

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398985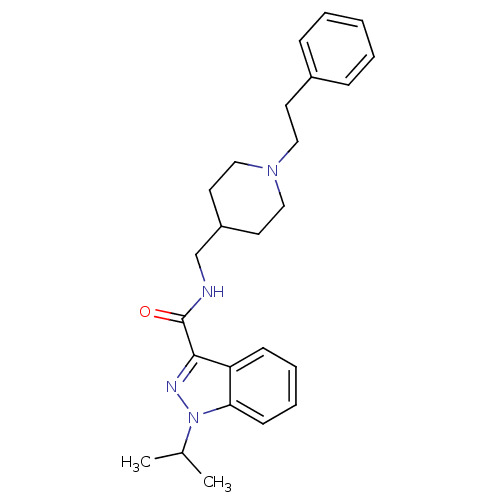 (CHEMBL2177130) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398983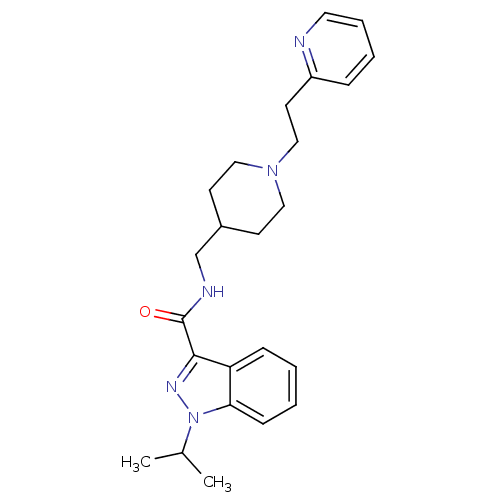 (CHEMBL2179697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026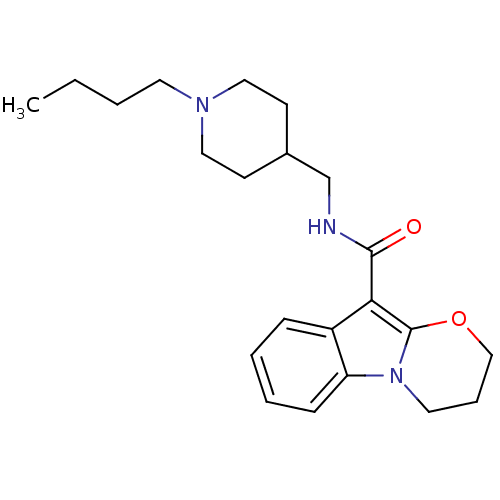 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398981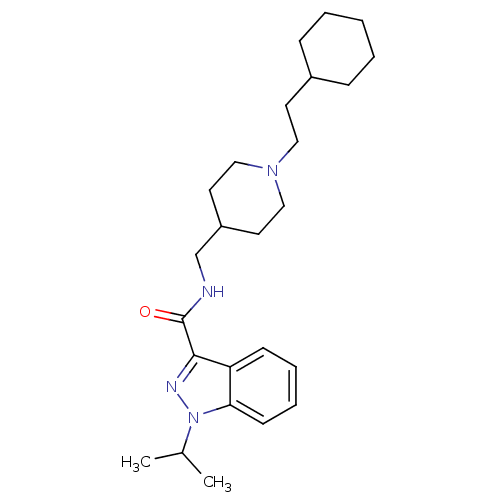 (CHEMBL2179699) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398975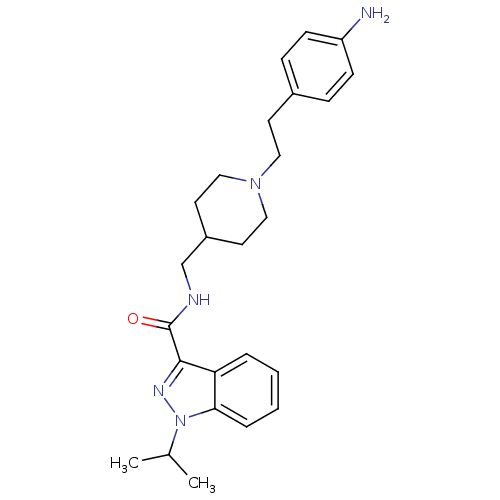 (CHEMBL2179706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398978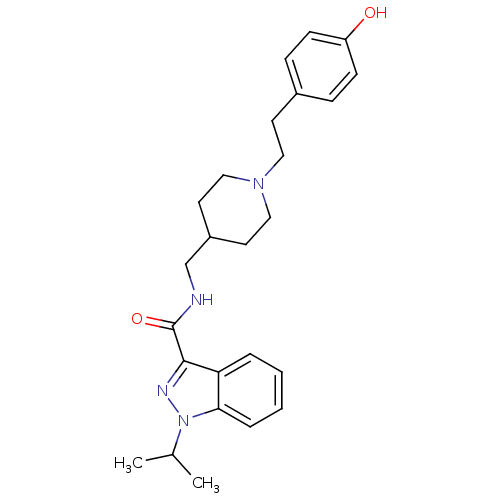 (CHEMBL2179704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398976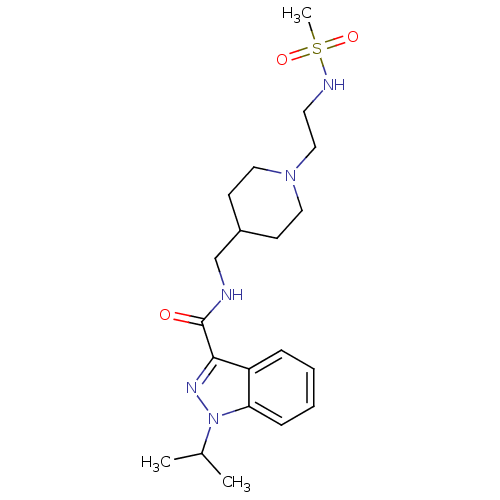 (CHEMBL2179702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398980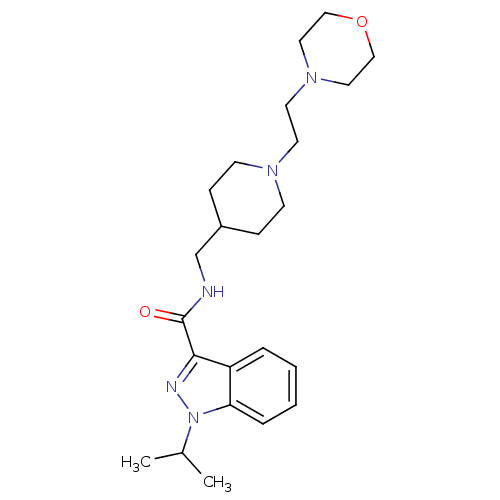 (CHEMBL2179700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398986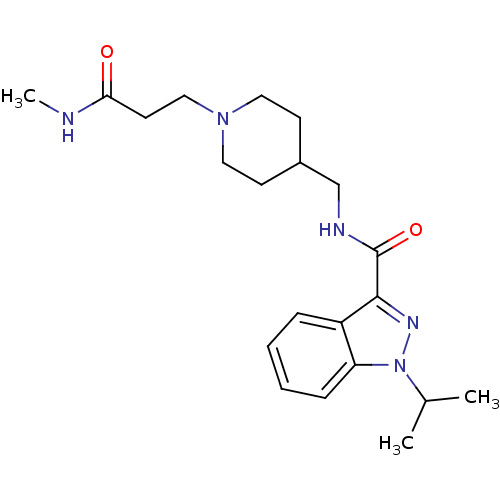 (CHEMBL2179703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398984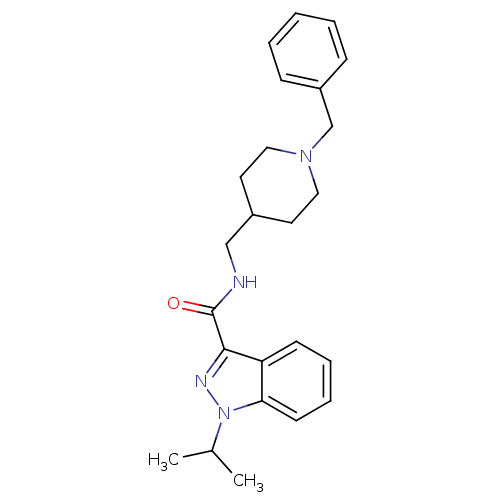 (CHEMBL2179696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398964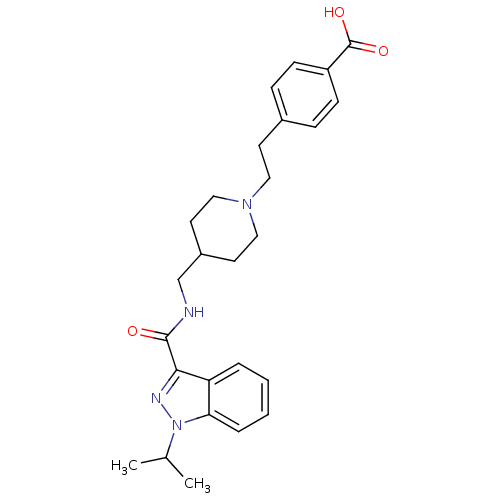 (CHEMBL2179707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398979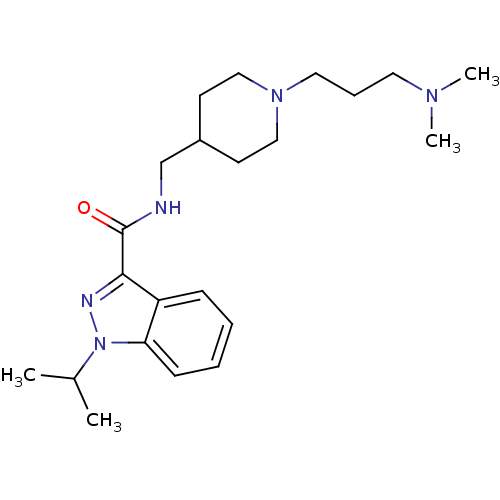 (CHEMBL2179701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398977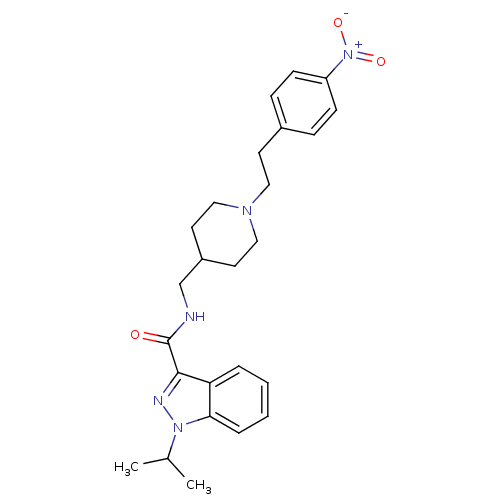 (CHEMBL2179705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50031942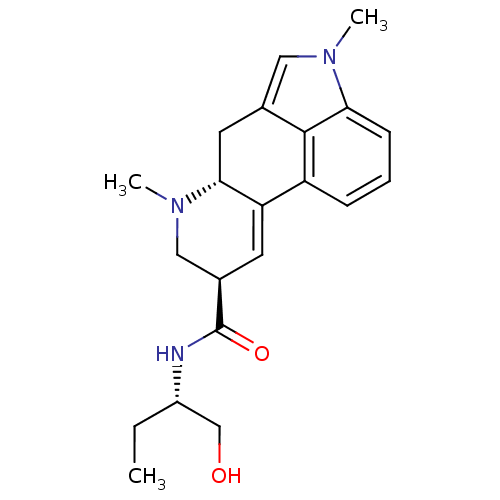 ((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398965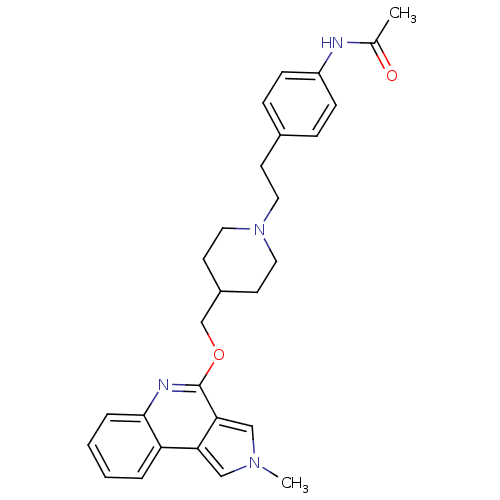 (CHEMBL2179670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398963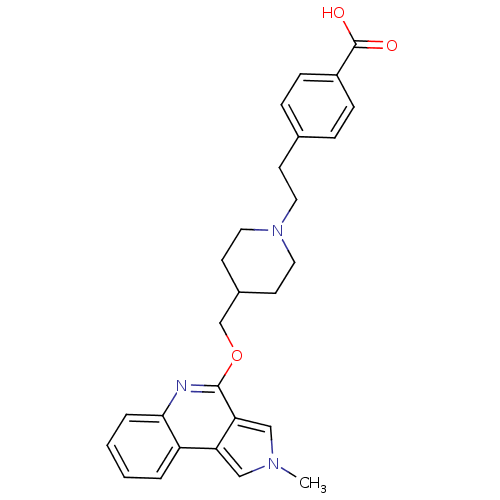 (CHEMBL2179674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398972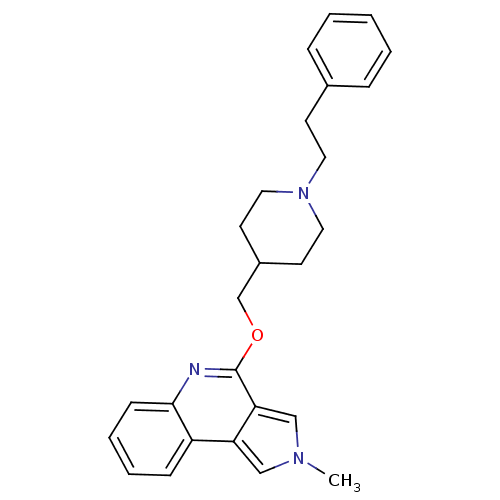 (CHEMBL2179678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398969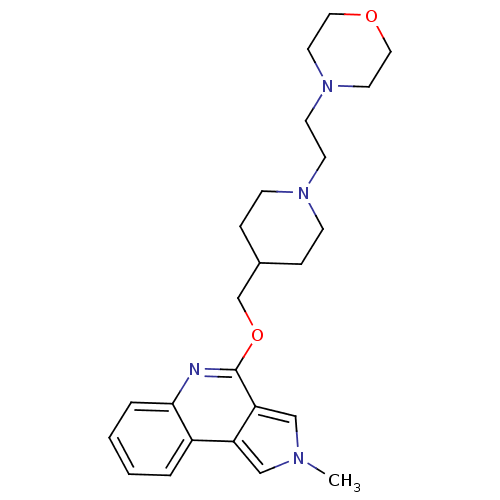 (CHEMBL2179676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50477178 (CHEMBL239063) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 3B reverse transcriptase | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50467700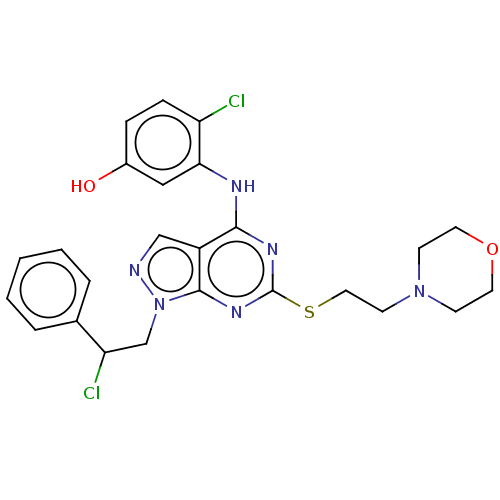 (CHEMBL4286927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human c-Src using KVEKIGEGTYGVVYK as substrate in presence of [gamma32P]ATP by scintillation counting method | Bioorg Med Chem Lett 28: 3454-3457 (2018) Article DOI: 10.1016/j.bmcl.2018.09.024 BindingDB Entry DOI: 10.7270/Q2HT2S18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50467697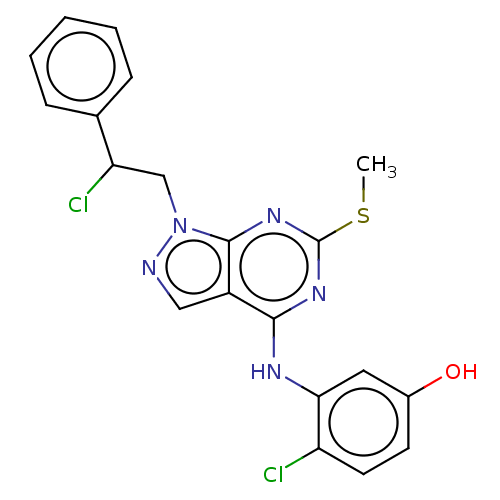 (CHEMBL4277981) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human c-Src using KVEKIGEGTYGVVYK as substrate in presence of [gamma32P]ATP by scintillation counting method | Bioorg Med Chem Lett 28: 3454-3457 (2018) Article DOI: 10.1016/j.bmcl.2018.09.024 BindingDB Entry DOI: 10.7270/Q2HT2S18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2325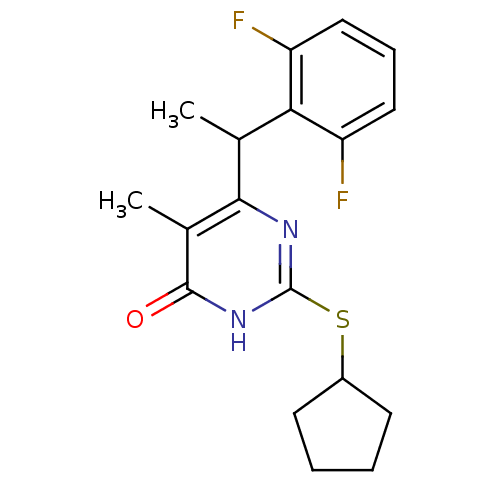 (2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 3B reverse transcriptase | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50436581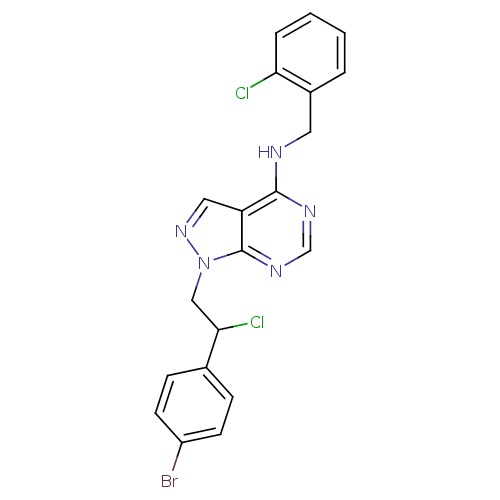 (CHEMBL2397805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to recombinant wild type human Abl using abitide as substrate | J Med Chem 56: 5382-94 (2014) Article DOI: 10.1021/jm400233w BindingDB Entry DOI: 10.7270/Q29G5P63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398968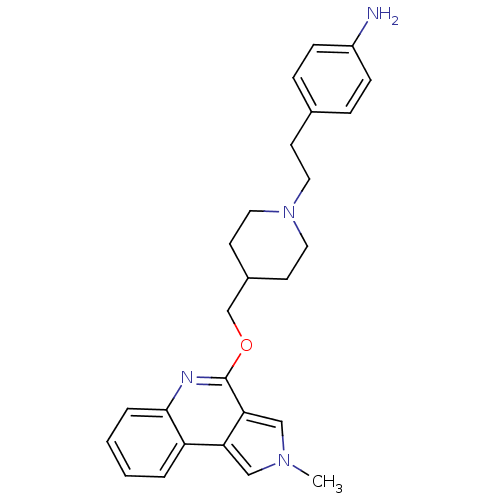 (CHEMBL2179675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102904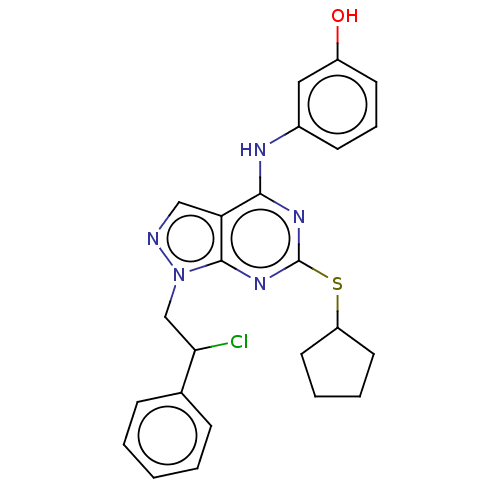 (CHEMBL3394091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398973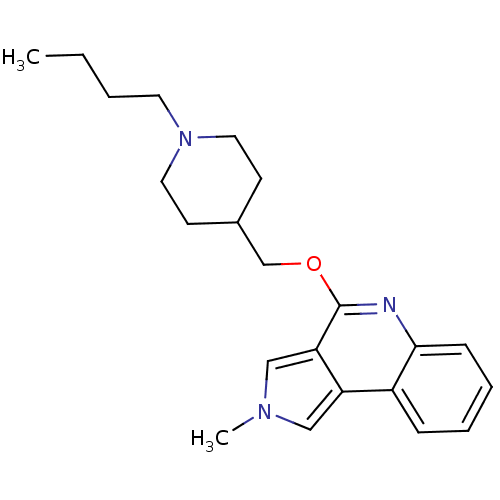 (CHEMBL2179679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50477201 (CHEMBL394990) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase V106A mutant | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA binary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102271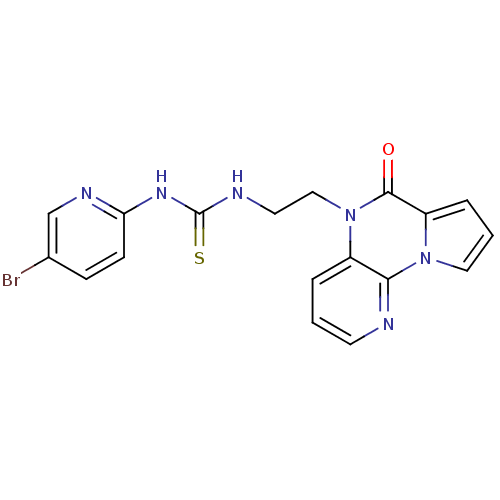 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-oxo-4H-5,9,9b-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102265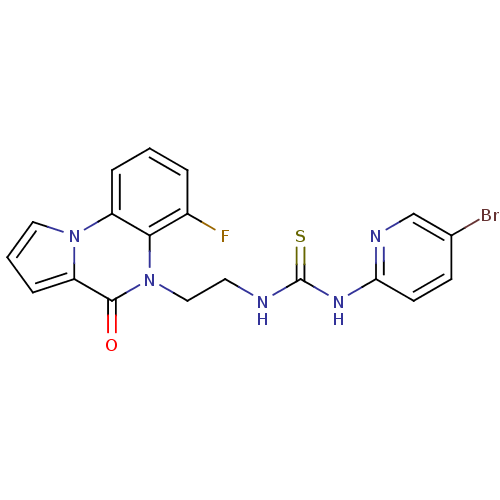 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase-DNA-dNTP ternary complex | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398978 (CHEMBL2179704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50477179 (CHEMBL399016) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase V106A mutant | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50339536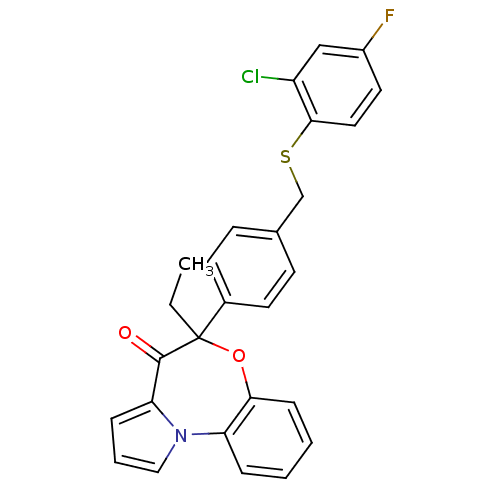 ((+/-)-6-Ethyl-6-[4-[(2-chloro-4-fluorophenylthio)m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
2-Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type reverse transcriptase/poly(rA)/oligo(dT) DNA/dTTP ternary complex | Bioorg Med Chem Lett 21: 3935-8 (2011) Article DOI: 10.1016/j.bmcl.2011.05.020 BindingDB Entry DOI: 10.7270/Q2319ZQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50479681 (CHEMBL478258) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 free reverse transcriptase | J Med Chem 52: 840-51 (2009) Article DOI: 10.1021/jm801330n BindingDB Entry DOI: 10.7270/Q22V2JXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102908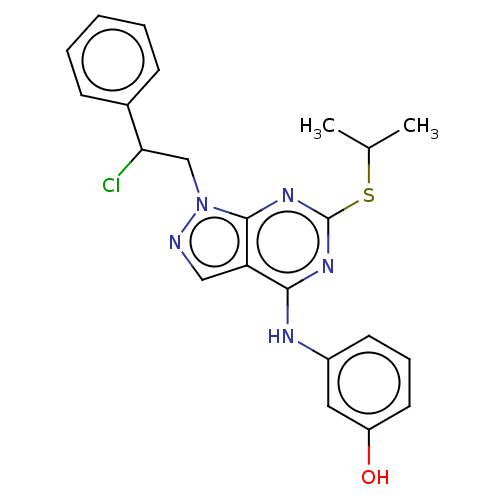 (CHEMBL3393071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50477179 (CHEMBL399016) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase V179D mutant | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398967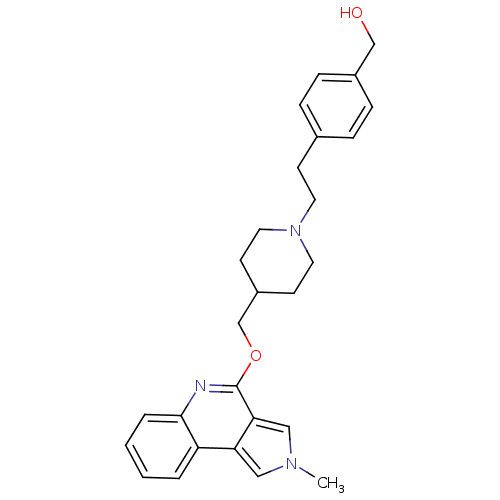 (CHEMBL2179672) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50477199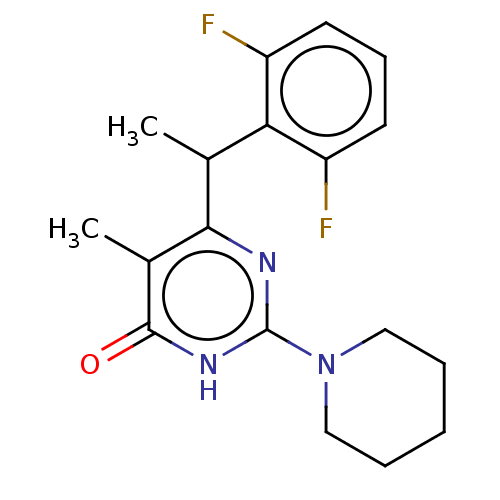 (CHEMBL239342) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase V179D mutant | J Med Chem 50: 5412-24 (2007) Article DOI: 10.1021/jm070811e BindingDB Entry DOI: 10.7270/Q2H99803 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061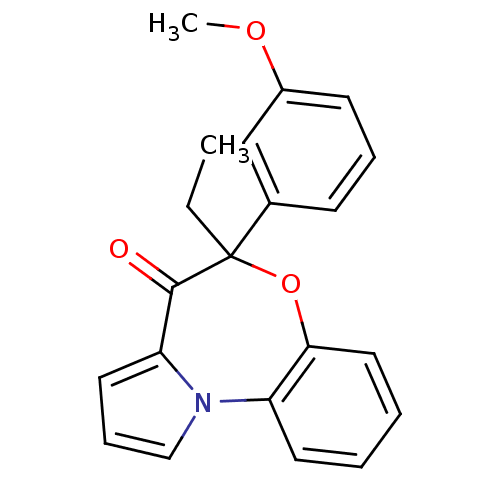 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution V106A | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50082061 ((+/-)-5-ethyl-5-(3-methoxy-phenyl)-6-oxa-10b-aza-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against HIV-1 mutant Reverse transcriptase containing the single amino acid substitution L100I | J Med Chem 42: 4462-70 (1999) BindingDB Entry DOI: 10.7270/Q20R9NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl by filter-binding assay | Eur J Med Chem 43: 2665-76 (2008) Article DOI: 10.1016/j.ejmech.2008.01.034 BindingDB Entry DOI: 10.7270/Q23J3CS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition of human recombinant c-Abl by filter-binding assay | J Med Chem 54: 2610-26 (2011) Article DOI: 10.1021/jm1012819 BindingDB Entry DOI: 10.7270/Q28K79F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit&aagrov; degli Studi di Siena Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 1207-11 (2008) Article DOI: 10.1016/j.bmcl.2007.11.112 BindingDB Entry DOI: 10.7270/Q23R0RF2 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102268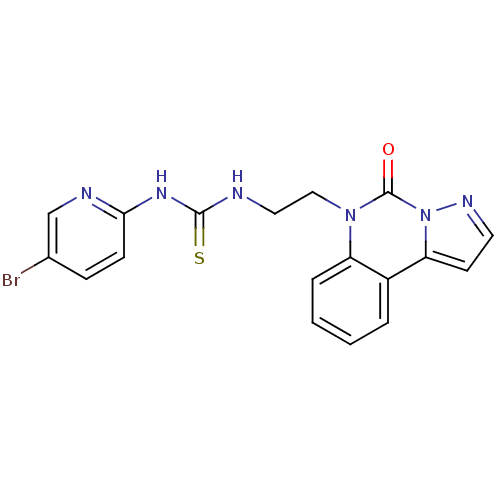 (1-(5-Bromo-pyridin-2-yl)-3-[2-(5-oxo-pyrazolo[1,5-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50102265 (1-(5-Bromo-pyridin-2-yl)-3-[2-(6-fluoro-4-oxo-4H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50262718 (CHEMBL476304 | N-(5-(4-methoxybenzyl)thiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Abl kinase (unknown origin) by cell free assay | Bioorg Med Chem Lett 18: 4328-31 (2008) Article DOI: 10.1016/j.bmcl.2008.06.082 BindingDB Entry DOI: 10.7270/Q2M908GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50467698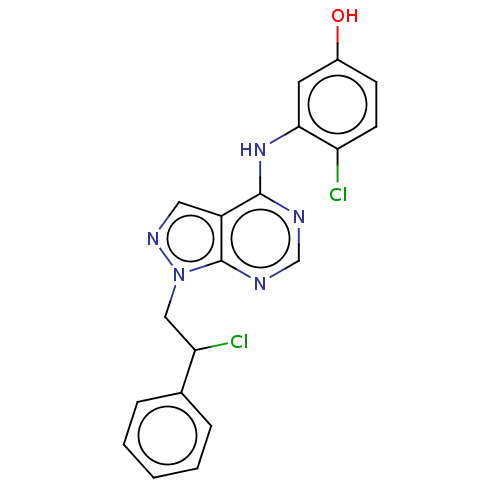 (CHEMBL4286320) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of human c-Src using KVEKIGEGTYGVVYK as substrate in presence of [gamma32P]ATP by scintillation counting method | Bioorg Med Chem Lett 28: 3454-3457 (2018) Article DOI: 10.1016/j.bmcl.2018.09.024 BindingDB Entry DOI: 10.7270/Q2HT2S18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50354489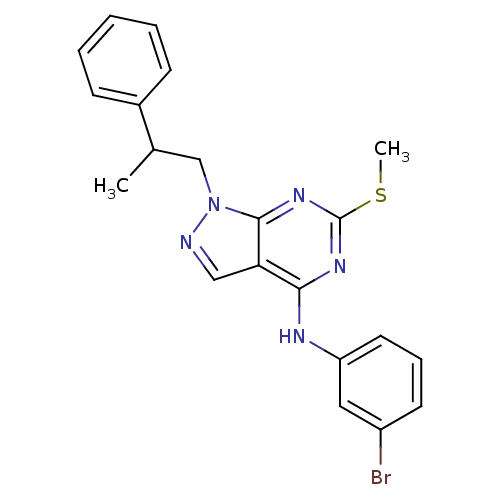 (CHEMBL1836680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena Curated by ChEMBL | Assay Description Inhibition human recombinant cSRC using KVEKIGEGTYGVVYK peptide substrate in presence of [gamma-32P]-ATP | Bioorg Med Chem Lett 21: 5928-33 (2011) Article DOI: 10.1016/j.bmcl.2011.07.079 BindingDB Entry DOI: 10.7270/Q29G5N6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2300 total ) | Next | Last >> |