 Found 81 hits with Last Name = 'marquart' and Initial = 'al'
Found 81 hits with Last Name = 'marquart' and Initial = 'al' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
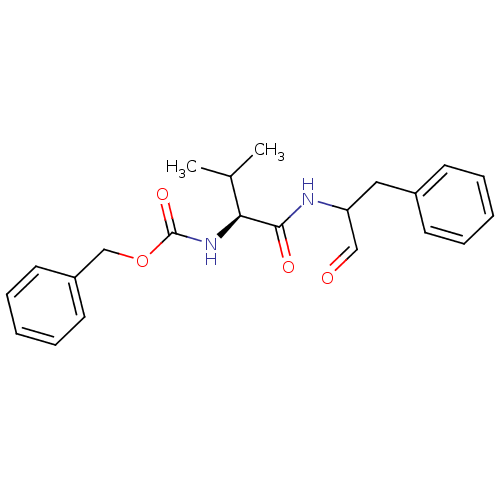
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073850

((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080206
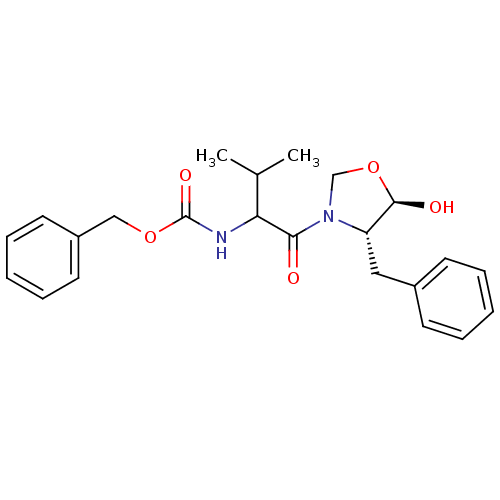
(CHEMBL311735 | [1-((4S,5R)-4-Benzyl-5-hydroxy-oxaz...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H28N2O5/c1-16(2)20(24-23(28)29-14-18-11-7-4-8-12-18)21(26)25-15-30-22(27)19(25)13-17-9-5-3-6-10-17/h3-12,16,19-20,22,27H,13-15H2,1-2H3,(H,24,28)/t19-,20?,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282635
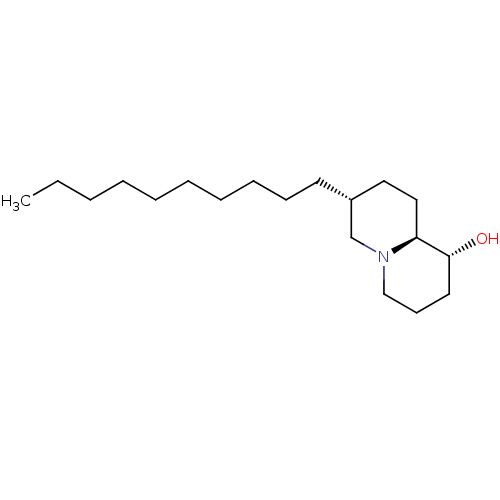
((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50080206

(CHEMBL311735 | [1-((4S,5R)-4-Benzyl-5-hydroxy-oxaz...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H28N2O5/c1-16(2)20(24-23(28)29-14-18-11-7-4-8-12-18)21(26)25-15-30-22(27)19(25)13-17-9-5-3-6-10-17/h3-12,16,19-20,22,27H,13-15H2,1-2H3,(H,24,28)/t19-,20?,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against bovine cathepsin B. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080209
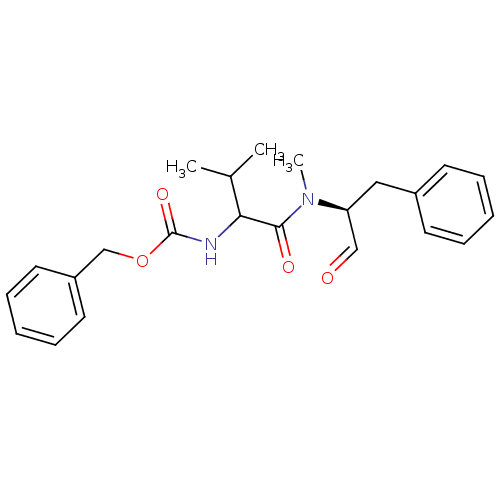
(CHEMBL78324 | {1-[((S)-1-Formyl-2-phenyl-ethyl)-me...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(C)[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O4/c1-17(2)21(24-23(28)29-16-19-12-8-5-9-13-19)22(27)25(3)20(15-26)14-18-10-6-4-7-11-18/h4-13,15,17,20-21H,14,16H2,1-3H3,(H,24,28)/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080207

(CHEMBL80142 | {1-[((S)-1-Formyl-2-phenyl-ethyl)-hy...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(CO)[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O5/c1-17(2)21(24-23(29)30-15-19-11-7-4-8-12-19)22(28)25(16-27)20(14-26)13-18-9-5-3-6-10-18/h3-12,14,17,20-21,27H,13,15-16H2,1-2H3,(H,24,29)/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282634
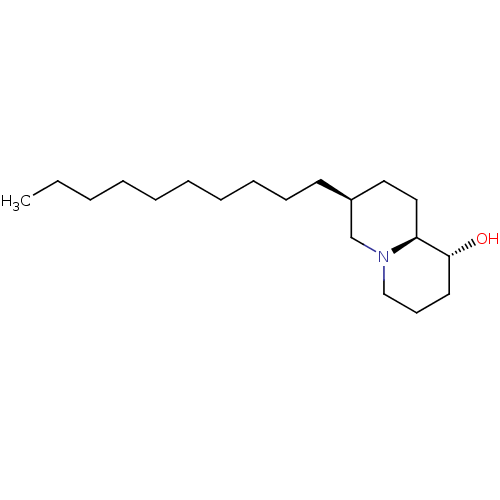
((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50366611
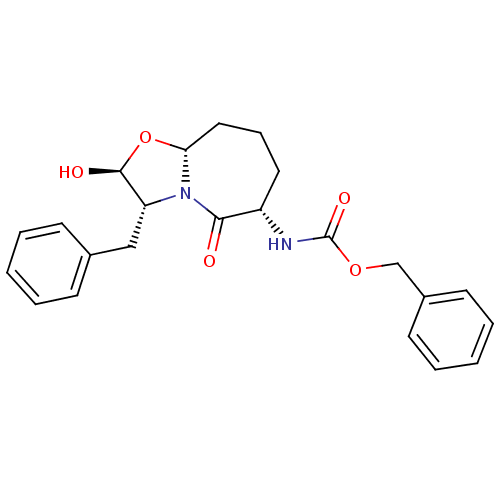
(CHEMBL1794816)Show SMILES O[C@H]1O[C@@H]2CCC[C@H](NC(=O)OCc3ccccc3)C(=O)N2[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H26N2O5/c26-21-18(24-23(28)29-15-17-10-5-2-6-11-17)12-7-13-20-25(21)19(22(27)30-20)14-16-8-3-1-4-9-16/h1-6,8-11,18-20,22,27H,7,12-15H2,(H,24,28)/t18-,19+,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080210
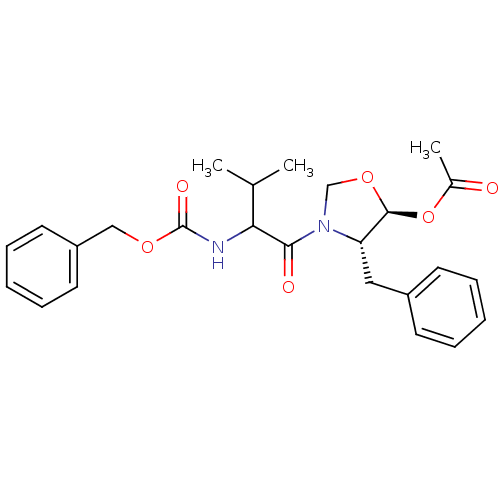
(Acetic acid (4S,5S)-4-benzyl-3-(2-benzyloxycarbony...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](OC(C)=O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C25H30N2O6/c1-17(2)22(26-25(30)31-15-20-12-8-5-9-13-20)23(29)27-16-32-24(33-18(3)28)21(27)14-19-10-6-4-7-11-19/h4-13,17,21-22,24H,14-16H2,1-3H3,(H,26,30)/t21-,22?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073851
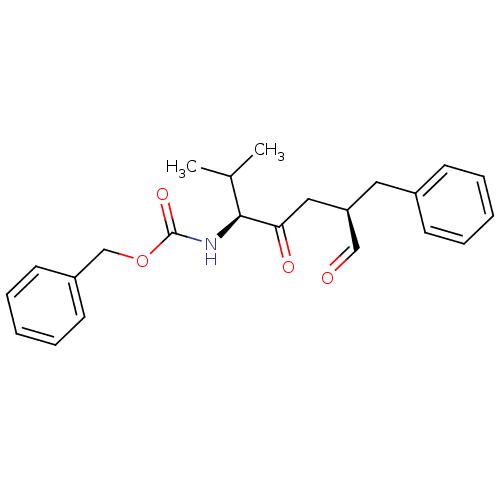
(((1S,4R)-4-Benzyl-1-isopropyl-2,5-dioxo-pentyl)-ca...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)C[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H27NO4/c1-17(2)22(24-23(27)28-16-19-11-7-4-8-12-19)21(26)14-20(15-25)13-18-9-5-3-6-10-18/h3-12,15,17,20,22H,13-14,16H2,1-2H3,(H,24,27)/t20-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50366612
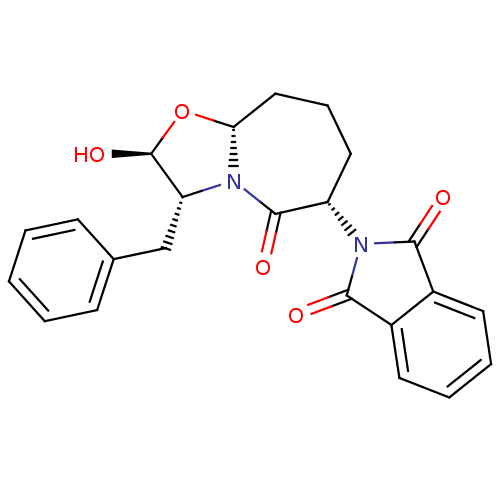
(CHEMBL1794817)Show SMILES O[C@H]1O[C@@H]2CCC[C@H](N3C(=O)c4ccccc4C3=O)C(=O)N2[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H22N2O5/c26-20-15-9-4-5-10-16(15)21(27)25(20)17-11-6-12-19-24(22(17)28)18(23(29)30-19)13-14-7-2-1-3-8-14/h1-5,7-10,17-19,23,29H,6,11-13H2/t17-,18+,19+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073849

(((S)-1-Isopropyl-4-methyl-2,5-dioxo-pentyl)-carbam...)Show InChI InChI=1S/C17H23NO4/c1-12(2)16(15(20)9-13(3)10-19)18-17(21)22-11-14-7-5-4-6-8-14/h4-8,10,12-13,16H,9,11H2,1-3H3,(H,18,21)/t13?,16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080208
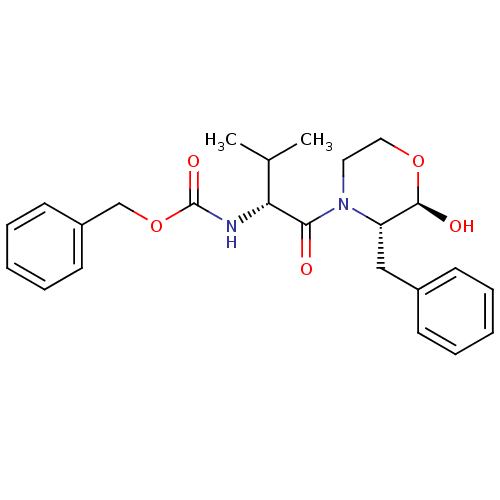
(CHEMBL73869 | [(R)-1-((2R,3S)-3-Benzyl-2-hydroxy-m...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N1CCO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C24H30N2O5/c1-17(2)21(25-24(29)31-16-19-11-7-4-8-12-19)22(27)26-13-14-30-23(28)20(26)15-18-9-5-3-6-10-18/h3-12,17,20-21,23,28H,13-16H2,1-2H3,(H,25,29)/t20-,21+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699
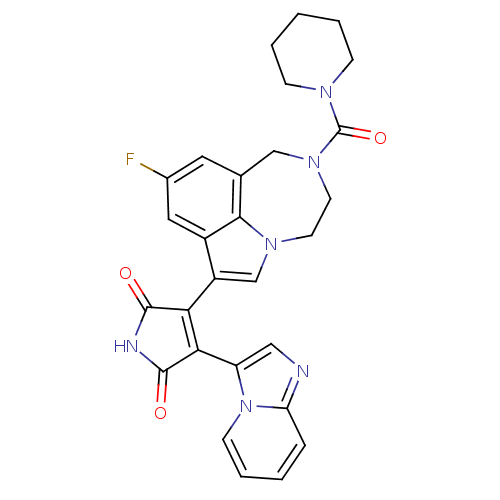
(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698
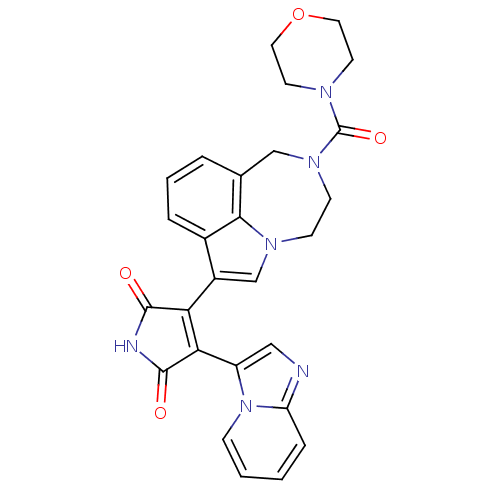
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
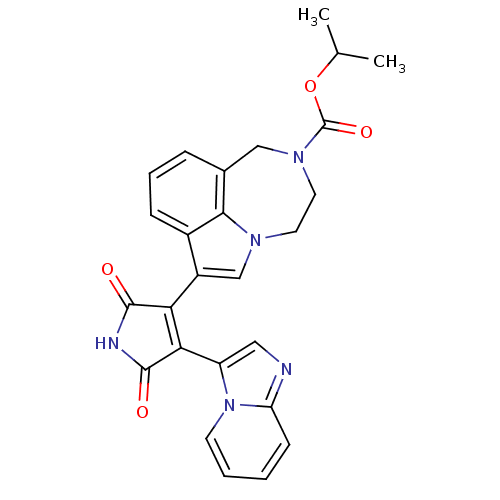
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701
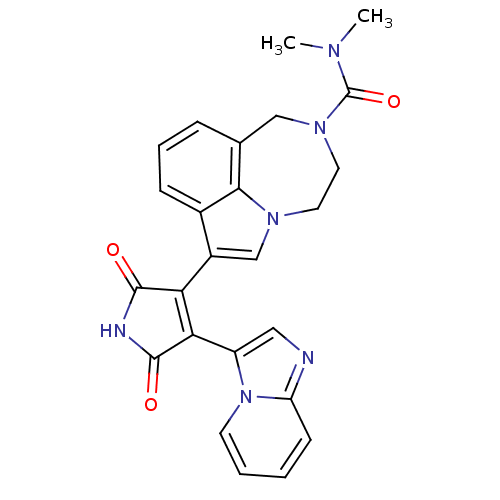
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150697

(3-Imidazo[1,2-a]pyridin-3-yl-4-[9-methyl-2-(piperi...)Show SMILES Cc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C29H28N6O3/c1-18-13-19-16-34(29(38)32-8-4-2-5-9-32)12-11-33-17-21(20(14-18)26(19)33)24-25(28(37)31-27(24)36)22-15-30-23-7-3-6-10-35(22)23/h3,6-7,10,13-15,17H,2,4-5,8-9,11-12,16H2,1H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700

(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150697

(3-Imidazo[1,2-a]pyridin-3-yl-4-[9-methyl-2-(piperi...)Show SMILES Cc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C29H28N6O3/c1-18-13-19-16-34(29(38)32-8-4-2-5-9-32)12-11-33-17-21(20(14-18)26(19)33)24-25(28(37)31-27(24)36)22-15-30-23-7-3-6-10-35(22)23/h3,6-7,10,13-15,17H,2,4-5,8-9,11-12,16H2,1H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50059889
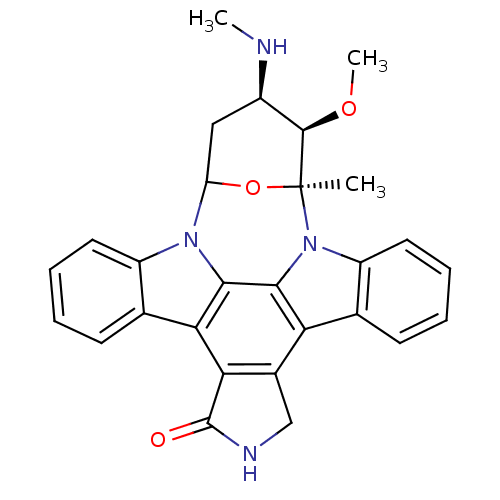
((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282635

((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase (OSC) |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150697

(3-Imidazo[1,2-a]pyridin-3-yl-4-[9-methyl-2-(piperi...)Show SMILES Cc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C29H28N6O3/c1-18-13-19-16-34(29(38)32-8-4-2-5-9-32)12-11-33-17-21(20(14-18)26(19)33)24-25(28(37)31-27(24)36)22-15-30-23-7-3-6-10-35(22)23/h3,6-7,10,13-15,17H,2,4-5,8-9,11-12,16H2,1H3,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282634

((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150700

(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Protein kinase C beta 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TGF-beta type II receptor |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 4 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 4 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 4 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TGF-beta type II receptor |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-2
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human TGF-beta type II receptor |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50150700

(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 4 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50150700

(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDK-1 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 4 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Cyclin-dependent kinase 2 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDK-1 |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data