 Found 137 hits with Last Name = 'manning' and Initial = 'g'
Found 137 hits with Last Name = 'manning' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782
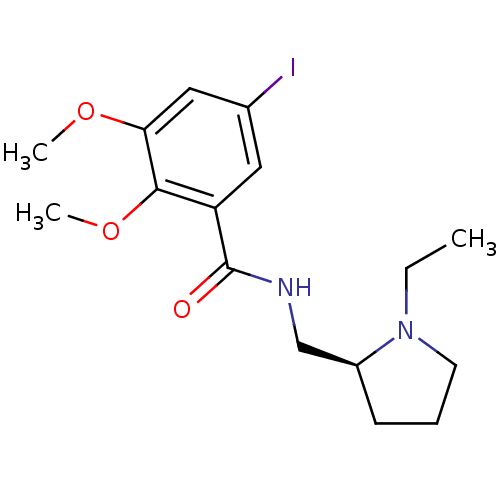
(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096231
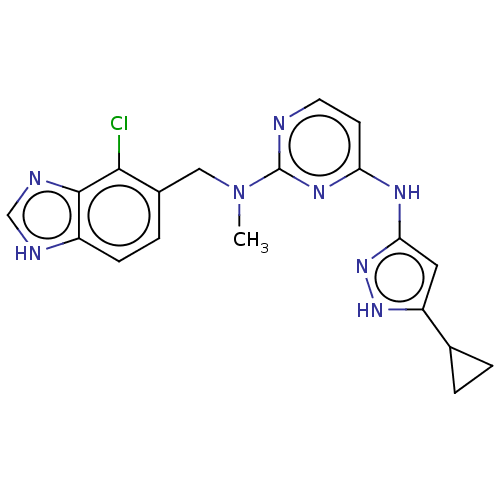
(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096231

(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096233
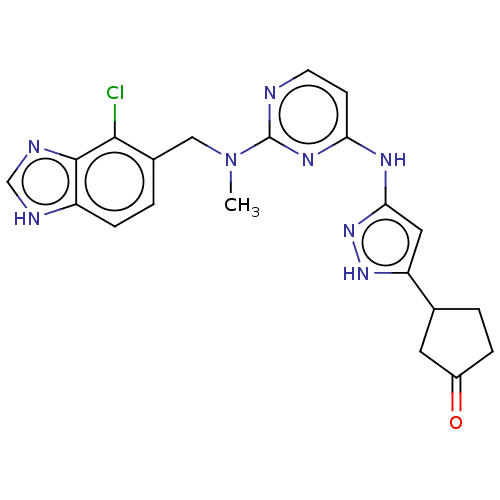
(CHEMBL3593824)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CCC(=O)C2)n1 Show InChI InChI=1S/C34H50BrN5O6/c1-5-21(3)30-34(45)39-17-11-10-14-29(39)33(44)36-26(13-9-7-8-12-24(41)6-2)31(42)37-27(32(43)38-30)18-22-20-40(46-4)28-16-15-23(35)19-25(22)28/h15-16,19,21-22,26-27,29-30H,5-14,17-18,20H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t21?,22?,26-,27-,29+,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096233

(CHEMBL3593824)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CCC(=O)C2)n1 Show InChI InChI=1S/C34H50BrN5O6/c1-5-21(3)30-34(45)39-17-11-10-14-29(39)33(44)36-26(13-9-7-8-12-24(41)6-2)31(42)37-27(32(43)38-30)18-22-20-40(46-4)28-16-15-23(35)19-25(22)28/h15-16,19,21-22,26-27,29-30H,5-14,17-18,20H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t21?,22?,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096228
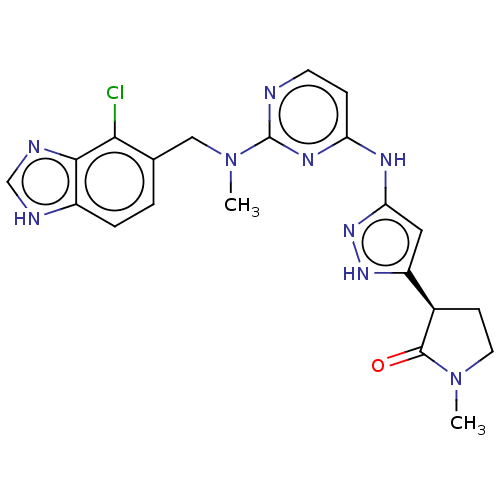
(CHEMBL3594175)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r| Show InChI InChI=1S/C36H53N5O6/c1-5-14-26(42)15-8-7-9-17-28-34(44)38-29(21-25-22-41(47-4)30-18-11-10-16-27(25)30)35(45)39-33(24(3)6-2)32(43)23-40-20-13-12-19-31(40)36(46)37-28/h10-11,16,18,22,24,28-29,31,33H,5-9,12-15,17,19-21,23H2,1-4H3,(H,37,46)(H,38,44)(H,39,45)/t24?,28-,29-,31+,33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096239

(CHEMBL3594180)Show SMILES CCN1CC[C@@H](C1=O)c1cc(Nc2ccnc(n2)N(C)C[C@H]2CC[C@H](N)CC2)n[nH]1 |r,wU:21.22,5.8,wD:24.26,(4.87,-11.79,;3.88,-11.04,;4.08,-9.51,;5.43,-8.77,;5.14,-7.26,;3.62,-7.09,;2.96,-8.46,;1.74,-8.68,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;-2.67,1.54,;-2.67,2.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C41H55N5O7/c1-6-25(3)36-41(51)46-20-14-13-19-35(46)40(50)43-33(18-10-8-9-15-27(47)7-2)38(48)44-34(39(49)45-36)24-31-30-16-11-12-17-32(30)42-37(31)26-21-28(52-4)23-29(22-26)53-5/h11-12,16-17,21-23,25,33-36,42H,6-10,13-15,18-20,24H2,1-5H3,(H,43,50)(H,44,48)(H,45,49)/t25?,33-,34-,35+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096232

(CHEMBL3593823)Show SMILES Clc1c(CNc2nccc(Nc3cc([nH]n3)C3CCCO3)n2)ccc2[nH]cnc12 Show InChI InChI=1S/C33H48N4O6/c1-4-22(3)29-33(43)37-19-13-12-18-28(37)32(42)34-26(17-11-7-10-16-24(38)5-2)30(40)35-27(31(41)36-29)21-25(39)20-23-14-8-6-9-15-23/h6,8-9,14-15,22,26-29H,4-5,7,10-13,16-21H2,1-3H3,(H,34,42)(H,35,40)(H,36,41)/t22?,26-,27-,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096232

(CHEMBL3593823)Show SMILES Clc1c(CNc2nccc(Nc3cc([nH]n3)C3CCCO3)n2)ccc2[nH]cnc12 Show InChI InChI=1S/C33H48N4O6/c1-4-22(3)29-33(43)37-19-13-12-18-28(37)32(42)34-26(17-11-7-10-16-24(38)5-2)30(40)35-27(31(41)36-29)21-25(39)20-23-14-8-6-9-15-23/h6,8-9,14-15,22,26-29H,4-5,7,10-13,16-21H2,1-3H3,(H,34,42)(H,35,40)(H,36,41)/t22?,26-,27-,28+,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096234
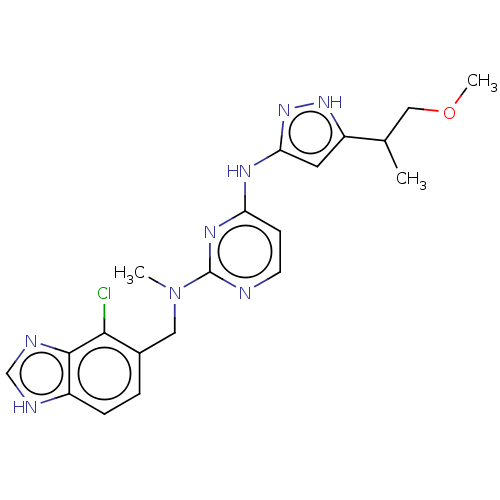
(CHEMBL3593825)Show SMILES COCC(C)c1cc(Nc2ccnc(n2)N(C)Cc2ccc3[nH]cnc3c2Cl)n[nH]1 Show InChI InChI=1S/C36H51N5O6/c1-5-20-40-22-26(25-16-11-12-18-28(25)40)32(43)31-35(46)38-30(23(4)6-2)36(47)41-21-14-13-19-29(41)34(45)37-27(33(44)39-31)17-10-8-9-15-24(42)7-3/h11-12,16,18,22-23,27,29-31H,5-10,13-15,17,19-21H2,1-4H3,(H,37,45)(H,38,46)(H,39,44)/t23?,27-,29+,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096229

(CHEMBL3594177)Show SMILES CN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r,wU:3.2,21.22,wD:6.6,(-2.67,2.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.14,-7.26,;5.43,-8.77,;4.08,-9.51,;3.92,-10.74,;2.96,-8.46,;1.74,-8.68,;-1.33,-.77,)| Show InChI InChI=1S/C38H54N6O5/c1-4-25(3)32-38(49)44-23-15-12-20-31(44)36(47)40-30(19-9-6-8-16-26(45)5-2)35(46)42-33(37(48)41-32)28-24-39-29-18-11-10-17-27(29)34(28)43-21-13-7-14-22-43/h10-11,17-18,24-25,30-33H,4-9,12-16,19-23H2,1-3H3,(H,40,47)(H,41,48)(H,42,46)/t25?,30-,31+,32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096240
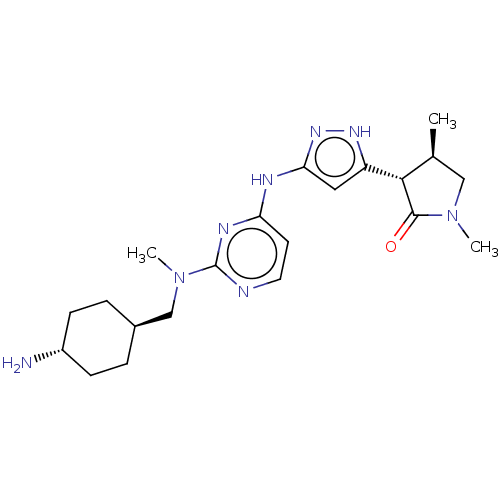
(CHEMBL3594181)Show SMILES C[C@H]1CN(C)C(=O)[C@@H]1c1cc(Nc2ccnc(n2)N(C)C[C@H]2CC[C@H](N)CC2)n[nH]1 |r,wU:21.22,7.8,wD:24.26,1.0,(1.74,-8.68,;2.96,-8.46,;4.08,-9.51,;5.43,-8.77,;6.54,-9.3,;5.14,-7.26,;5.98,-6.36,;3.62,-7.09,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;-2.67,1.54,;-2.67,2.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C34H49N5O5/c1-5-22(3)30-34(44)39-19-13-12-18-29(39)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-38(4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22?,26-,27-,29+,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096230

(CHEMBL3594178)Show SMILES CCN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r,wU:4.3,22.23,wD:7.7,(-3.74,3.69,;-2.68,3.08,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.14,-7.26,;5.43,-8.77,;4.08,-9.51,;3.92,-10.74,;2.96,-8.46,;1.74,-8.68,;-1.33,-.77,)| Show InChI InChI=1S/C32H46N4O6/c1-4-21(3)27-32(42)36-19-13-12-18-25(36)30(40)33-24(17-11-7-10-16-23(37)5-2)29(39)35-28(31(41)34-27)26(38)20-22-14-8-6-9-15-22/h6,8-9,14-15,21,24-25,27-28H,4-5,7,10-13,16-20H2,1-3H3,(H,33,40)(H,34,41)(H,35,39)/t21?,24-,25+,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096237

(CHEMBL3594176)Show SMILES CN1CC[C@@H](C1=O)c1cc(Nc2ccnc(NC[C@H]3CC[C@H](N)CC3)n2)n[nH]1 |r,wU:18.18,4.7,wD:21.22,(3.92,-10.74,;4.08,-9.51,;5.43,-8.77,;5.14,-7.26,;3.62,-7.09,;2.96,-8.46,;1.74,-8.68,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,-.77,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C35H51N5O5/c1-5-23(4)31-35(45)40-20-14-13-19-30(40)34(44)36-27(17-10-8-9-15-25(41)6-2)32(42)37-28(33(43)38-31)21-24-22-39(7-3)29-18-12-11-16-26(24)29/h11-12,16,18,22-23,27-28,30-31H,5-10,13-15,17,19-21H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t23?,27-,28-,30+,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096234

(CHEMBL3593825)Show SMILES COCC(C)c1cc(Nc2ccnc(n2)N(C)Cc2ccc3[nH]cnc3c2Cl)n[nH]1 Show InChI InChI=1S/C36H51N5O6/c1-5-20-40-22-26(25-16-11-12-18-28(25)40)32(43)31-35(46)38-30(23(4)6-2)36(47)41-21-14-13-19-29(41)34(45)37-27(33(44)39-31)17-10-8-9-15-24(42)7-3/h11-12,16,18,22-23,27,29-31H,5-10,13-15,17,19-21H2,1-4H3,(H,37,45)(H,38,46)(H,39,44)/t23?,27-,29+,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096231

(CHEMBL3580975)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C30H50N4O6/c1-7-19(3)25-29(40)34-17-13-12-16-23(34)28(39)31-21(15-11-9-10-14-20(35)8-2)26(37)32-22(27(38)33-25)18-24(36)30(4,5)6/h19,21-23,25H,7-18H2,1-6H3,(H,31,39)(H,32,37)(H,33,38)/t19?,21-,22-,23+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096229

(CHEMBL3594177)Show SMILES CN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r,wU:3.2,21.22,wD:6.6,(-2.67,2.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.14,-7.26,;5.43,-8.77,;4.08,-9.51,;3.92,-10.74,;2.96,-8.46,;1.74,-8.68,;-1.33,-.77,)| Show InChI InChI=1S/C38H54N6O5/c1-4-25(3)32-38(49)44-23-15-12-20-31(44)36(47)40-30(19-9-6-8-16-26(45)5-2)35(46)42-33(37(48)41-32)28-24-39-29-18-11-10-17-27(29)34(28)43-21-13-7-14-22-43/h10-11,17-18,24-25,30-33H,4-9,12-16,19-23H2,1-3H3,(H,40,47)(H,41,48)(H,42,46)/t25?,30-,31+,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096238

(CHEMBL3594179)Show SMILES CN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)C2CCCN(C)C2=O)n1 |r,wU:3.2,wD:6.6,(-2.67,2.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.17,-7.12,;5.9,-8.48,;5.1,-9.79,;3.56,-9.75,;2.91,-10.8,;2.82,-8.4,;1.59,-8.37,;-1.33,-.77,)| Show InChI InChI=1S/C33H45N5O6/c1-4-20(3)27-33(44)38-18-12-11-17-26(38)31(42)35-25(16-8-6-7-13-21(39)5-2)30(41)37-28(32(43)36-27)23-19-34-24-15-10-9-14-22(24)29(23)40/h9-10,14-15,19-20,25-28H,4-8,11-13,16-18H2,1-3H3,(H,34,40)(H,35,42)(H,36,43)(H,37,41)/t20?,25-,26+,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 362 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096235
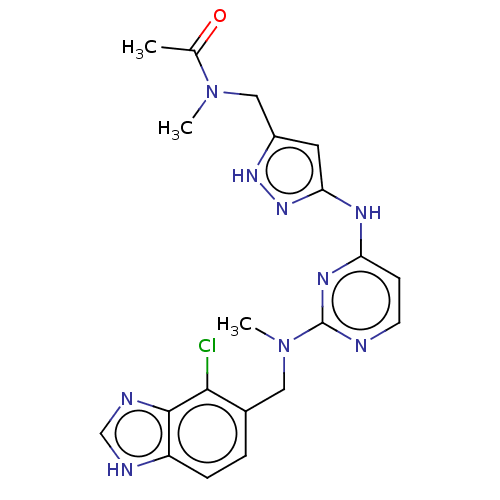
(CHEMBL3594173)Show SMILES CN(Cc1cc(Nc2ccnc(n2)N(C)Cc2ccc3[nH]cnc3c2Cl)n[nH]1)C(C)=O Show InChI InChI=1S/C34H47N5O6/c1-5-21(3)28-34(45)39-18-11-10-14-27(39)32(43)35-25(13-9-7-8-12-24(40)6-2)31(42)37-29(33(44)36-28)30(41)23-15-16-26-22(20-23)17-19-38(26)4/h15-17,19-21,25,27-29H,5-14,18H2,1-4H3,(H,35,43)(H,36,44)(H,37,42)/t21?,25-,27+,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM84637
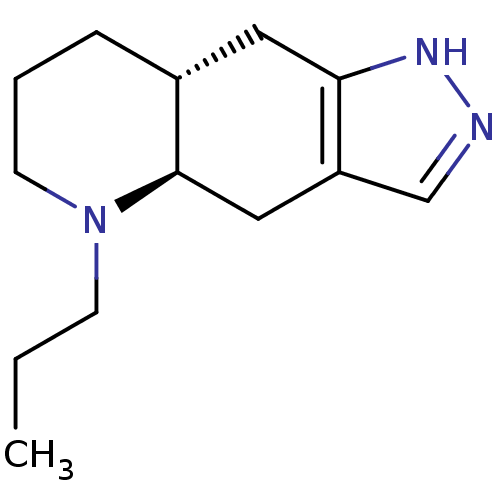
(CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 519 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50096236
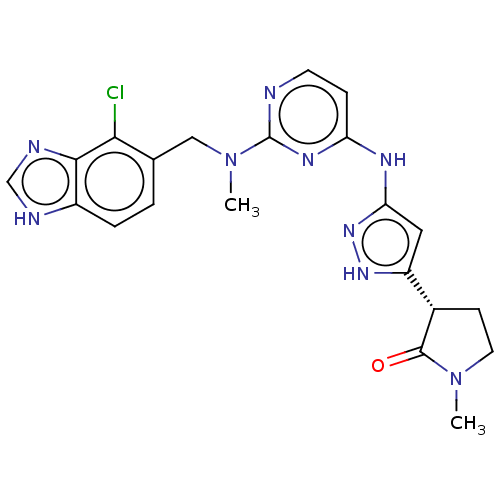
(CHEMBL3594174)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)[C@@H]2CCN(C)C2=O)n1 |r| Show InChI InChI=1S/C33H45N5O6/c1-4-20(3)27-33(44)38-18-12-11-17-26(38)31(42)35-25(16-8-6-7-13-21(39)5-2)30(41)37-28(32(43)36-27)29(40)23-19-34-24-15-10-9-14-22(23)24/h9-10,14-15,19-20,25-28,34H,4-8,11-13,16-18H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t20?,25-,26+,27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 552 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 555 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096234

(CHEMBL3593825)Show SMILES COCC(C)c1cc(Nc2ccnc(n2)N(C)Cc2ccc3[nH]cnc3c2Cl)n[nH]1 Show InChI InChI=1S/C36H51N5O6/c1-5-20-40-22-26(25-16-11-12-18-28(25)40)32(43)31-35(46)38-30(23(4)6-2)36(47)41-21-14-13-19-29(41)34(45)37-27(33(44)39-31)17-10-8-9-15-24(42)7-3/h11-12,16,18,22-23,27,29-31H,5-10,13-15,17,19-21H2,1-4H3,(H,37,45)(H,38,46)(H,39,44)/t23?,27-,29+,30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096230

(CHEMBL3594178)Show SMILES CCN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r,wU:4.3,22.23,wD:7.7,(-3.74,3.69,;-2.68,3.08,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.14,-7.26,;5.43,-8.77,;4.08,-9.51,;3.92,-10.74,;2.96,-8.46,;1.74,-8.68,;-1.33,-.77,)| Show InChI InChI=1S/C32H46N4O6/c1-4-21(3)27-32(42)36-19-13-12-18-25(36)30(40)33-24(17-11-7-10-16-23(37)5-2)29(39)35-28(31(41)34-27)26(38)20-22-14-8-6-9-15-22/h6,8-9,14-15,21,24-25,27-28H,4-5,7,10-13,16-20H2,1-3H3,(H,33,40)(H,34,41)(H,35,39)/t21?,24-,25+,27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096233

(CHEMBL3593824)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)C2CCC(=O)C2)n1 Show InChI InChI=1S/C34H50BrN5O6/c1-5-21(3)30-34(45)39-17-11-10-14-29(39)33(44)36-26(13-9-7-8-12-24(41)6-2)31(42)37-27(32(43)38-30)18-22-20-40(46-4)28-16-15-23(35)19-25(22)28/h15-16,19,21-22,26-27,29-30H,5-14,17-18,20H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t21?,22?,26-,27-,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM35256
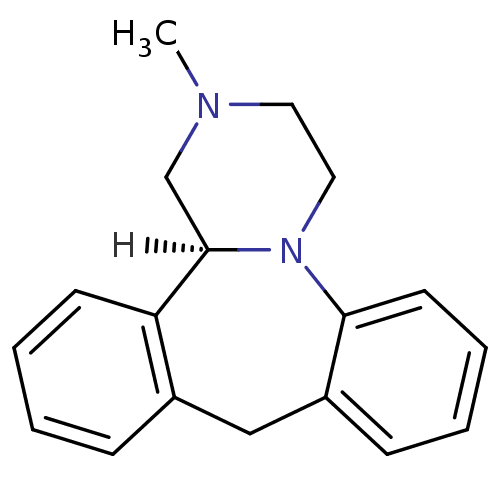
((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM84637

(CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 734 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096239

(CHEMBL3594180)Show SMILES CCN1CC[C@@H](C1=O)c1cc(Nc2ccnc(n2)N(C)C[C@H]2CC[C@H](N)CC2)n[nH]1 |r,wU:21.22,5.8,wD:24.26,(4.87,-11.79,;3.88,-11.04,;4.08,-9.51,;5.43,-8.77,;5.14,-7.26,;3.62,-7.09,;2.96,-8.46,;1.74,-8.68,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;-2.67,1.54,;-2.67,2.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C41H55N5O7/c1-6-25(3)36-41(51)46-20-14-13-19-35(46)40(50)43-33(18-10-8-9-15-27(47)7-2)38(48)44-34(39(49)45-36)24-31-30-16-11-12-17-32(30)42-37(31)26-21-28(52-4)23-29(22-26)53-5/h11-12,16-17,21-23,25,33-36,42H,6-10,13-15,18-20,24H2,1-5H3,(H,43,50)(H,44,48)(H,45,49)/t25?,33-,34-,35+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50096235

(CHEMBL3594173)Show SMILES CN(Cc1cc(Nc2ccnc(n2)N(C)Cc2ccc3[nH]cnc3c2Cl)n[nH]1)C(C)=O Show InChI InChI=1S/C34H47N5O6/c1-5-21(3)28-34(45)39-18-11-10-14-27(39)32(43)35-25(13-9-7-8-12-24(40)6-2)31(42)37-29(33(44)36-28)30(41)23-15-16-26-22(20-23)17-19-38(26)4/h15-17,19-21,25,27-29H,5-14,18H2,1-4H3,(H,35,43)(H,36,44)(H,37,42)/t21?,25-,27+,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 893 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM84637

(CAS_85760-74-3 | CHEMBL240773 | NSC_54562 | QUINPI...)Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/t10-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096228

(CHEMBL3594175)Show SMILES CN(Cc1ccc2[nH]cnc2c1Cl)c1nccc(Nc2cc([nH]n2)[C@H]2CCN(C)C2=O)n1 |r| Show InChI InChI=1S/C36H53N5O6/c1-5-14-26(42)15-8-7-9-17-28-34(44)38-29(21-25-22-41(47-4)30-18-11-10-16-27(25)30)35(45)39-33(24(3)6-2)32(43)23-40-20-13-12-19-31(40)36(46)37-28/h10-11,16,18,22,24,28-29,31,33H,5-9,12-15,17,19-21,23H2,1-4H3,(H,37,46)(H,38,44)(H,39,45)/t24?,28-,29-,31+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096240

(CHEMBL3594181)Show SMILES C[C@H]1CN(C)C(=O)[C@@H]1c1cc(Nc2ccnc(n2)N(C)C[C@H]2CC[C@H](N)CC2)n[nH]1 |r,wU:21.22,7.8,wD:24.26,1.0,(1.74,-8.68,;2.96,-8.46,;4.08,-9.51,;5.43,-8.77,;6.54,-9.3,;5.14,-7.26,;5.98,-6.36,;3.62,-7.09,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;-2.67,1.54,;-2.67,2.77,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C34H49N5O5/c1-5-22(3)30-34(44)39-19-13-12-18-29(39)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-38(4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22?,26-,27-,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096237

(CHEMBL3594176)Show SMILES CN1CC[C@@H](C1=O)c1cc(Nc2ccnc(NC[C@H]3CC[C@H](N)CC3)n2)n[nH]1 |r,wU:18.18,4.7,wD:21.22,(3.92,-10.74,;4.08,-9.51,;5.43,-8.77,;5.14,-7.26,;3.62,-7.09,;2.96,-8.46,;1.74,-8.68,;2.99,-5.68,;1.49,-5.37,;1.34,-3.85,;0,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,-.77,;2.72,-3.21,;3.76,-4.35,)| Show InChI InChI=1S/C35H51N5O5/c1-5-23(4)31-35(45)40-20-14-13-19-30(40)34(44)36-27(17-10-8-9-15-25(41)6-2)32(42)37-28(33(43)38-31)21-24-22-39(7-3)29-18-12-11-16-26(24)29/h11-12,16,18,22-23,27-28,30-31H,5-10,13-15,17,19-21H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)/t23?,27-,28-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096232

(CHEMBL3593823)Show SMILES Clc1c(CNc2nccc(Nc3cc([nH]n3)C3CCCO3)n2)ccc2[nH]cnc12 Show InChI InChI=1S/C33H48N4O6/c1-4-22(3)29-33(43)37-19-13-12-18-28(37)32(42)34-26(17-11-7-10-16-24(38)5-2)30(40)35-27(31(41)36-29)21-25(39)20-23-14-8-6-9-15-23/h6,8-9,14-15,22,26-29H,4-5,7,10-13,16-21H2,1-3H3,(H,34,42)(H,35,40)(H,36,41)/t22?,26-,27-,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM35256

((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM35256

((S)-mianserin | Lerivon | MIANSERIN | MIANSERIN (+...)Show InChI InChI=1S/C18H20N2/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19/h2-9,18H,10-13H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50096238

(CHEMBL3594179)Show SMILES CN(C[C@H]1CC[C@H](N)CC1)c1nccc(Nc2cc([nH]n2)C2CCCN(C)C2=O)n1 |r,wU:3.2,wD:6.6,(-2.67,2.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-6.67,.76,;-8.01,1.53,;-8.01,3.07,;-9.07,3.69,;-6.67,3.84,;-5.34,3.07,;-1.33,.77,;,1.54,;1.33,.77,;1.33,-.77,;,-1.54,;0,-3.08,;1.34,-3.85,;1.49,-5.37,;2.99,-5.68,;3.76,-4.35,;2.72,-3.21,;3.62,-7.09,;5.17,-7.12,;5.9,-8.48,;5.1,-9.79,;3.56,-9.75,;2.91,-10.8,;2.82,-8.4,;1.59,-8.37,;-1.33,-.77,)| Show InChI InChI=1S/C33H45N5O6/c1-4-20(3)27-33(44)38-18-12-11-17-26(38)31(42)35-25(16-8-6-7-13-21(39)5-2)30(41)37-28(32(43)36-27)23-19-34-24-15-10-9-14-22(24)29(23)40/h9-10,14-15,19-20,25-28H,4-8,11-13,16-18H2,1-3H3,(H,34,40)(H,35,42)(H,36,43)(H,37,41)/t20?,25-,26+,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 (unknown origin) |
ACS Med Chem Lett 6: 711-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00151
BindingDB Entry DOI: 10.7270/Q24J0GWT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data