

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364189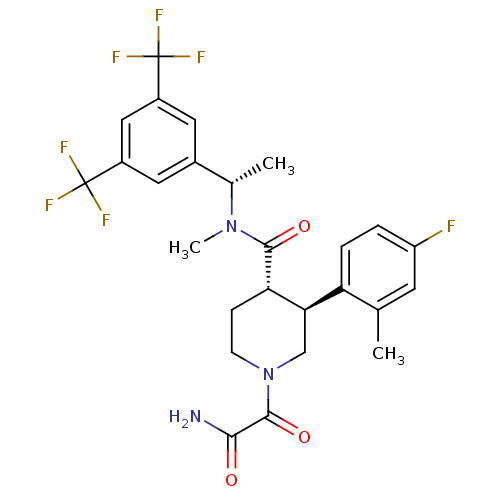 (CHEMBL1951813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190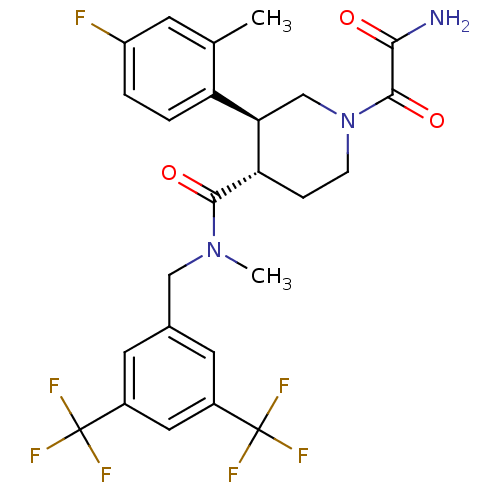 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364190 (CHEMBL1951810) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM47085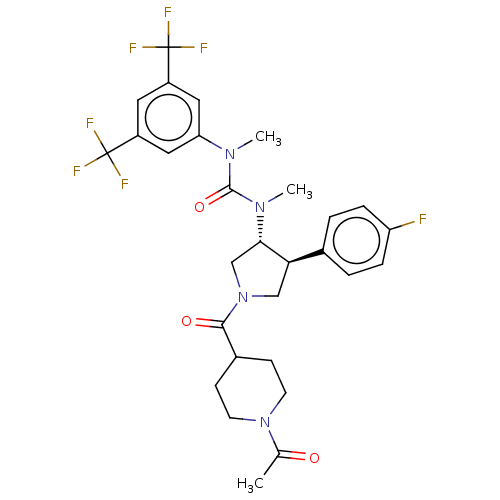 (US8592454, 71b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106978 (US8592454, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106983 (US8592454, 420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106974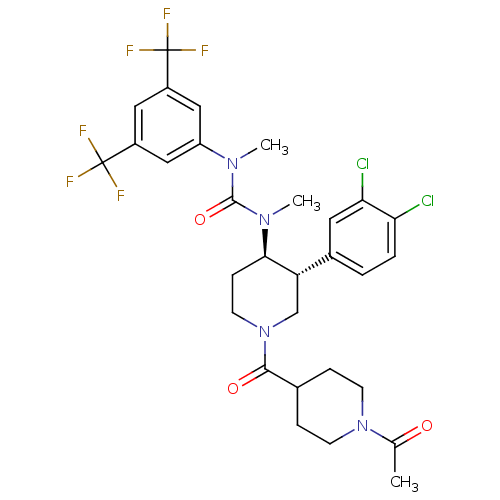 (US8592454, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106979 (US8592454, 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364202 (CHEMBL1951626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364195 (CHEMBL1951633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106982 (US8592454, 378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364197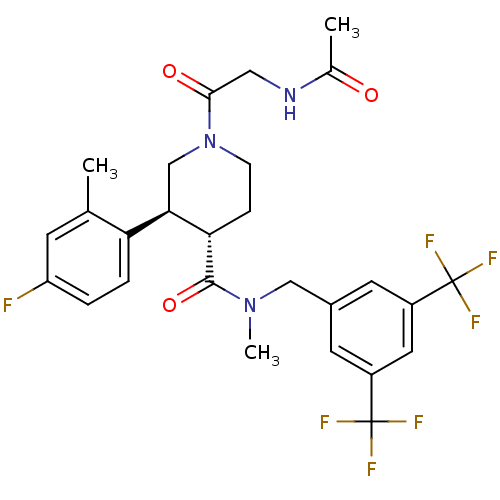 (CHEMBL1951631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364192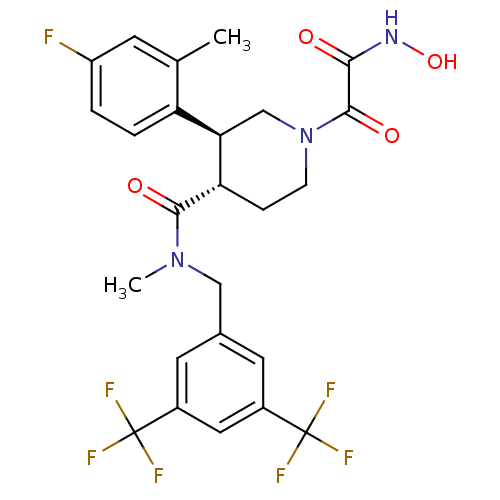 (CHEMBL1951811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364198 (CHEMBL1951630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364196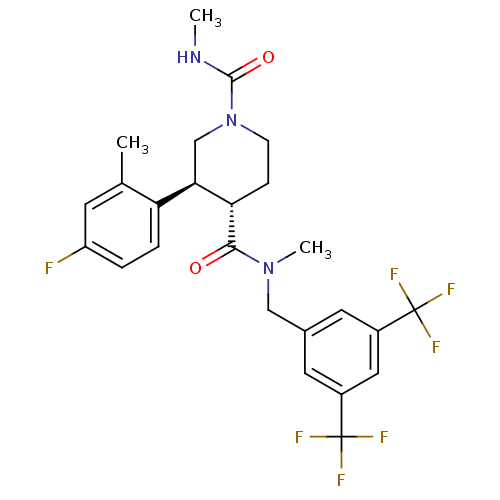 (CHEMBL1951632) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106980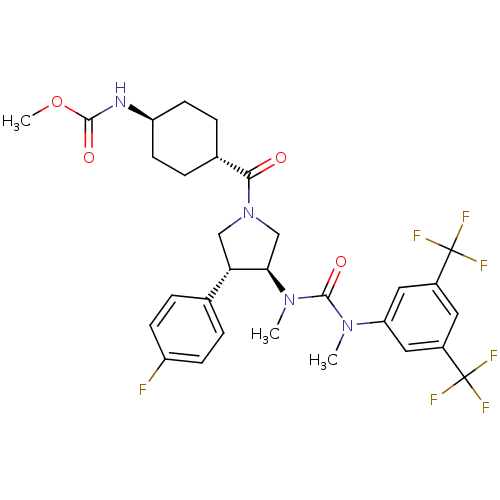 (US8592454, 233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364199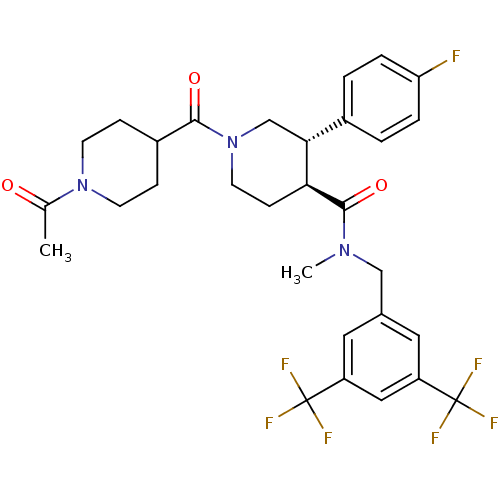 (CHEMBL1951629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364201 (CHEMBL1951627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106981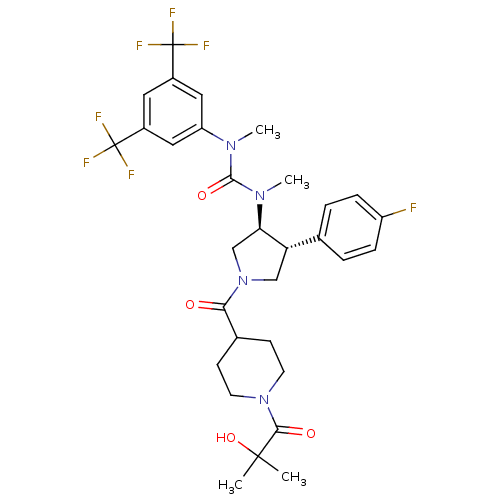 (US8592454, 308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106977 (US8592454, 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364194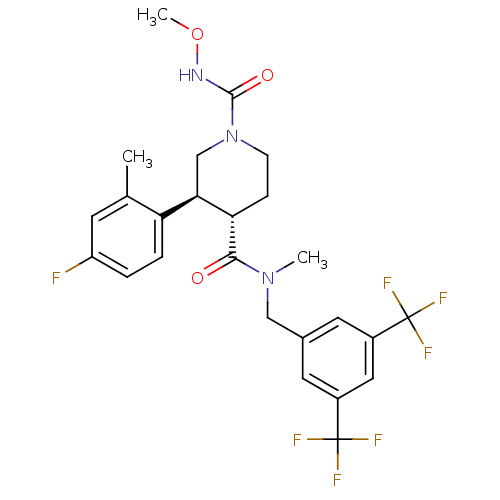 (CHEMBL1951634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106976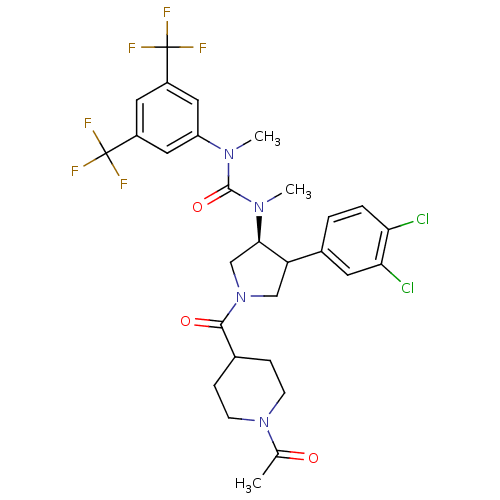 (US8592454, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364207 (CHEMBL1951621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364193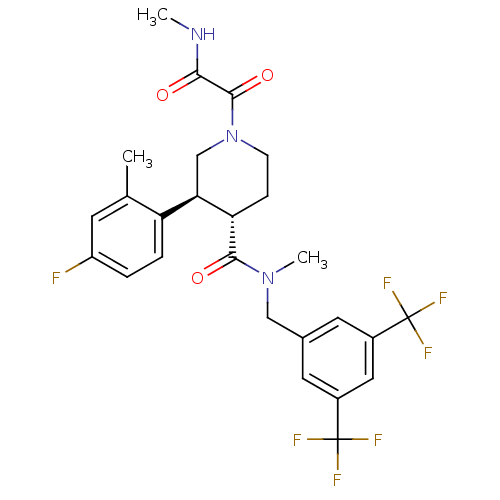 (CHEMBL1951635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM106973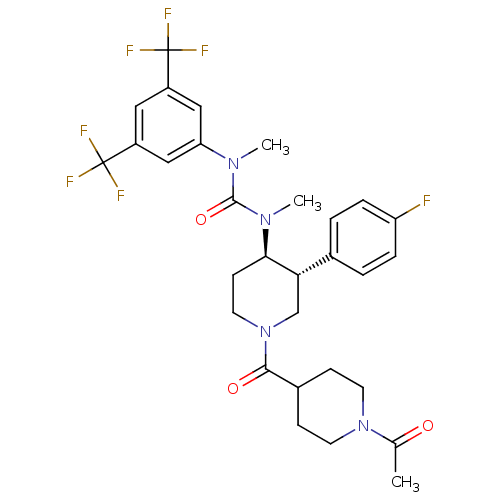 (US8592454, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364203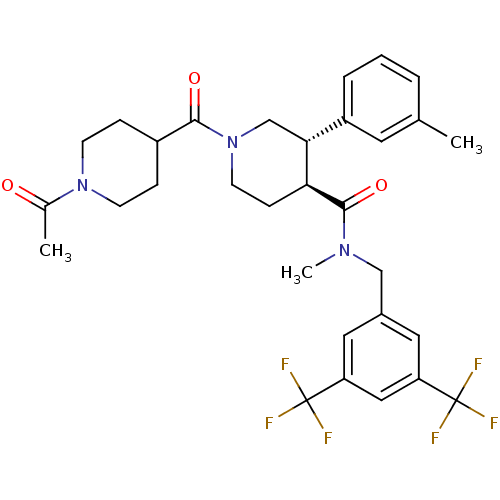 (CHEMBL1951625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364205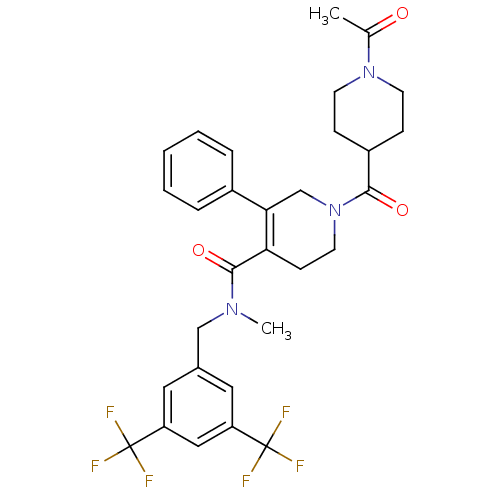 (CHEMBL1951623) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364204 (CHEMBL1951624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Mus musculus) | BDBM50247081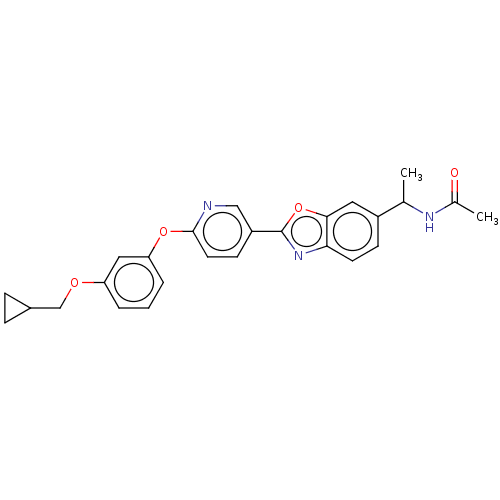 (CHEMBL4060253) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of mouse His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins followed by subst... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364188 (CHEMBL1951814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50364200 (CHEMBL1951628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from tachykinin NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 20: 962-77 (2012) Article DOI: 10.1016/j.bmc.2011.11.048 BindingDB Entry DOI: 10.7270/Q2NP24WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50254020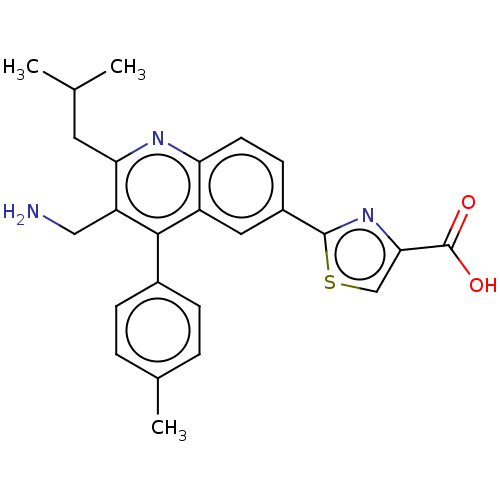 (CHEMBL4068446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins | Bioorg Med Chem Lett 27: 3565-3571 (2017) Article DOI: 10.1016/j.bmcl.2017.05.048 BindingDB Entry DOI: 10.7270/Q2PK0JKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130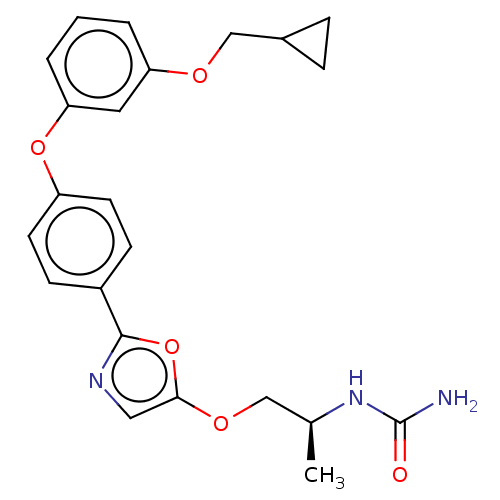 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114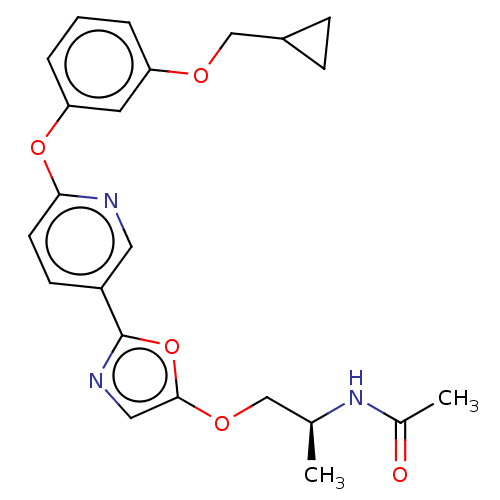 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112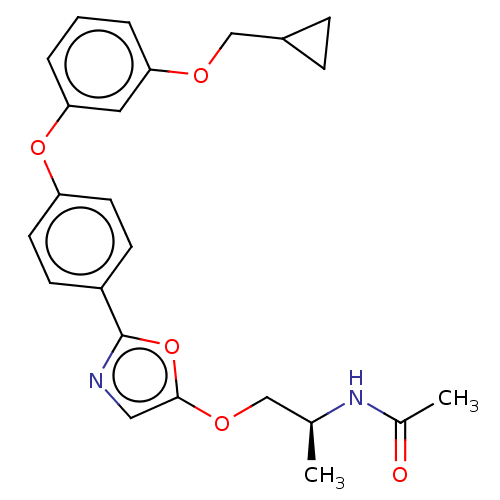 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50333177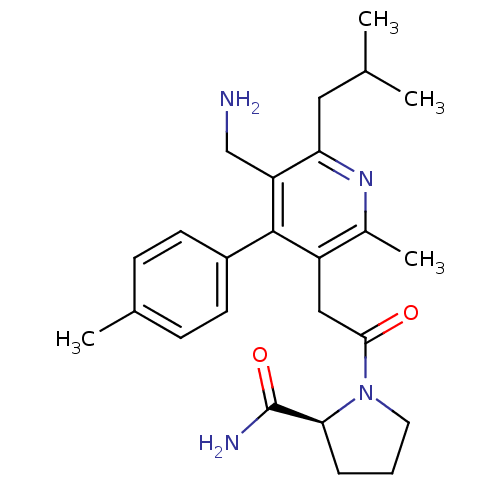 (1-{[5-(Aminomethyl)-2-methyl-4-(4-methylphenyl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry | Bioorg Med Chem 19: 172-85 (2011) Article DOI: 10.1016/j.bmc.2010.11.038 BindingDB Entry DOI: 10.7270/Q2TB174K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50336806 (1-(3-(aminomethyl)-2-isobutyl-4-p-tolylquinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco2 cells after 60 mins | J Med Chem 54: 831-50 (2012) Article DOI: 10.1021/jm101236h BindingDB Entry DOI: 10.7270/Q2QR4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50333175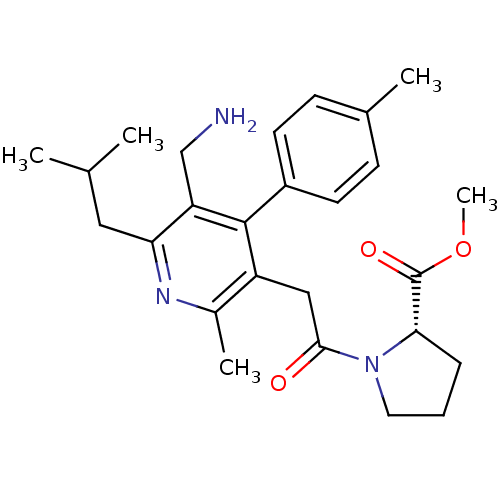 (CHEMBL1644848 | Methyl 1-{[5-(aminomethyl)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry | Bioorg Med Chem 19: 172-85 (2011) Article DOI: 10.1016/j.bmc.2010.11.038 BindingDB Entry DOI: 10.7270/Q2TB174K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267928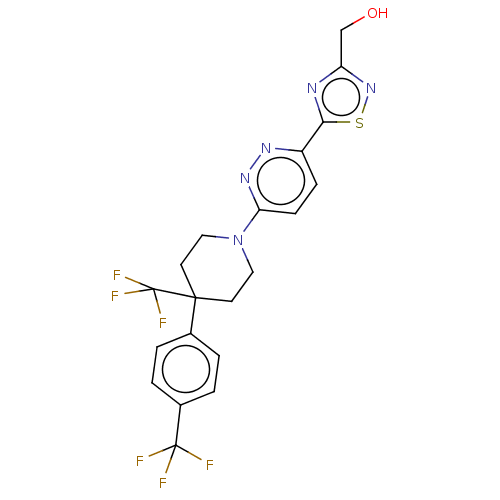 (CHEMBL4066506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50336806 (1-(3-(aminomethyl)-2-isobutyl-4-p-tolylquinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529222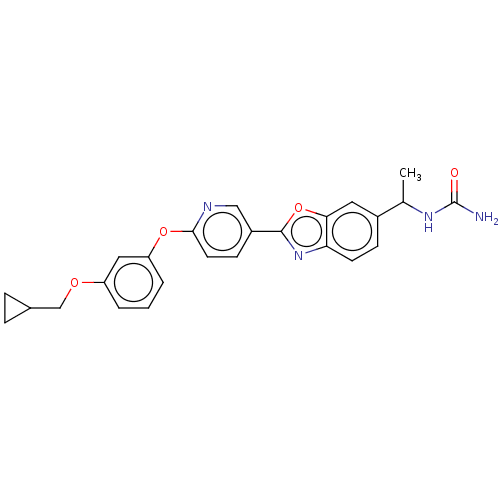 (CHEMBL4528475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349380 (CHEMBL1808468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50333167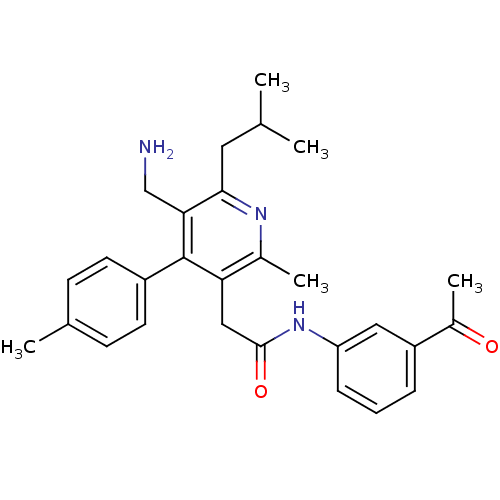 (3-({[5-(Aminomethyl)-2-methyl-4-(4-methylphenyl)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry | Bioorg Med Chem 19: 172-85 (2011) Article DOI: 10.1016/j.bmc.2010.11.038 BindingDB Entry DOI: 10.7270/Q2TB174K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM106976 (US8592454, 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Radioligand receptor binding inhibitory assay using human NK receptor. | US Patent US8592454 (2013) BindingDB Entry DOI: 10.7270/Q2XD10B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529224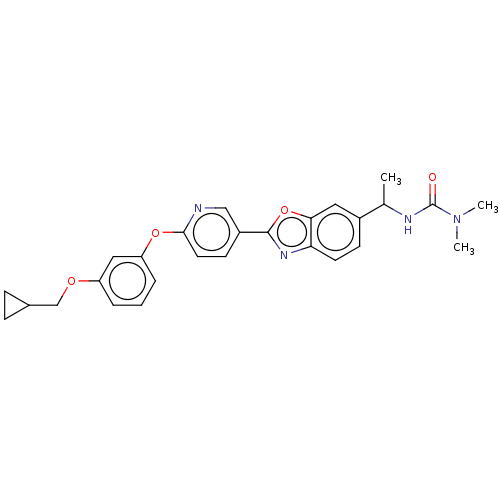 (CHEMBL4435891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253979 (CHEMBL4068477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human DPP4 purified from Caco2 cells pre-incubated for 15 mins before Gly-Pro-pNA substrate addition and measured after 60 mins | Bioorg Med Chem Lett 27: 3565-3571 (2017) Article DOI: 10.1016/j.bmcl.2017.05.048 BindingDB Entry DOI: 10.7270/Q2PK0JKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50349384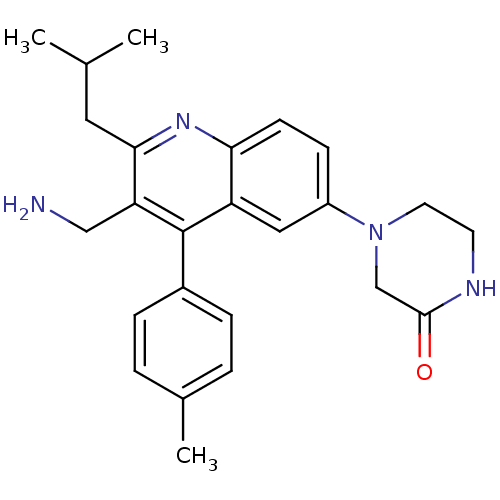 (CHEMBL1808473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase-4 expressed in Caco-2 cells using Gly-Pro-pNA.Tos as substrate after 60 mins by microplate reader analysis | Bioorg Med Chem 19: 4482-98 (2011) Article DOI: 10.1016/j.bmc.2011.06.032 BindingDB Entry DOI: 10.7270/Q2P26ZGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 499 total ) | Next | Last >> |