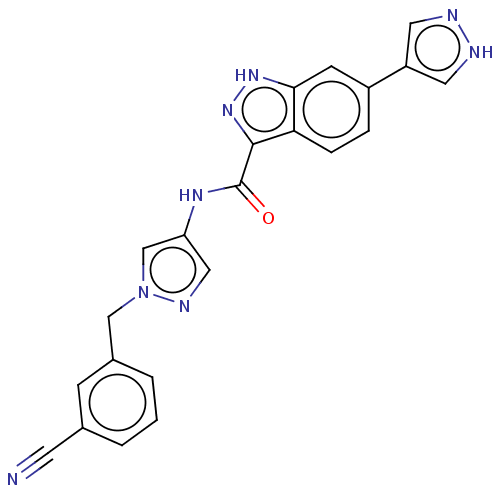 (CHEMBL3263036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175471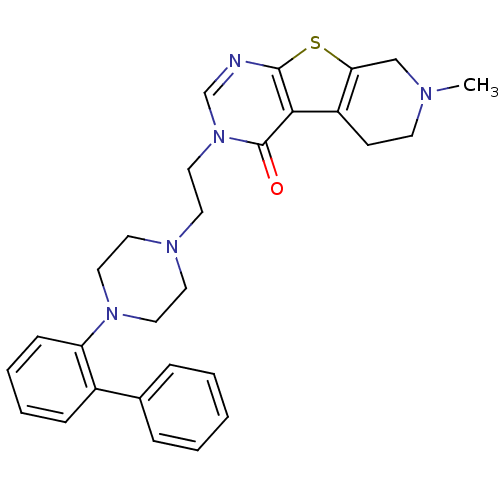 (3-[2-(4-biphenyl-2-yl-piperazin-1-yl)-ethyl]-7-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50175461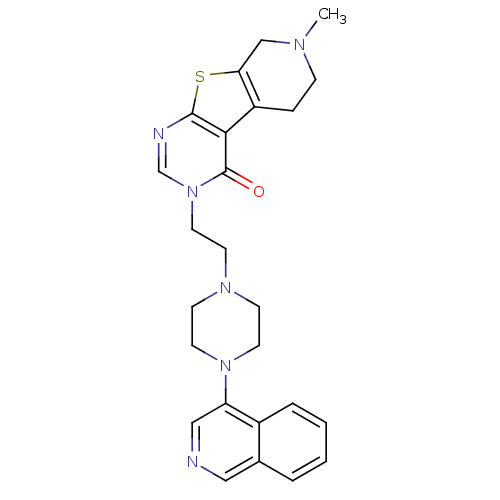 (3-[2-(4-isoquinolin-4-yl-piperazin-1-yl)-ethyl]-7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against dopamine D3 receptor | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50175464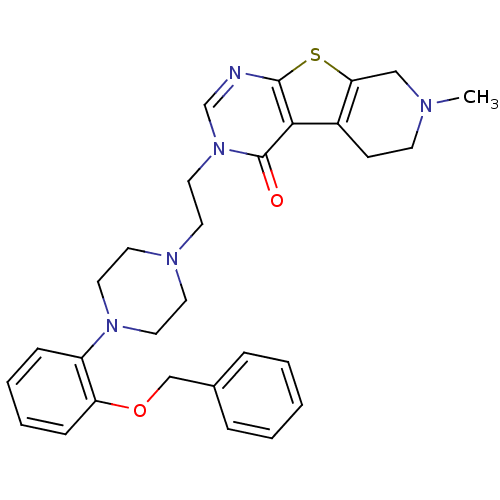 (3-{2-[4-(2-benzyloxy-phenyl)-piperazin-1-yl]-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant 5HT1B receptor co-expressed with G-protein chimera Gqo5 in HEK293 cells by FLIPR | Bioorg Med Chem Lett 15: 5567-73 (2005) Article DOI: 10.1016/j.bmcl.2005.04.077 BindingDB Entry DOI: 10.7270/Q2CR5SX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2308 total ) | Next | Last >> |