 Found 195 hits with Last Name = 'markland' and Initial = 'w'
Found 195 hits with Last Name = 'markland' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35641
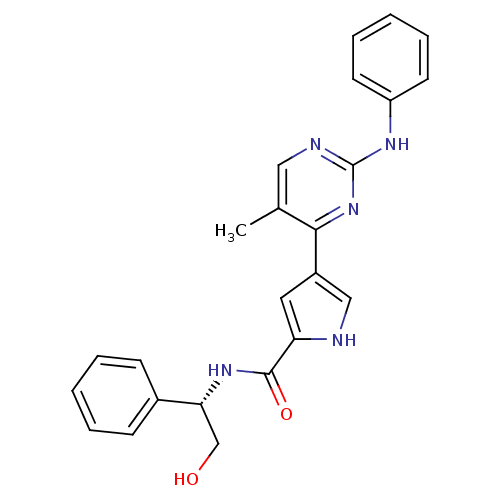
(erk000040 | pyrimidylpyrrole, 2)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-13-26-24(27-19-10-6-3-7-11-19)29-22(16)18-12-20(25-14-18)23(31)28-21(15-30)17-8-4-2-5-9-17/h2-14,21,25,30H,15H2,1H3,(H,28,31)(H,26,27,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35642
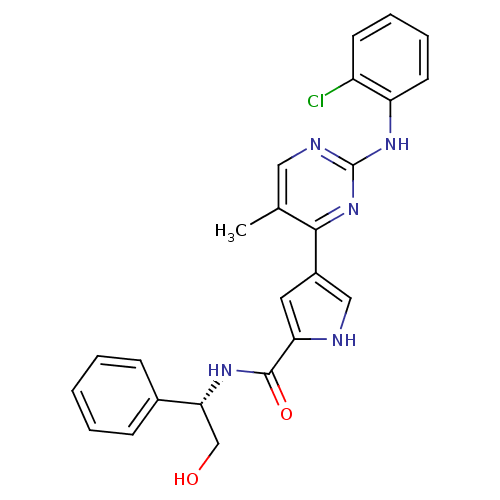
(pyrimidylpyrrole, 9a)Show SMILES Cc1cnc(Nc2ccccc2Cl)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H22ClN5O2/c1-15-12-27-24(29-19-10-6-5-9-18(19)25)30-22(15)17-11-20(26-13-17)23(32)28-21(14-31)16-7-3-2-4-8-16/h2-13,21,26,31H,14H2,1H3,(H,28,32)(H,27,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35653

(pyrimidylpyrrole, 11a)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H24ClN5O2/c1-15-6-3-4-9-20(15)30-25-28-12-16(2)23(31-25)18-11-21(27-13-18)24(33)29-22(14-32)17-7-5-8-19(26)10-17/h3-13,22,27,32H,14H2,1-2H3,(H,29,33)(H,28,30,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35647
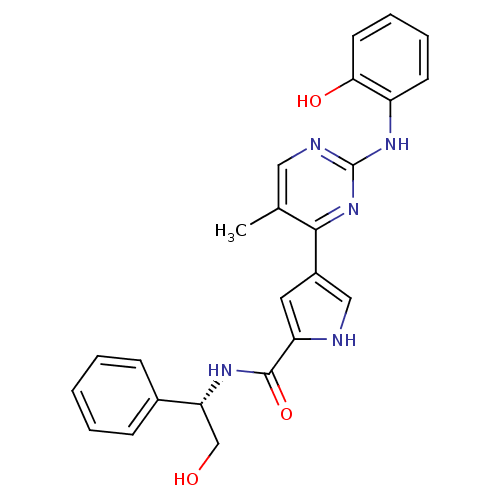
(erk000636 | pyrimidylpyrrole, 9f)Show SMILES Cc1cnc(Nc2ccccc2O)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O3/c1-15-12-26-24(28-18-9-5-6-10-21(18)31)29-22(15)17-11-19(25-13-17)23(32)27-20(14-30)16-7-3-2-4-8-16/h2-13,20,25,30-31H,14H2,1H3,(H,27,32)(H,26,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35663
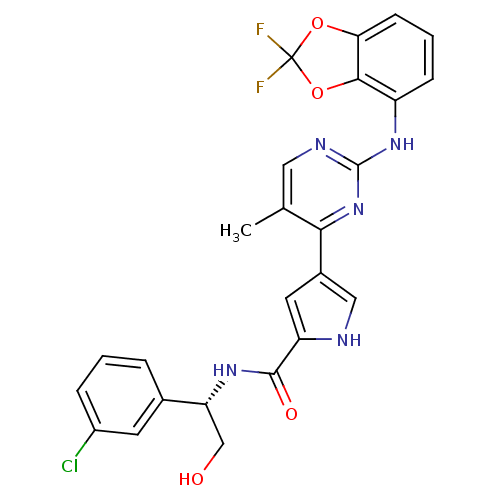
(pyrimidylpyrrole, 11k)Show SMILES Cc1cnc(Nc2cccc3OC(F)(F)Oc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H20ClF2N5O4/c1-13-10-30-24(32-17-6-3-7-20-22(17)37-25(27,28)36-20)33-21(13)15-9-18(29-11-15)23(35)31-19(12-34)14-4-2-5-16(26)8-14/h2-11,19,29,34H,12H2,1H3,(H,31,35)(H,30,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35662
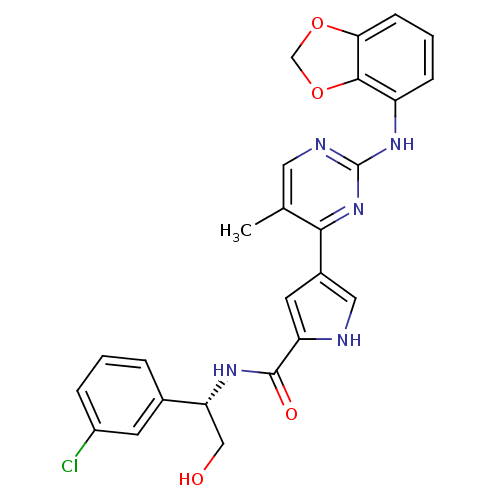
(pyrimidylpyrrole, 11j)Show SMILES Cc1cnc(Nc2cccc3OCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H22ClN5O4/c1-14-10-28-25(30-18-6-3-7-21-23(18)35-13-34-21)31-22(14)16-9-19(27-11-16)24(33)29-20(12-32)15-4-2-5-17(26)8-15/h2-11,20,27,32H,12-13H2,1H3,(H,29,33)(H,28,30,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35660
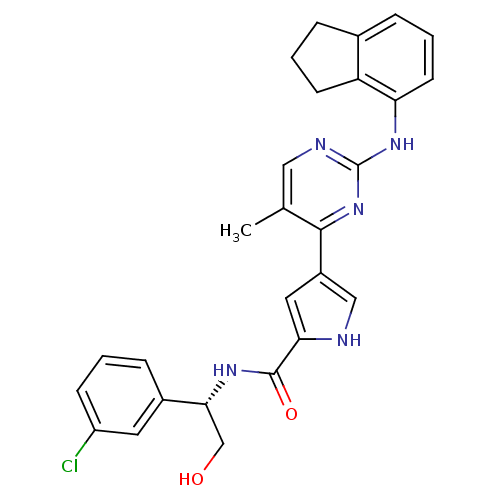
(pyrimidylpyrrole, 11h)Show SMILES Cc1cnc(Nc2cccc3CCCc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H26ClN5O2/c1-16-13-30-27(32-22-10-4-6-17-5-3-9-21(17)22)33-25(16)19-12-23(29-14-19)26(35)31-24(15-34)18-7-2-8-20(28)11-18/h2,4,6-8,10-14,24,29,34H,3,5,9,15H2,1H3,(H,31,35)(H,30,32,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35659
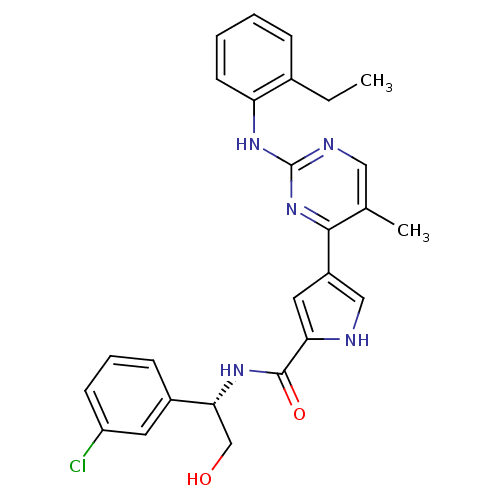
(pyrimidylpyrrole, 11g)Show SMILES CCc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H26ClN5O2/c1-3-17-7-4-5-10-21(17)31-26-29-13-16(2)24(32-26)19-12-22(28-14-19)25(34)30-23(15-33)18-8-6-9-20(27)11-18/h4-14,23,28,33H,3,15H2,1-2H3,(H,30,34)(H,29,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35657
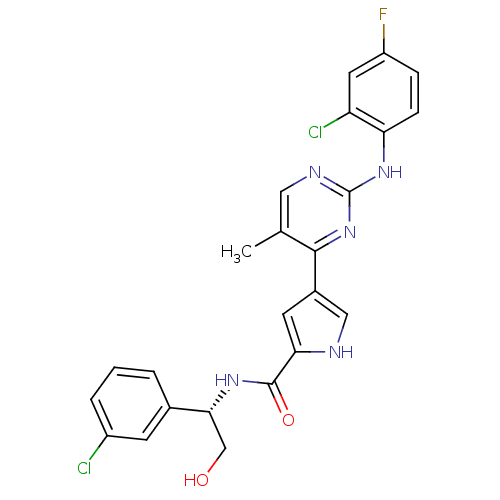
(pyrimidylpyrrole, 11e)Show SMILES Cc1cnc(Nc2ccc(F)cc2Cl)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H20Cl2FN5O2/c1-13-10-29-24(31-19-6-5-17(27)9-18(19)26)32-22(13)15-8-20(28-11-15)23(34)30-21(12-33)14-3-2-4-16(25)7-14/h2-11,21,28,33H,12H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35654

(erk000526 | pyrimidylpyrrole, 11b)Show SMILES Cc1cccc(Nc2ncc(C)c(n2)-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c1C |r| Show InChI InChI=1S/C26H26ClN5O2/c1-15-6-4-9-21(17(15)3)31-26-29-12-16(2)24(32-26)19-11-22(28-13-19)25(34)30-23(14-33)18-7-5-8-20(27)10-18/h4-13,23,28,33H,14H2,1-3H3,(H,30,34)(H,29,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35653

(pyrimidylpyrrole, 11a)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H24ClN5O2/c1-15-6-3-4-9-20(15)30-25-28-12-16(2)23(31-25)18-11-21(27-13-18)24(33)29-22(14-32)17-7-5-8-19(26)10-17/h3-13,22,27,32H,14H2,1-2H3,(H,29,33)(H,28,30,31)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35651
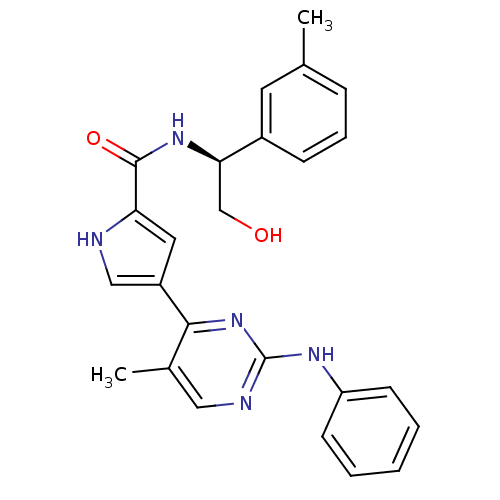
(pyrimidylpyrrole, 10d)Show SMILES Cc1cccc(c1)[C@@H](CO)NC(=O)c1cc(c[nH]1)-c1nc(Nc2ccccc2)ncc1C |r| Show InChI InChI=1S/C25H25N5O2/c1-16-7-6-8-18(11-16)22(15-31)29-24(32)21-12-19(14-26-21)23-17(2)13-27-25(30-23)28-20-9-4-3-5-10-20/h3-14,22,26,31H,15H2,1-2H3,(H,29,32)(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35650

(pyrimidylpyrrole, 10c)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H22ClN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35649
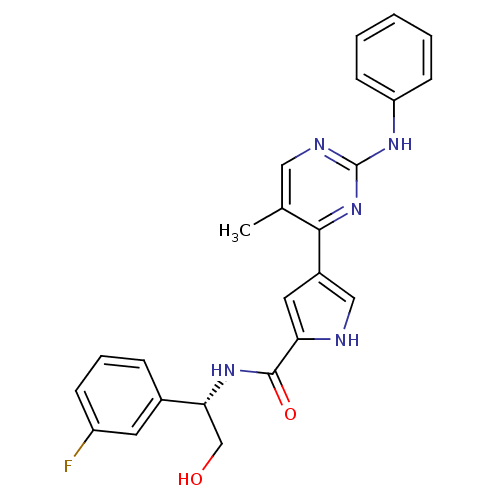
(erk000537 | pyrimidylpyrrole, 10b)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(F)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35645
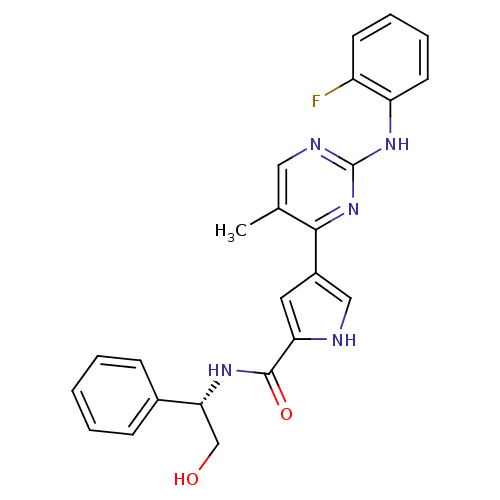
(pyrimidylpyrrole, 9d)Show SMILES Cc1cnc(Nc2ccccc2F)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-12-27-24(29-19-10-6-5-9-18(19)25)30-22(15)17-11-20(26-13-17)23(32)28-21(14-31)16-7-3-2-4-8-16/h2-13,21,26,31H,14H2,1H3,(H,28,32)(H,27,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35643

(pyrimidylpyrrole, 9b)Show SMILES Cc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O2/c1-16-8-6-7-11-20(16)29-25-27-13-17(2)23(30-25)19-12-21(26-14-19)24(32)28-22(15-31)18-9-4-3-5-10-18/h3-14,22,26,31H,15H2,1-2H3,(H,28,32)(H,27,29,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <2 | <-50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35644
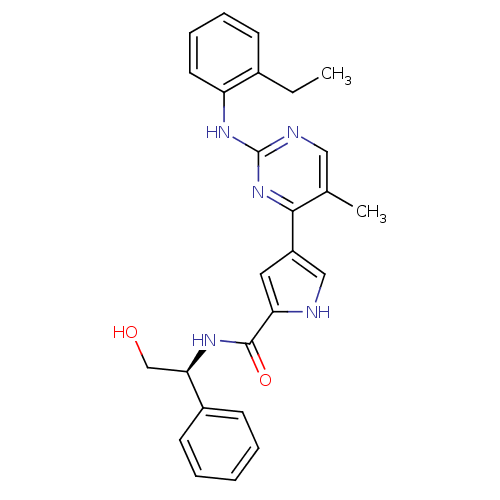
(erk000524 | pyrimidylpyrrole, 9c)Show SMILES CCc1ccccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C26H27N5O2/c1-3-18-9-7-8-12-21(18)30-26-28-14-17(2)24(31-26)20-13-22(27-15-20)25(33)29-23(16-32)19-10-5-4-6-11-19/h4-15,23,27,32H,3,16H2,1-2H3,(H,29,33)(H,28,30,31)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35665
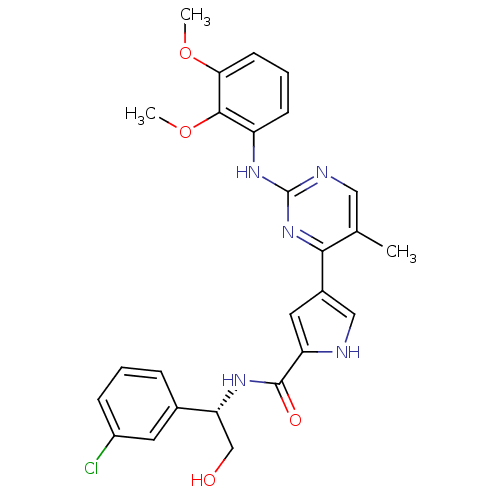
(erk000617 | pyrimidylpyrrole, 11m)Show SMILES COc1cccc(Nc2ncc(C)c(n2)-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c1OC |r| Show InChI InChI=1S/C26H26ClN5O4/c1-15-12-29-26(31-19-8-5-9-22(35-2)24(19)36-3)32-23(15)17-11-20(28-13-17)25(34)30-21(14-33)16-6-4-7-18(27)10-16/h4-13,21,28,33H,14H2,1-3H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35647

(erk000636 | pyrimidylpyrrole, 9f)Show SMILES Cc1cnc(Nc2ccccc2O)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O3/c1-15-12-26-24(28-18-9-5-6-10-21(18)31)29-22(15)17-11-19(25-13-17)23(32)27-20(14-30)16-7-3-2-4-8-16/h2-13,20,25,30-31H,14H2,1H3,(H,27,32)(H,26,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275934
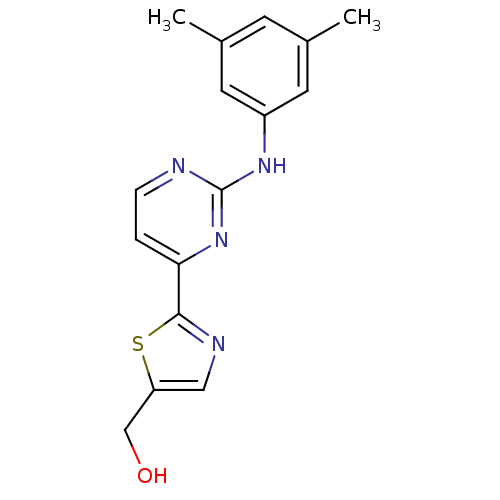
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-8-13(9-21)22-15/h3-8,21H,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35646

(erk000506 | pyrimidylpyrrole, 9e)Show SMILES Cc1cnc(Nc2ccccc2C(F)(F)F)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C25H22F3N5O2/c1-15-12-30-24(32-19-10-6-5-9-18(19)25(26,27)28)33-22(15)17-11-20(29-13-17)23(35)31-21(14-34)16-7-3-2-4-8-16/h2-13,21,29,34H,14H2,1H3,(H,31,35)(H,30,32,33)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35649

(erk000537 | pyrimidylpyrrole, 10b)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(F)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35656
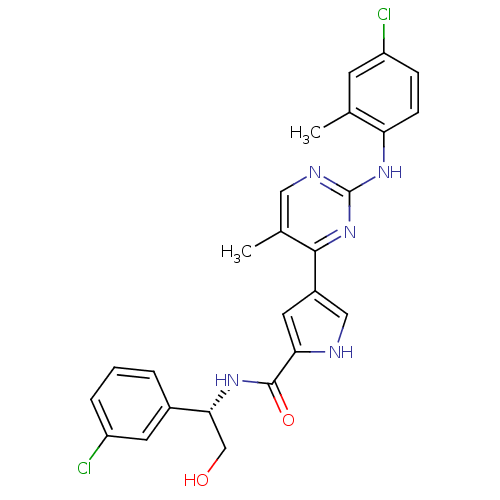
(erk000650 | pyrimidylpyrrole, 11d)Show SMILES Cc1cc(Cl)ccc1Nc1ncc(C)c(n1)-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H23Cl2N5O2/c1-14-8-19(27)6-7-20(14)31-25-29-11-15(2)23(32-25)17-10-21(28-12-17)24(34)30-22(13-33)16-4-3-5-18(26)9-16/h3-12,22,28,33H,13H2,1-2H3,(H,30,34)(H,29,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35658
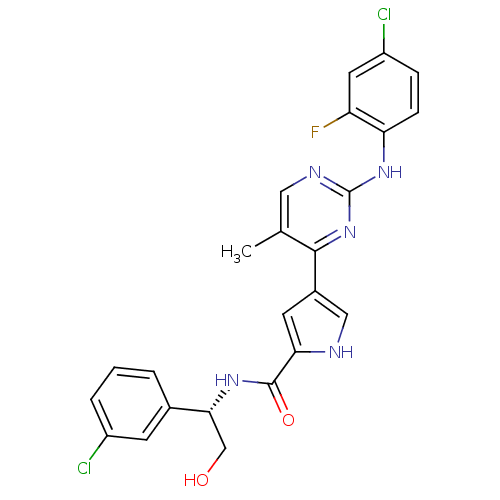
(erk000651 | pyrimidylpyrrole, 11f)Show SMILES Cc1cnc(Nc2ccc(Cl)cc2F)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H20Cl2FN5O2/c1-13-10-29-24(31-19-6-5-17(26)9-18(19)27)32-22(13)15-8-20(28-11-15)23(34)30-21(12-33)14-3-2-4-16(25)7-14/h2-11,21,28,33H,12H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35641

(erk000040 | pyrimidylpyrrole, 2)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-13-26-24(27-19-10-6-3-7-11-19)29-22(16)18-12-20(25-14-18)23(31)28-21(15-30)17-8-4-2-5-9-17/h2-14,21,25,30H,15H2,1H3,(H,28,31)(H,26,27,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276015
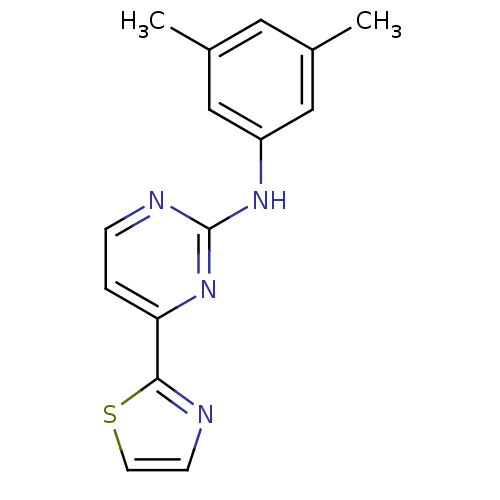
(CHEMBL509161 | N-(3,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-7-11(2)9-12(8-10)18-15-17-4-3-13(19-15)14-16-5-6-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275935
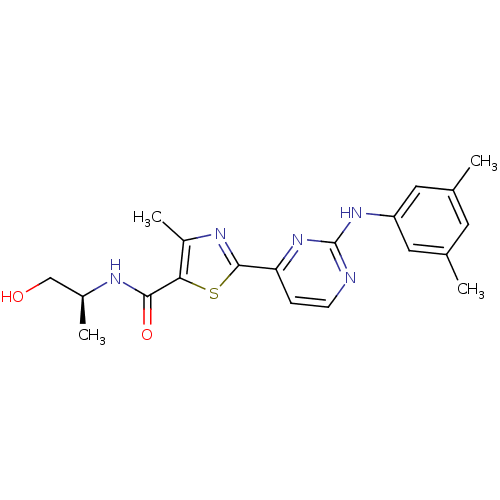
((S)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275358
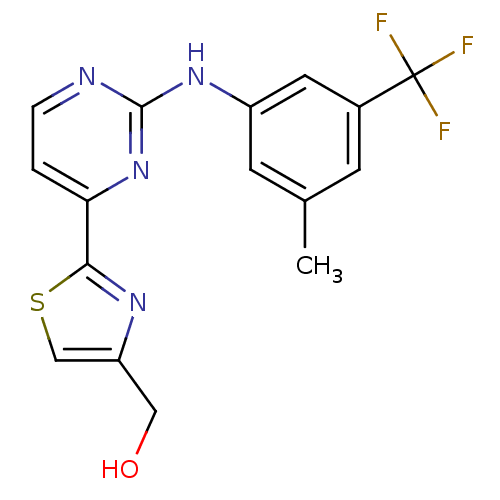
((2-(2-(3-methyl-5-(trifluoromethyl)phenylamino)pyr...)Show SMILES Cc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-4-10(16(17,18)19)6-11(5-9)22-15-20-3-2-13(23-15)14-21-12(7-24)8-25-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276065
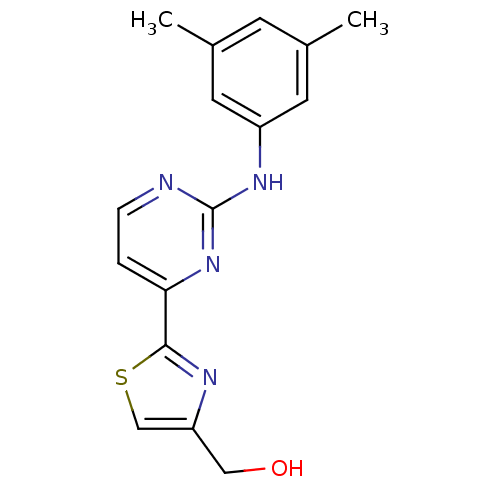
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-13(8-21)9-22-15/h3-7,9,21H,8H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM35664
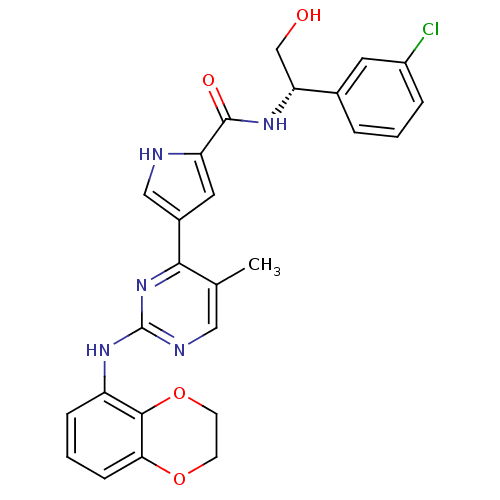
(erk000628 | pyrimidylpyrrole, 11l)Show SMILES Cc1cnc(Nc2cccc3OCCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H24ClN5O4/c1-15-12-29-26(31-19-6-3-7-22-24(19)36-9-8-35-22)32-23(15)17-11-20(28-13-17)25(34)30-21(14-33)16-4-2-5-18(27)10-16/h2-7,10-13,21,28,33H,8-9,14H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35661
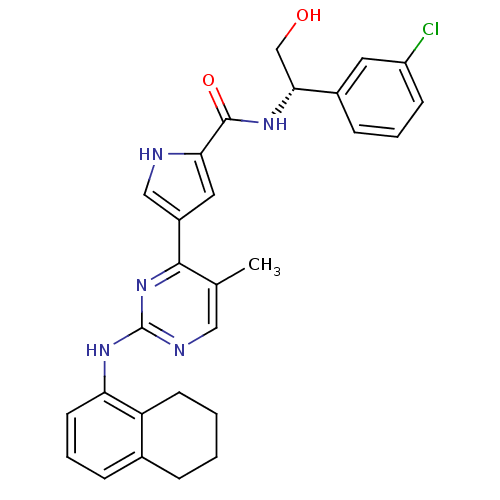
(erk000595 | pyrimidylpyrrole, 11i)Show SMILES Cc1cnc(Nc2cccc3CCCCc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H28ClN5O2/c1-17-14-31-28(33-23-11-5-7-18-6-2-3-10-22(18)23)34-26(17)20-13-24(30-15-20)27(36)32-25(16-35)19-8-4-9-21(29)12-19/h4-5,7-9,11-15,25,30,35H,2-3,6,10,16H2,1H3,(H,32,36)(H,31,33,34)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276018
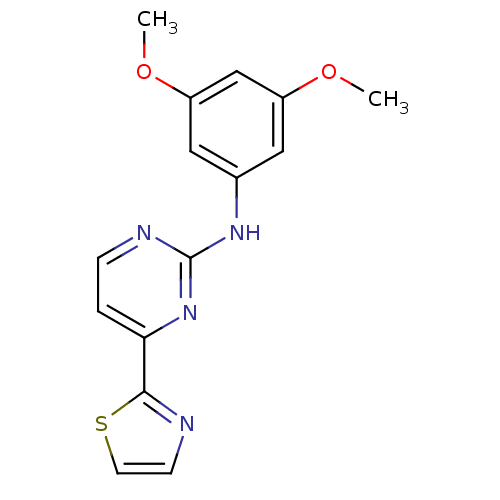
(1-(3-(cyclopenta-1,3-dienyl)benzyl)-3,5-diethylben...)Show InChI InChI=1S/C15H14N4O2S/c1-20-11-7-10(8-12(9-11)21-2)18-15-17-4-3-13(19-15)14-16-5-6-22-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM35662

(pyrimidylpyrrole, 11j)Show SMILES Cc1cnc(Nc2cccc3OCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H22ClN5O4/c1-14-10-28-25(30-18-6-3-7-21-23(18)35-13-34-21)31-22(14)16-9-19(27-11-16)24(33)29-20(12-32)15-4-2-5-17(26)8-15/h2-11,20,27,32H,12-13H2,1H3,(H,29,33)(H,28,30,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM35641

(erk000040 | pyrimidylpyrrole, 2)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-13-26-24(27-19-10-6-3-7-11-19)29-22(16)18-12-20(25-14-18)23(31)28-21(15-30)17-8-4-2-5-9-17/h2-14,21,25,30H,15H2,1H3,(H,28,31)(H,26,27,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35664

(erk000628 | pyrimidylpyrrole, 11l)Show SMILES Cc1cnc(Nc2cccc3OCCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H24ClN5O4/c1-15-12-29-26(31-19-6-3-7-22-24(19)36-9-8-35-22)32-23(15)17-11-20(28-13-17)25(34)30-21(14-33)16-4-2-5-18(27)10-16/h2-7,10-13,21,28,33H,8-9,14H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM35641

(erk000040 | pyrimidylpyrrole, 2)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1ccccc1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-13-26-24(27-19-10-6-3-7-11-19)29-22(16)18-12-20(25-14-18)23(31)28-21(15-30)17-8-4-2-5-9-17/h2-14,21,25,30H,15H2,1H3,(H,28,31)(H,26,27,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM35650

(pyrimidylpyrrole, 10c)Show SMILES Cc1cnc(Nc2ccccc2)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H22ClN5O2/c1-15-12-27-24(28-19-8-3-2-4-9-19)30-22(15)17-11-20(26-13-17)23(32)29-21(14-31)16-6-5-7-18(25)10-16/h2-13,21,26,31H,14H2,1H3,(H,29,32)(H,27,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249284
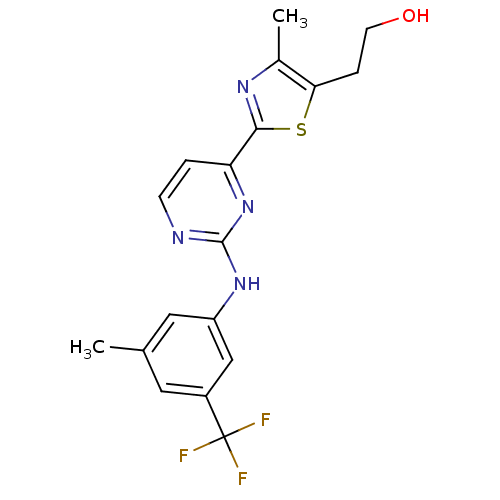
(2-(4-methyl-2-(2-(3-methyl-5-(trifluoromethyl)phen...)Show SMILES Cc1nc(sc1CCO)-c1ccnc(Nc2cc(C)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C18H17F3N4OS/c1-10-7-12(18(19,20)21)9-13(8-10)24-17-22-5-3-14(25-17)16-23-11(2)15(27-16)4-6-26/h3,5,7-9,26H,4,6H2,1-2H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276064
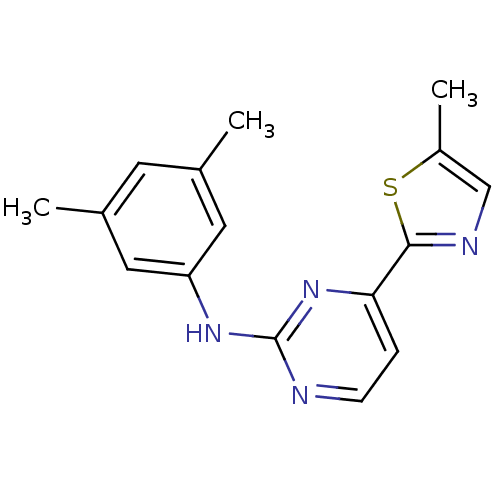
(CHEMBL470780 | N-(3,5-dimethylphenyl)-4-(5-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-9-12(3)21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275357
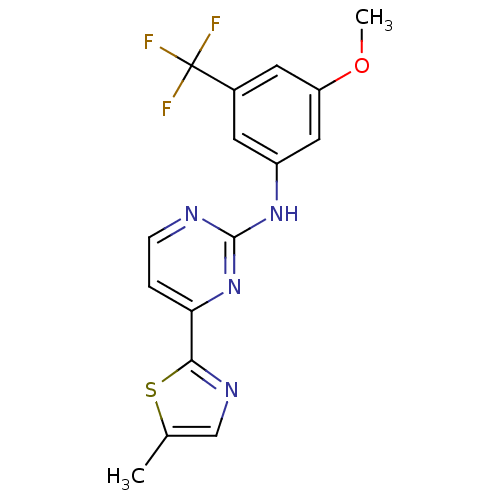
(CHEMBL451450 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show SMILES COc1cc(Nc2nccc(n2)-c2ncc(C)s2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-8-21-14(25-9)13-3-4-20-15(23-13)22-11-5-10(16(17,18)19)6-12(7-11)24-2/h3-8H,1-2H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35651

(pyrimidylpyrrole, 10d)Show SMILES Cc1cccc(c1)[C@@H](CO)NC(=O)c1cc(c[nH]1)-c1nc(Nc2ccccc2)ncc1C |r| Show InChI InChI=1S/C25H25N5O2/c1-16-7-6-8-18(11-16)22(15-31)29-24(32)21-12-19(14-26-21)23-17(2)13-27-25(30-23)28-20-9-4-3-5-10-20/h3-14,22,26,31H,15H2,1-2H3,(H,29,32)(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249286
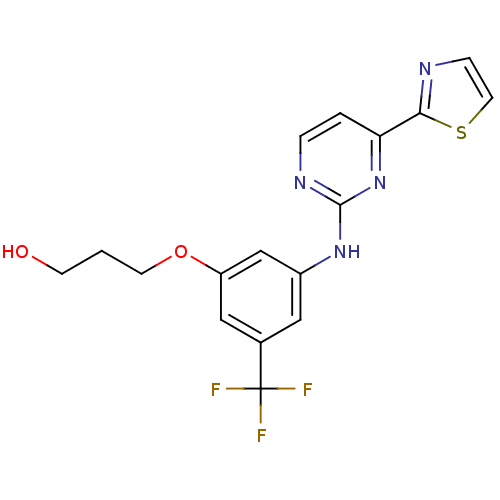
(3-(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trif...)Show SMILES OCCCOc1cc(Nc2nccc(n2)-c2nccs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c18-17(19,20)11-8-12(10-13(9-11)26-6-1-5-25)23-16-22-3-2-14(24-16)15-21-4-7-27-15/h2-4,7-10,25H,1,5-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276016
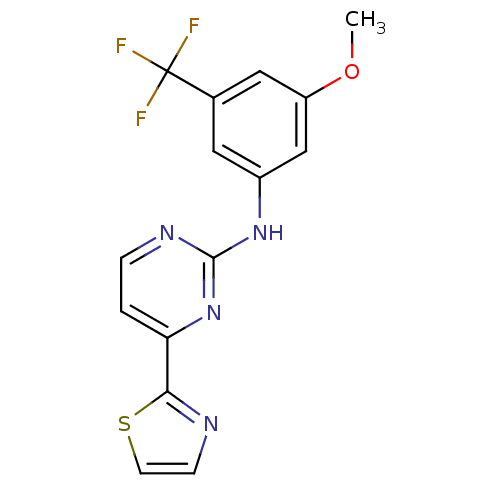
(CHEMBL512319 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show InChI InChI=1S/C15H11F3N4OS/c1-23-11-7-9(15(16,17)18)6-10(8-11)21-14-20-3-2-12(22-14)13-19-4-5-24-13/h2-8H,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249318
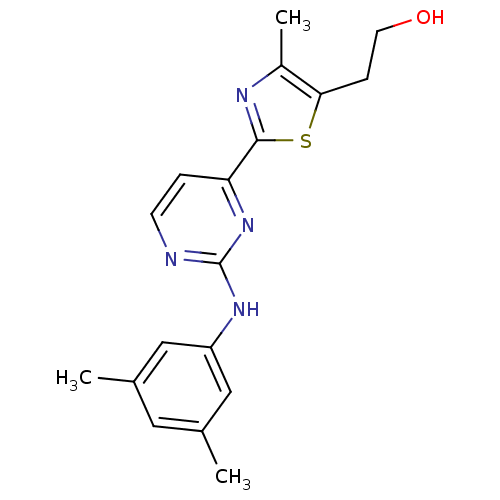
(2-(2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-4...)Show InChI InChI=1S/C18H20N4OS/c1-11-8-12(2)10-14(9-11)21-18-19-6-4-15(22-18)17-20-13(3)16(24-17)5-7-23/h4,6,8-10,23H,5,7H2,1-3H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35662

(pyrimidylpyrrole, 11j)Show SMILES Cc1cnc(Nc2cccc3OCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H22ClN5O4/c1-14-10-28-25(30-18-6-3-7-21-23(18)35-13-34-21)31-22(14)16-9-19(27-11-16)24(33)29-20(12-32)15-4-2-5-17(26)8-15/h2-11,20,27,32H,12-13H2,1H3,(H,29,33)(H,28,30,31)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275355
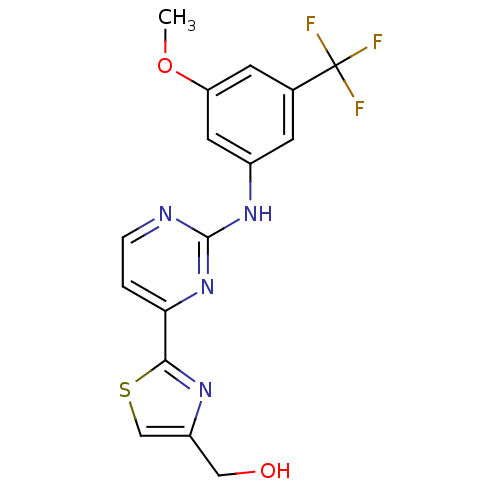
((2-(2-(3-methoxy-5-(trifluoromethyl)phenylamino)py...)Show SMILES COc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4O2S/c1-25-12-5-9(16(17,18)19)4-10(6-12)22-15-20-3-2-13(23-15)14-21-11(7-24)8-26-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM35655

(erk000598 | pyrimidylpyrrole, 11c)Show SMILES Cc1cnc(Nc2cccc(F)c2C)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H23ClFN5O2/c1-14-11-29-25(31-20-8-4-7-19(27)15(20)2)32-23(14)17-10-21(28-12-17)24(34)30-22(13-33)16-5-3-6-18(26)9-16/h3-12,22,28,33H,13H2,1-2H3,(H,30,34)(H,29,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM35654

(erk000526 | pyrimidylpyrrole, 11b)Show SMILES Cc1cccc(Nc2ncc(C)c(n2)-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c1C |r| Show InChI InChI=1S/C26H26ClN5O2/c1-15-6-4-9-21(17(15)3)31-26-29-12-16(2)24(32-26)19-11-22(28-13-19)25(34)30-23(14-33)18-7-5-8-20(27)10-18/h4-13,23,28,33H,14H2,1-3H3,(H,30,34)(H,29,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276017
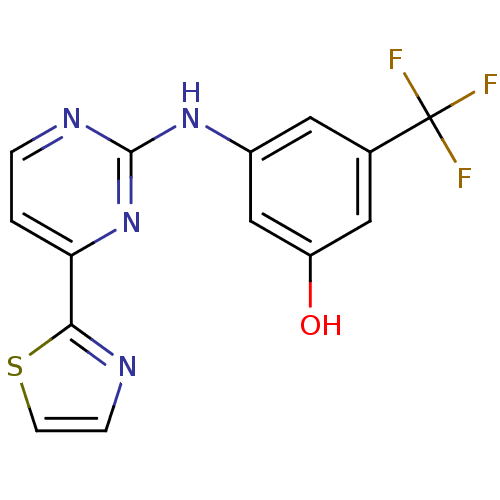
(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trifluo...)Show InChI InChI=1S/C14H9F3N4OS/c15-14(16,17)8-5-9(7-10(22)6-8)20-13-19-2-1-11(21-13)12-18-3-4-23-12/h1-7,22H,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM35664

(erk000628 | pyrimidylpyrrole, 11l)Show SMILES Cc1cnc(Nc2cccc3OCCOc23)nc1-c1c[nH]c(c1)C(=O)N[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H24ClN5O4/c1-15-12-29-26(31-19-6-3-7-22-24(19)36-9-8-35-22)32-23(15)17-11-20(28-13-17)25(34)30-21(14-33)16-4-2-5-18(27)10-16/h2-7,10-13,21,28,33H,8-9,14H2,1H3,(H,30,34)(H,29,31,32)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex
| Assay Description
A coupled spectrophotometric assay was used in which ADP generated by kinase was converted to ATP with the concomitant production of pyruvate from PE... |
J Med Chem 52: 6362-8 (2009)
Article DOI: 10.1021/jm900630q
BindingDB Entry DOI: 10.7270/Q2D798SS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data