 Found 1468 hits with Last Name = 'madan' and Initial = 'a'
Found 1468 hits with Last Name = 'madan' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
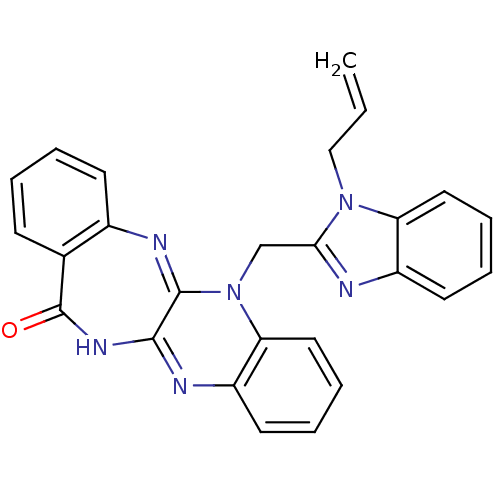
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
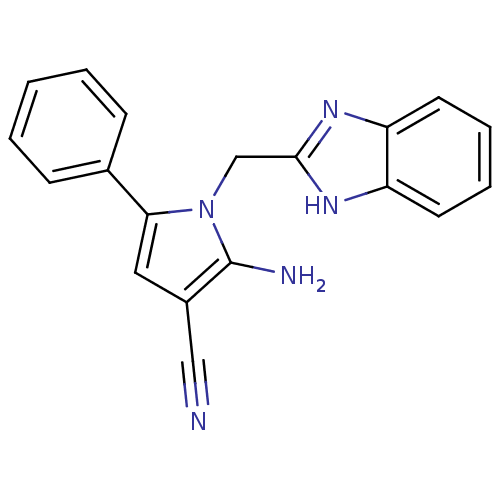
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
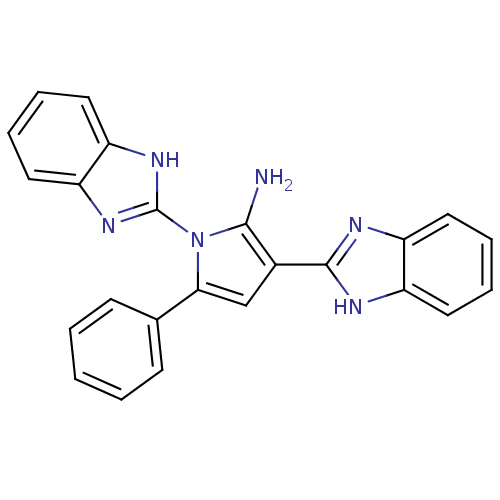
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
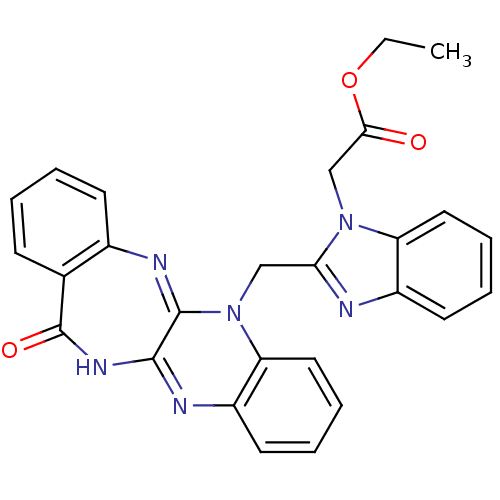
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
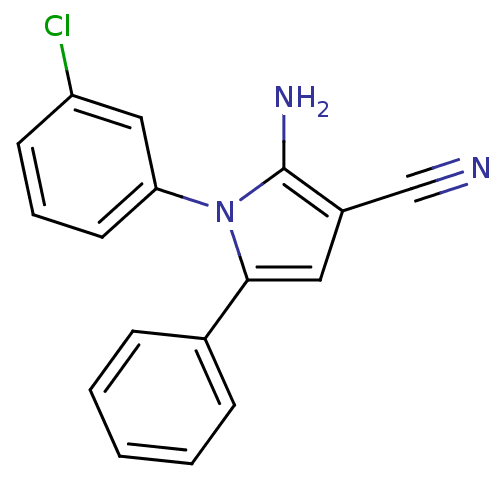
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
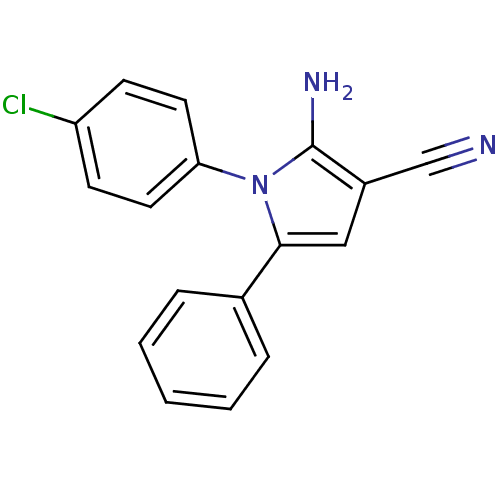
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
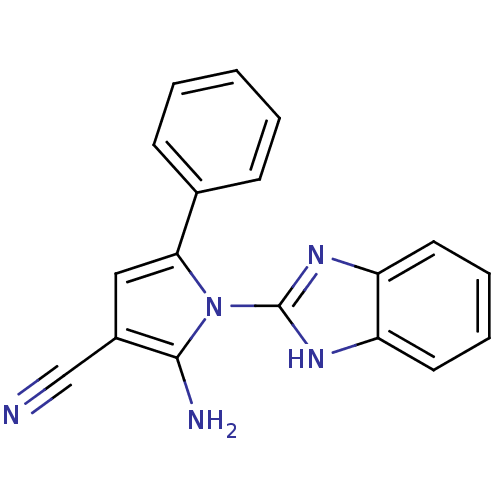
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
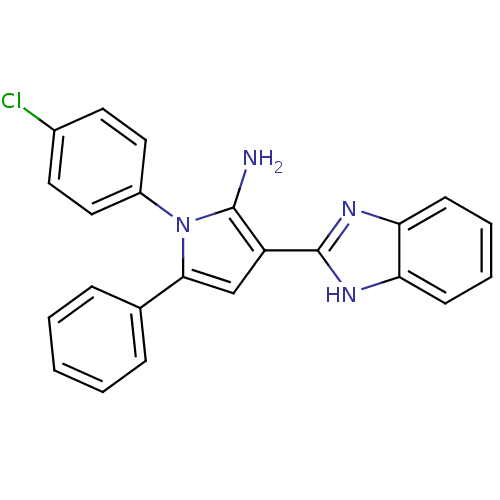
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
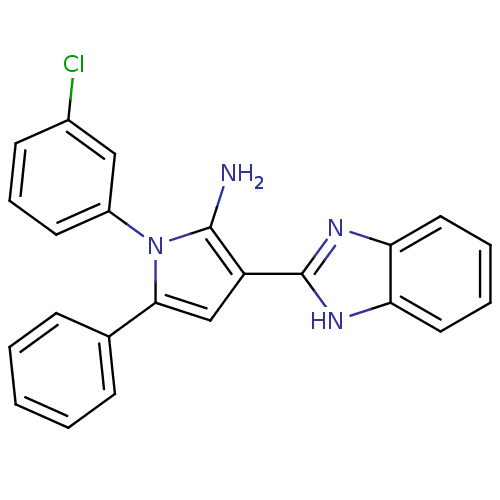
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
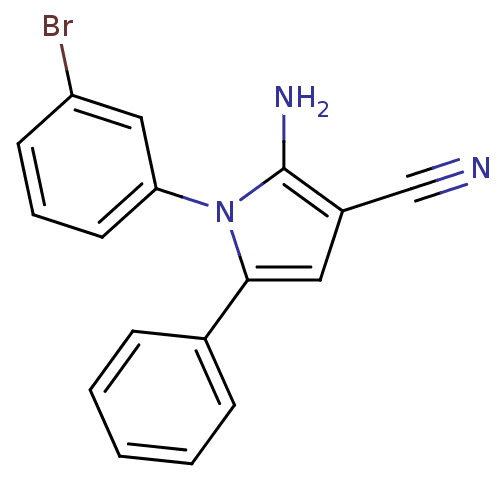
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
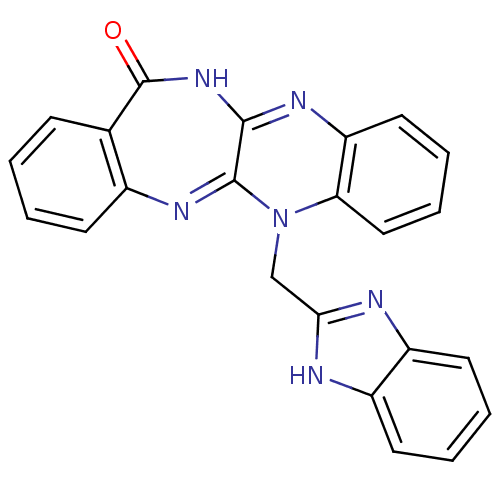
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
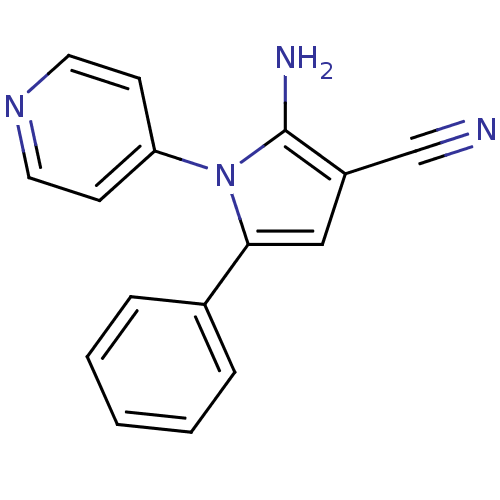
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
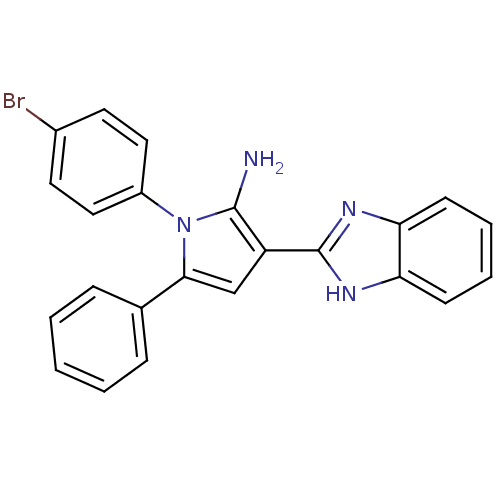
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
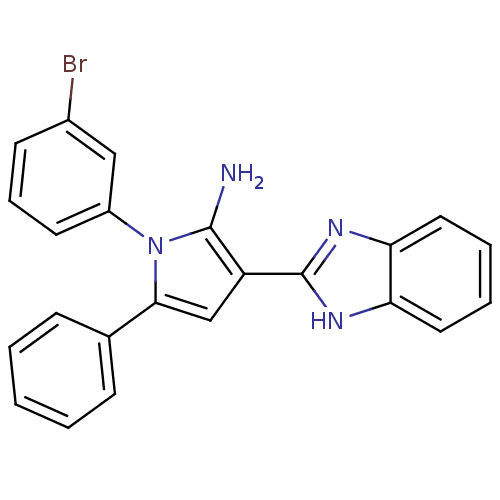
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297306
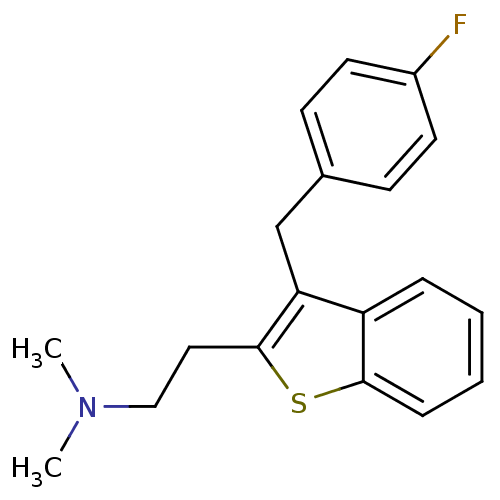
(CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...)Show InChI InChI=1S/C19H20FNS/c1-21(2)12-11-19-17(13-14-7-9-15(20)10-8-14)16-5-3-4-6-18(16)22-19/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297304
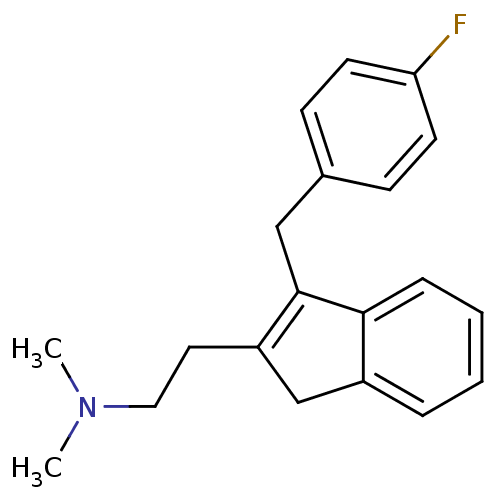
(CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...)Show InChI InChI=1S/C20H22FN/c1-22(2)12-11-17-14-16-5-3-4-6-19(16)20(17)13-15-7-9-18(21)10-8-15/h3-10H,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
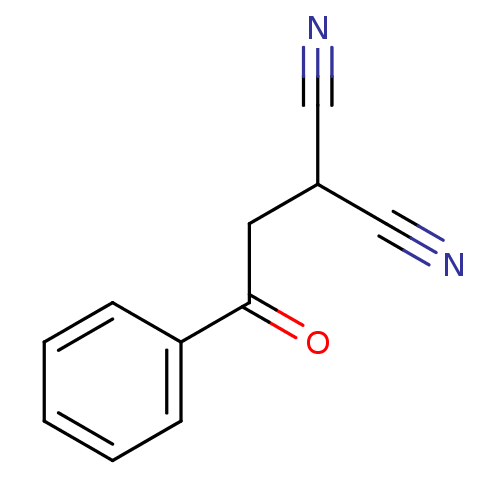
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315206
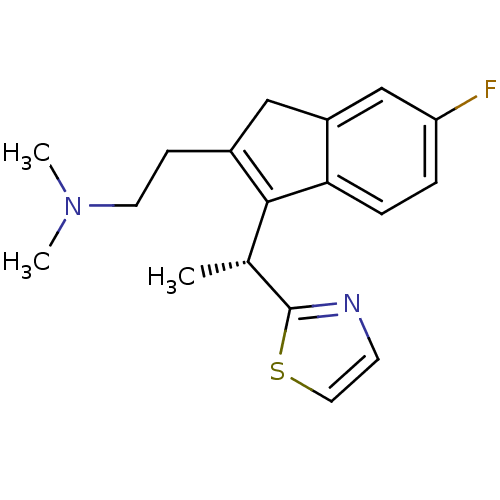
((R)-2-(6-fluoro-3-(1-(thiazol-2-yl)ethyl)-1H-inden...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2cc(F)ccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H21FN2S/c1-12(18-20-7-9-22-18)17-13(6-8-21(2)3)10-14-11-15(19)4-5-16(14)17/h4-5,7,9,11-12H,6,8,10H2,1-3H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50297310
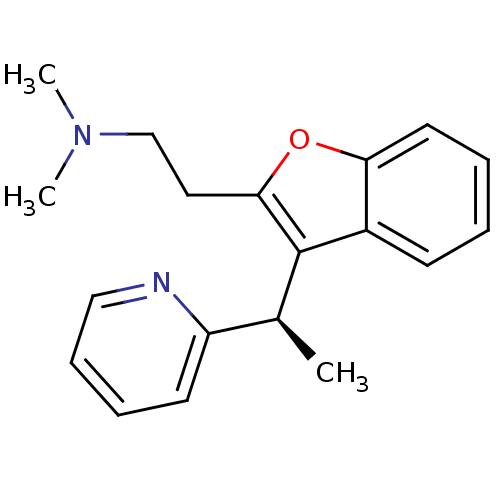
((-)-Dimethyl-{2-[3-((R)-1-pyridin-2-yl-ethyl)-benz...)Show InChI InChI=1S/C19H22N2O/c1-14(16-9-6-7-12-20-16)19-15-8-4-5-10-17(15)22-18(19)11-13-21(2)3/h4-10,12,14H,11,13H2,1-3H3/t14-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylscopolamine from human muscarinic M1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297307
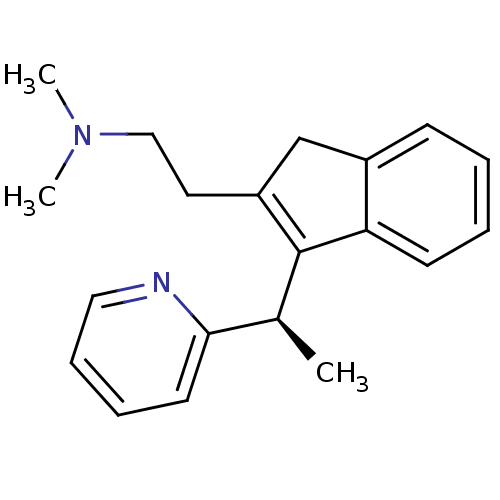
(CHEMBL564226 | R-dimethindene)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1ccccn1 |r,c:2| Show InChI InChI=1S/C20H24N2/c1-15(19-10-6-7-12-21-19)20-17(11-13-22(2)3)14-16-8-4-5-9-18(16)20/h4-10,12,15H,11,13-14H2,1-3H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50316938
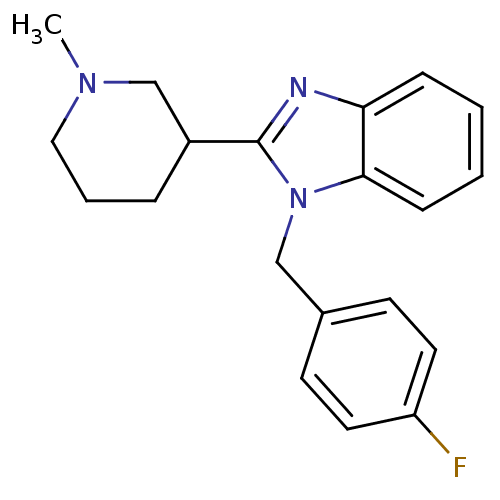
(1-(4-fluorobenzyl)-2-(1-methylpiperidin-3-yl)-1H-b...)Show InChI InChI=1S/C20H22FN3/c1-23-12-4-5-16(14-23)20-22-18-6-2-3-7-19(18)24(20)13-15-8-10-17(21)11-9-15/h2-3,6-11,16H,4-5,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor |
Bioorg Med Chem Lett 22: 421-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.115
BindingDB Entry DOI: 10.7270/Q2J103KS |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50261243
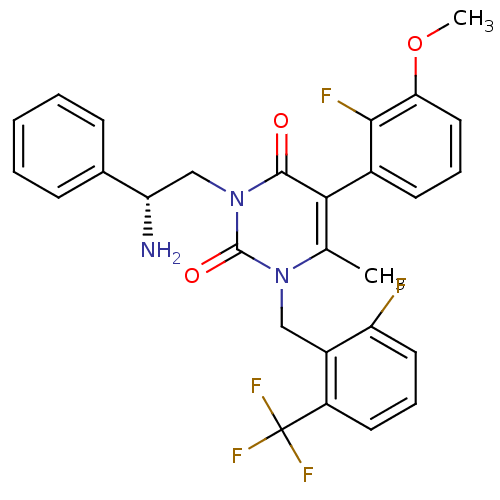
((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1c(C)n(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r,wD:29.31,(2.33,6.39,;1,5.62,;1.01,4.08,;2.35,3.31,;2.35,1.77,;1.02,.99,;-.31,1.77,;-.32,3.3,;-1.66,4.06,;-1.64,1,;-1.64,-.54,;-.31,-1.32,;-2.97,-1.31,;-2.97,-2.85,;-1.64,-3.62,;-.32,-2.85,;-1.66,-2.08,;1.02,-3.61,;1.02,-5.16,;-.31,-5.93,;-1.65,-5.16,;-2.98,-5.92,;-4.32,-6.68,;-2.22,-7.26,;-3.74,-4.59,;-4.3,-.54,;-5.63,-1.32,;-4.3,1,;-5.64,1.76,;-6.97,.99,;-6.96,-.55,;-8.3,1.75,;-8.3,3.29,;-9.64,4.06,;-10.97,3.28,;-10.96,1.74,;-9.62,.98,;-2.97,1.77,;-2.97,3.31,)| Show InChI InChI=1S/C28H24F5N3O3/c1-16-24(18-10-6-13-23(39-2)25(18)30)26(37)36(15-22(34)17-8-4-3-5-9-17)27(38)35(16)14-19-20(28(31,32)33)11-7-12-21(19)29/h3-13,22H,14-15,34H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 51: 7478-85 (2009)
Article DOI: 10.1021/jm8006454
BindingDB Entry DOI: 10.7270/Q2S46RT8 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50162007
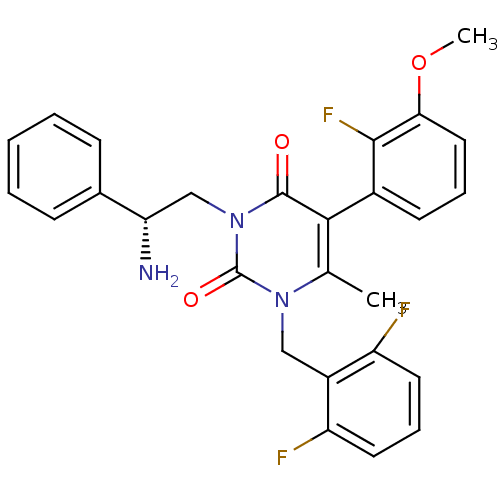
((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...)Show SMILES COc1cccc(c1F)-c1c(C)n(Cc2c(F)cccc2F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r,wD:26.28,(6.65,3.5,;5.31,2.74,;3.98,3.51,;3.98,5.05,;2.64,5.83,;1.31,5.06,;1.31,3.52,;2.64,2.75,;2.64,1.21,;-.02,2.76,;-.02,1.22,;1.3,.46,;-1.35,.46,;-1.35,-1.08,;-.01,-1.85,;1.31,-1.08,;2.64,-.3,;2.64,-1.85,;2.65,-3.39,;1.31,-4.16,;-.02,-3.39,;-1.36,-4.16,;-2.68,1.22,;-4.01,.45,;-2.68,2.76,;-4.01,3.53,;-5.34,2.75,;-5.34,1.21,;-6.68,3.52,;-6.68,5.06,;-8.02,5.83,;-9.36,5.04,;-9.35,3.51,;-8.01,2.75,;-1.35,3.54,;-1.35,5.08,)| Show InChI InChI=1S/C27H24F3N3O3/c1-16-24(18-10-6-13-23(36-2)25(18)30)26(34)33(15-22(31)17-8-4-3-5-9-17)27(35)32(16)14-19-20(28)11-7-12-21(19)29/h3-13,22H,14-15,31H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 51: 7478-85 (2009)
Article DOI: 10.1021/jm8006454
BindingDB Entry DOI: 10.7270/Q2S46RT8 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166450
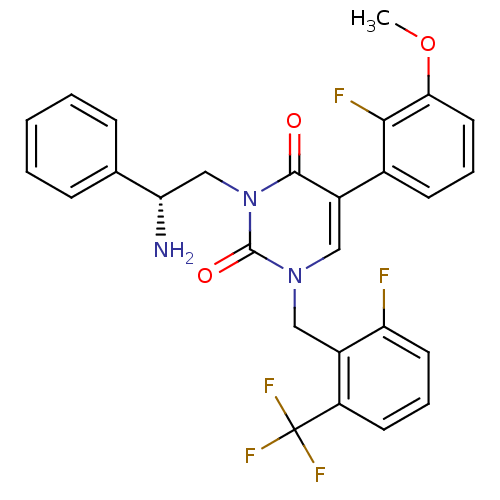
((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H22F5N3O3/c1-38-23-12-5-9-17(24(23)29)18-13-34(14-19-20(27(30,31)32)10-6-11-21(19)28)26(37)35(25(18)36)15-22(33)16-7-3-2-4-8-16/h2-13,22H,14-15,33H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Pro-N-Et-GnRH from human cloned GnRH receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 4503-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.059
BindingDB Entry DOI: 10.7270/Q2QR4WZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297305
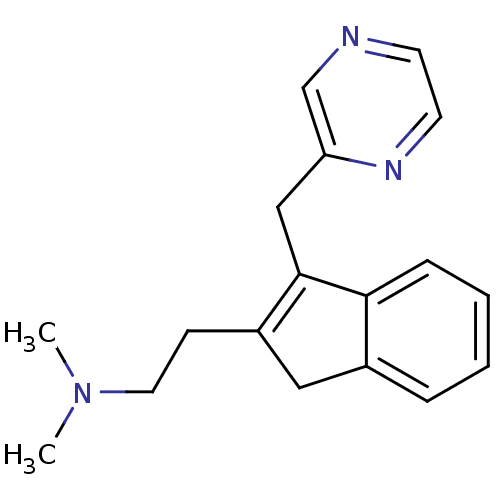
(CHEMBL559251 | Dimethyl-[2-(3-pyrazin-2ylmethyl-1H...)Show InChI InChI=1S/C18H21N3/c1-21(2)10-7-15-11-14-5-3-4-6-17(14)18(15)12-16-13-19-8-9-20-16/h3-6,8-9,13H,7,10-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50188823
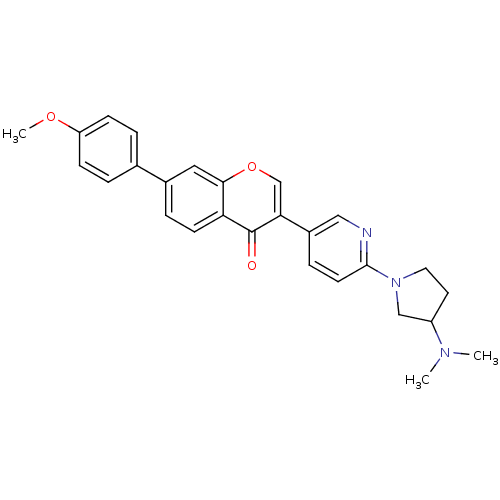
(3-(6-(3-(dimethylamino)pyrrolidin-1-yl)pyridin-3-y...)Show SMILES COc1ccc(cc1)-c1ccc2c(c1)occ(-c1ccc(nc1)N1CCC(C1)N(C)C)c2=O Show InChI InChI=1S/C27H27N3O3/c1-29(2)21-12-13-30(16-21)26-11-7-20(15-28-26)24-17-33-25-14-19(6-10-23(25)27(24)31)18-4-8-22(32-3)9-5-18/h4-11,14-15,17,21H,12-13,16H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human MCH1R |
Bioorg Med Chem Lett 16: 4237-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.075
BindingDB Entry DOI: 10.7270/Q2N29XRN |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50224184
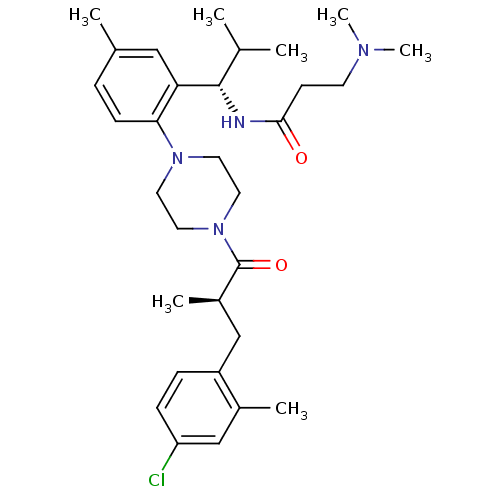
(1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methy...)Show SMILES CC(C)[C@H](NC(=O)CCN(C)C)c1cc(C)ccc1N1CCN(CC1)C(=O)[C@H](C)Cc1ccc(Cl)cc1C |r| Show InChI InChI=1S/C31H45ClN4O2/c1-21(2)30(33-29(37)12-13-34(6)7)27-18-22(3)8-11-28(27)35-14-16-36(17-15-35)31(38)24(5)19-25-9-10-26(32)20-23(25)4/h8-11,18,20-21,24,30H,12-17,19H2,1-7H3,(H,33,37)/t24-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-MSH from human MC4R expressed in HEK293 cells |
J Med Chem 50: 5249-52 (2007)
Article DOI: 10.1021/jm070806a
BindingDB Entry DOI: 10.7270/Q29C6X6B |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50221119
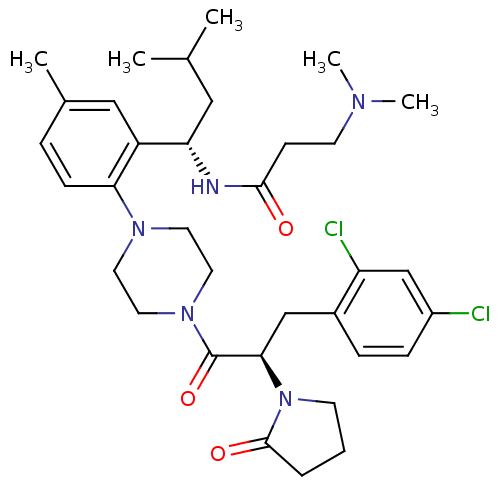
(CHEMBL236521 | N-((S)-1-(2-(4-((R)-3-(2,4-dichloro...)Show SMILES CC(C)C[C@H](NC(=O)CCN(C)C)c1cc(C)ccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1Cl)N1CCCC1=O Show InChI InChI=1S/C34H47Cl2N5O3/c1-23(2)19-29(37-32(42)12-14-38(4)5)27-20-24(3)8-11-30(27)39-15-17-40(18-16-39)34(44)31(41-13-6-7-33(41)43)21-25-9-10-26(35)22-28(25)36/h8-11,20,22-23,29,31H,6-7,12-19,21H2,1-5H3,(H,37,42)/t29-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDPMSH from human MC4R expressed in HEK293 cells |
J Med Chem 50: 6356-66 (2007)
Article DOI: 10.1021/jm701137s
BindingDB Entry DOI: 10.7270/Q2P84BM9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50297304

(CHEMBL560741 | {2-[3-(4-Fluoro-benzyl)-1H-inden-2-...)Show InChI InChI=1S/C20H22FN/c1-22(2)12-11-17-14-16-5-3-4-6-19(16)20(17)13-15-7-9-18(21)10-8-15/h3-10H,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166450

((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H22F5N3O3/c1-38-23-12-5-9-17(24(23)29)18-13-34(14-19-20(27(30,31)32)10-6-11-21(19)28)26(37)35(25(18)36)15-22(33)16-7-3-2-4-8-16/h2-13,22H,14-15,33H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 51: 7478-85 (2009)
Article DOI: 10.1021/jm8006454
BindingDB Entry DOI: 10.7270/Q2S46RT8 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166450

((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H22F5N3O3/c1-38-23-12-5-9-17(24(23)29)18-13-34(14-19-20(27(30,31)32)10-6-11-21(19)28)26(37)35(25(18)36)15-22(33)16-7-3-2-4-8-16/h2-13,22H,14-15,33H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GnRHR |
Bioorg Med Chem Lett 18: 3301-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.036
BindingDB Entry DOI: 10.7270/Q2JD4WKM |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315205
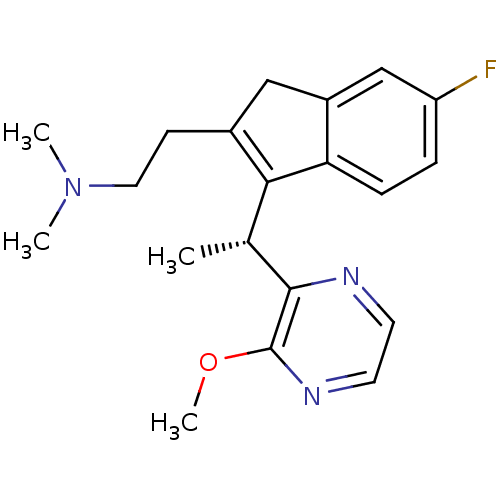
((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(F)ccc12 |r,c:11| Show InChI InChI=1S/C20H24FN3O/c1-13(19-20(25-4)23-9-8-22-19)18-14(7-10-24(2)3)11-15-12-16(21)5-6-17(15)18/h5-6,8-9,12-13H,7,10-11H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of histamine H1 receptor |
Bioorg Med Chem Lett 20: 5874-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.117
BindingDB Entry DOI: 10.7270/Q26W9BDC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50190927
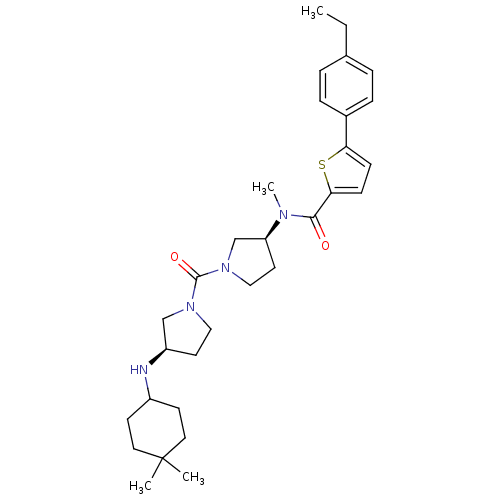
(CHEMBL214513 | N-((S)-1-((R)-3-(4,4-dimethylcycloh...)Show SMILES CCc1ccc(cc1)-c1ccc(s1)C(=O)N(C)[C@H]1CCN(C1)C(=O)N1CC[C@H](C1)NC1CCC(C)(C)CC1 Show InChI InChI=1S/C31H44N4O2S/c1-5-22-6-8-23(9-7-22)27-10-11-28(38-27)29(36)33(4)26-15-19-35(21-26)30(37)34-18-14-25(20-34)32-24-12-16-31(2,3)17-13-24/h6-11,24-26,32H,5,12-21H2,1-4H3/t25-,26+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 by competitive binding assay |
Bioorg Med Chem Lett 16: 4922-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.049
BindingDB Entry DOI: 10.7270/Q2TM7BX5 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315205

((R)-2-(6-fluoro-3-(1-(3-methoxypyrazin-2-yl)ethyl)...)Show SMILES COc1nccnc1[C@H](C)C1=C(CCN(C)C)Cc2cc(F)ccc12 |r,c:11| Show InChI InChI=1S/C20H24FN3O/c1-13(19-20(25-4)23-9-8-22-19)18-14(7-10-24(2)3)11-15-12-16(21)5-6-17(15)18/h5-6,8-9,12-13H,7,10-11H2,1-4H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50221120
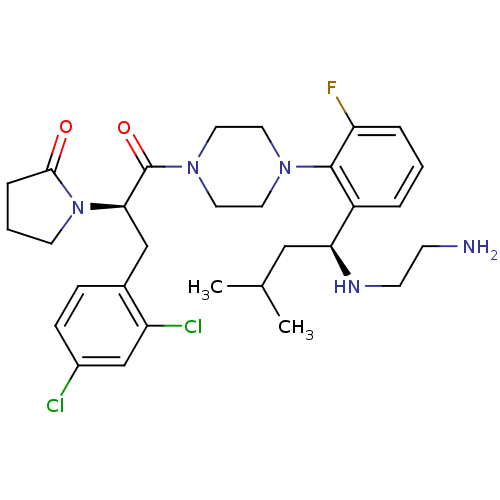
(1-((R)-1-(4-(2-((S)-1-(2-aminoethylamino)-3-methyl...)Show SMILES CC(C)C[C@H](NCCN)c1cccc(F)c1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1Cl)N1CCCC1=O Show InChI InChI=1S/C30H40Cl2FN5O2/c1-20(2)17-26(35-11-10-34)23-5-3-6-25(33)29(23)36-13-15-37(16-14-36)30(40)27(38-12-4-7-28(38)39)18-21-8-9-22(31)19-24(21)32/h3,5-6,8-9,19-20,26-27,35H,4,7,10-18,34H2,1-2H3/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mouse MC4R |
J Med Chem 50: 6356-66 (2007)
Article DOI: 10.1021/jm701137s
BindingDB Entry DOI: 10.7270/Q2P84BM9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50314264
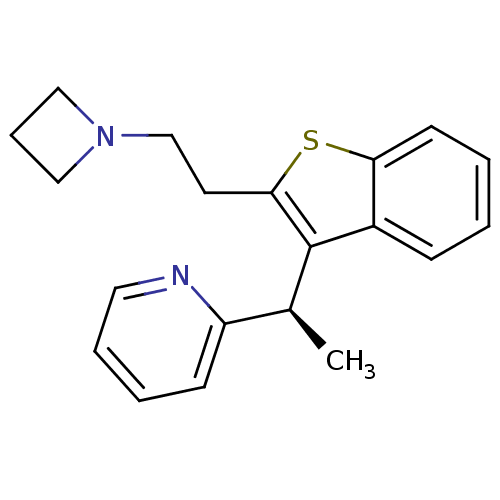
((-)-(R)-2-(1-(2-(2-(azetidin-1-yl)ethyl)benzo[b]th...)Show SMILES C[C@H](c1c(CCN2CCC2)sc2ccccc12)c1ccccn1 |r| Show InChI InChI=1S/C20H22N2S/c1-15(17-8-4-5-11-21-17)20-16-7-2-3-9-18(16)23-19(20)10-14-22-12-6-13-22/h2-5,7-9,11,15H,6,10,12-14H2,1H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK cells assessed as blockade of potassium tail current by standard patch clamp analysis |
Bioorg Med Chem Lett 20: 2316-20 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.134
BindingDB Entry DOI: 10.7270/Q2P55PG0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50214688
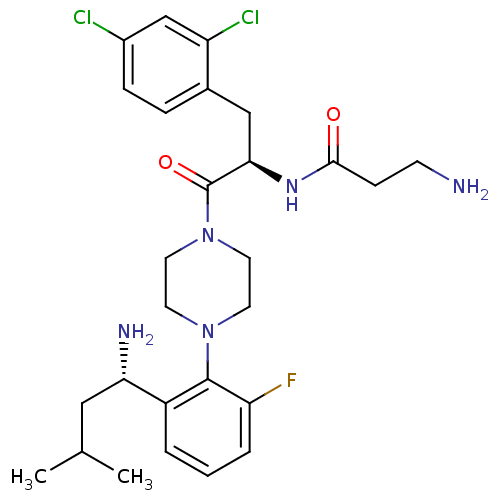
(1-{4-[(1R)-2-(amino-3-methylbutyl)-6-fluorophenyl]...)Show SMILES CC(C)C[C@H](N)c1cccc(F)c1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1Cl)NC(=O)CCN Show InChI InChI=1S/C27H36Cl2FN5O2/c1-17(2)14-23(32)20-4-3-5-22(30)26(20)34-10-12-35(13-11-34)27(37)24(33-25(36)8-9-31)15-18-6-7-19(28)16-21(18)29/h3-7,16-17,23-24H,8-15,31-32H2,1-2H3,(H,33,36)/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-MSH from MC4R receptor in HEK293 cells |
Bioorg Med Chem 15: 5166-76 (2007)
Article DOI: 10.1016/j.bmc.2007.05.026
BindingDB Entry DOI: 10.7270/Q2FF3S2P |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50214682
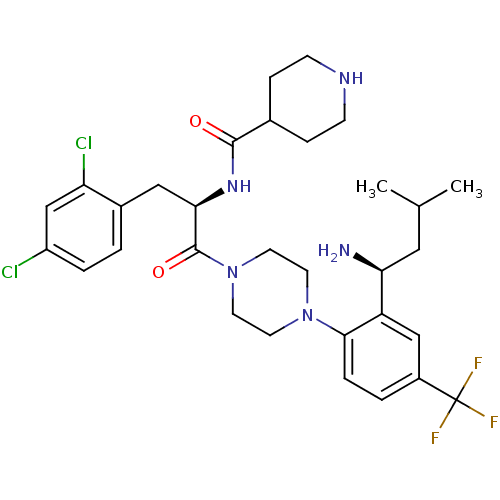
(1-{4-[(1S)-2-(1-amino-3-methylbutyl)-4-trifluorome...)Show SMILES CC(C)C[C@H](N)c1cc(ccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1CCNCC1)C(F)(F)F Show InChI InChI=1S/C31H40Cl2F3N5O2/c1-19(2)15-26(37)24-17-22(31(34,35)36)4-6-28(24)40-11-13-41(14-12-40)30(43)27(16-21-3-5-23(32)18-25(21)33)39-29(42)20-7-9-38-10-8-20/h3-6,17-20,26-27,38H,7-16,37H2,1-2H3,(H,39,42)/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-MSH from MC4R receptor in HEK293 cells |
Bioorg Med Chem 15: 5166-76 (2007)
Article DOI: 10.1016/j.bmc.2007.05.026
BindingDB Entry DOI: 10.7270/Q2FF3S2P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50361005
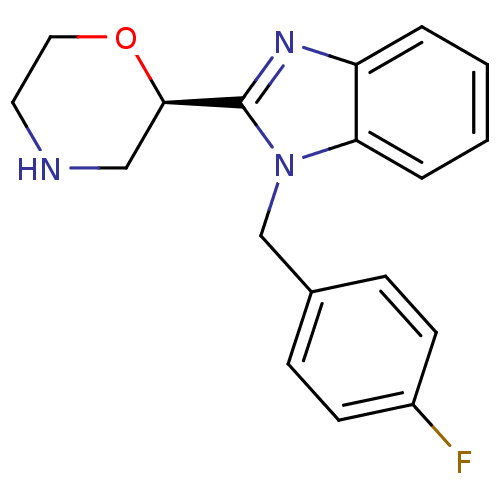
(CHEMBL1935442)Show SMILES Fc1ccc(Cn2c(nc3ccccc23)[C@H]2CNCCO2)cc1 |r| Show InChI InChI=1S/C18H18FN3O/c19-14-7-5-13(6-8-14)12-22-16-4-2-1-3-15(16)21-18(22)17-11-20-9-10-23-17/h1-8,17,20H,9-12H2/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor |
Bioorg Med Chem Lett 22: 421-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.115
BindingDB Entry DOI: 10.7270/Q2J103KS |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315198
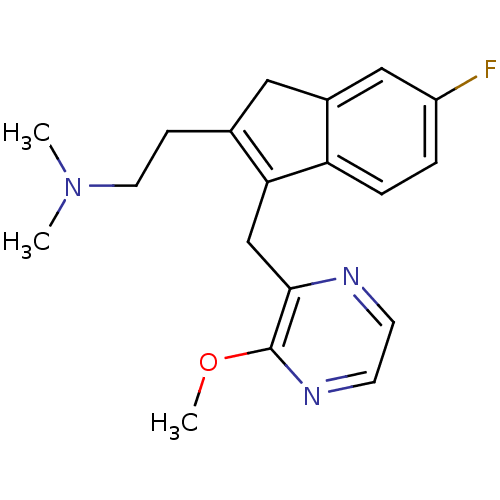
(2-(6-fluoro-3-((3-methoxypyrazin-2-yl)methyl)-1H-i...)Show SMILES COc1nccnc1CC1=C(CCN(C)C)Cc2cc(F)ccc12 |c:10| Show InChI InChI=1S/C19H22FN3O/c1-23(2)9-6-13-10-14-11-15(20)4-5-16(14)17(13)12-18-19(24-3)22-8-7-21-18/h4-5,7-8,11H,6,9-10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50316936
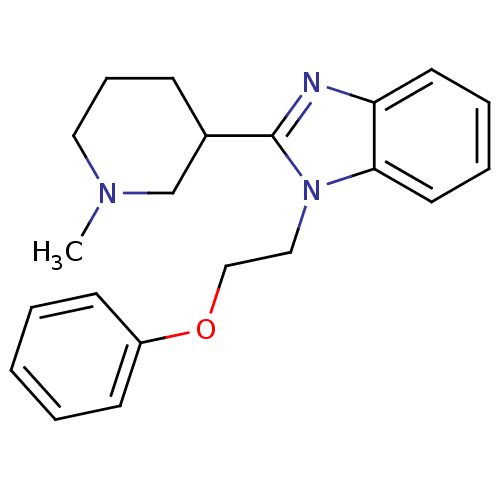
(2-(1-methylpiperidin-3-yl)-1-(2-phenoxyethyl)-1H-b...)Show InChI InChI=1S/C21H25N3O/c1-23-13-7-8-17(16-23)21-22-19-11-5-6-12-20(19)24(21)14-15-25-18-9-3-2-4-10-18/h2-6,9-12,17H,7-8,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor |
Bioorg Med Chem Lett 22: 421-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.115
BindingDB Entry DOI: 10.7270/Q2J103KS |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50315191
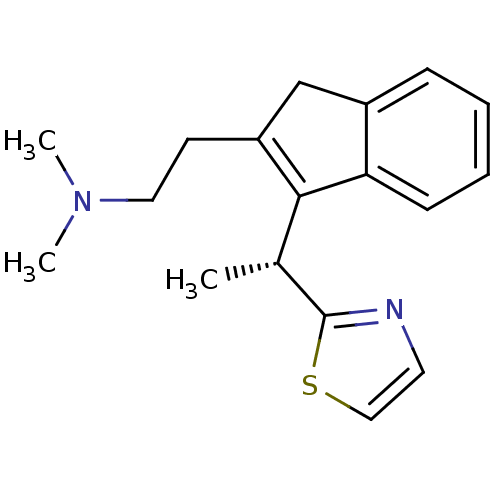
((R)-N,N-dimethyl-2-(3-(1-(thiazol-2-yl)ethyl)-1H-i...)Show SMILES C[C@H](C1=C(CCN(C)C)Cc2ccccc12)c1nccs1 |r,c:2| Show InChI InChI=1S/C18H22N2S/c1-13(18-19-9-11-21-18)17-15(8-10-20(2)3)12-14-6-4-5-7-16(14)17/h4-7,9,11,13H,8,10,12H2,1-3H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H1 receptor |
Bioorg Med Chem Lett 20: 2629-33 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.055
BindingDB Entry DOI: 10.7270/Q2NG4QSV |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50221120

(1-((R)-1-(4-(2-((S)-1-(2-aminoethylamino)-3-methyl...)Show SMILES CC(C)C[C@H](NCCN)c1cccc(F)c1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1Cl)N1CCCC1=O Show InChI InChI=1S/C30H40Cl2FN5O2/c1-20(2)17-26(35-11-10-34)23-5-3-6-25(33)29(23)36-13-15-37(16-14-36)30(40)27(38-12-4-7-28(38)39)18-21-8-9-22(31)19-24(21)32/h3,5-6,8-9,19-20,26-27,35H,4,7,10-18,34H2,1-2H3/t26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDPMSH from human MC4R expressed in HEK293 cells |
J Med Chem 50: 6356-66 (2007)
Article DOI: 10.1021/jm701137s
BindingDB Entry DOI: 10.7270/Q2P84BM9 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166451
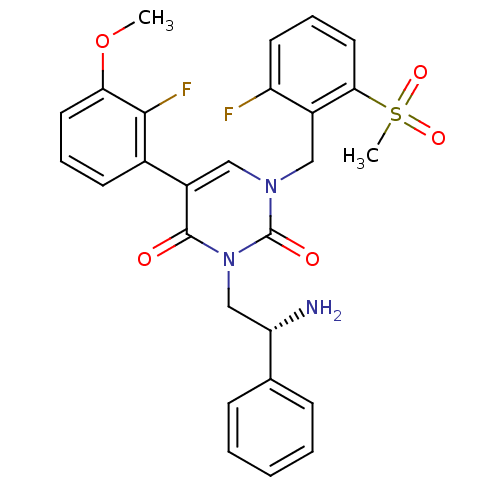
((R)-1-(2-fluoro-6-(methylsulfonyl)benzyl)-3-(2-ami...)Show SMILES COc1cccc(c1F)-c1cn(Cc2c(F)cccc2S(C)(=O)=O)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H25F2N3O5S/c1-37-23-12-6-10-18(25(23)29)19-14-31(15-20-21(28)11-7-13-24(20)38(2,35)36)27(34)32(26(19)33)16-22(30)17-8-4-3-5-9-17/h3-14,22H,15-16,30H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GnRHR |
Bioorg Med Chem Lett 18: 3301-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.036
BindingDB Entry DOI: 10.7270/Q2JD4WKM |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50190919
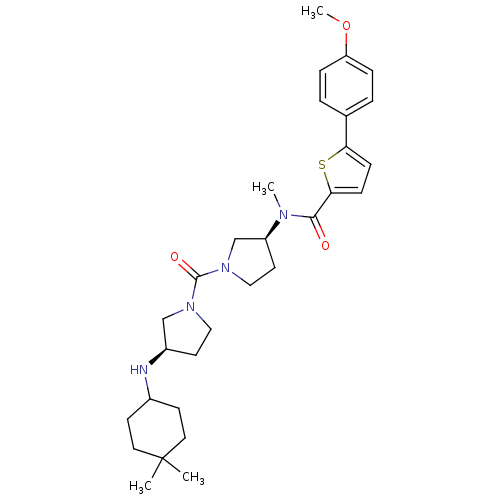
(CHEMBL386454 | N-((S)-1-((R)-3-(4,4-dimethylcycloh...)Show SMILES COc1ccc(cc1)-c1ccc(s1)C(=O)N(C)[C@H]1CCN(C1)C(=O)N1CC[C@H](C1)NC1CCC(C)(C)CC1 Show InChI InChI=1S/C30H42N4O3S/c1-30(2)15-11-22(12-16-30)31-23-13-17-33(19-23)29(36)34-18-14-24(20-34)32(3)28(35)27-10-9-26(38-27)21-5-7-25(37-4)8-6-21/h5-10,22-24,31H,11-20H2,1-4H3/t23-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to MCHR1 by competitive binding assay |
Bioorg Med Chem Lett 16: 4922-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.049
BindingDB Entry DOI: 10.7270/Q2TM7BX5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data