 Found 18 hits with Last Name = 'marzec' and Initial = 'u'
Found 18 hits with Last Name = 'marzec' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14714
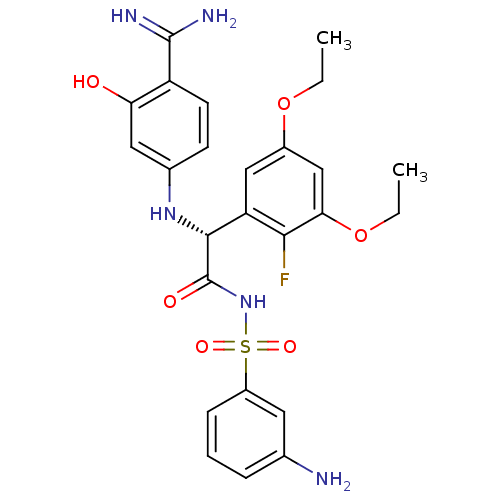
((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180400
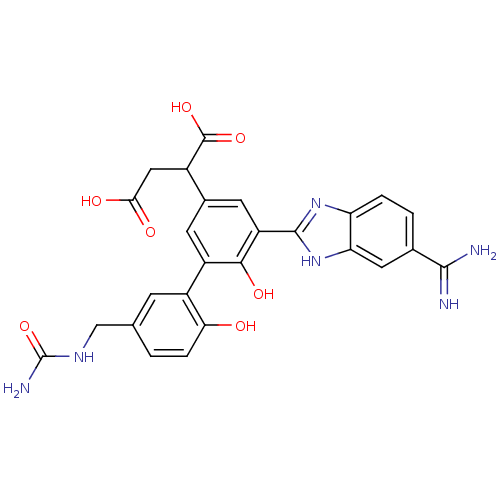
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H24N6O7/c27-23(28)12-2-3-18-19(8-12)32-24(31-18)17-7-13(14(25(37)38)9-21(34)35)6-16(22(17)36)15-5-11(1-4-20(15)33)10-30-26(29)39/h1-8,14,33,36H,9-10H2,(H3,27,28)(H,31,32)(H,34,35)(H,37,38)(H3,29,30,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM14898
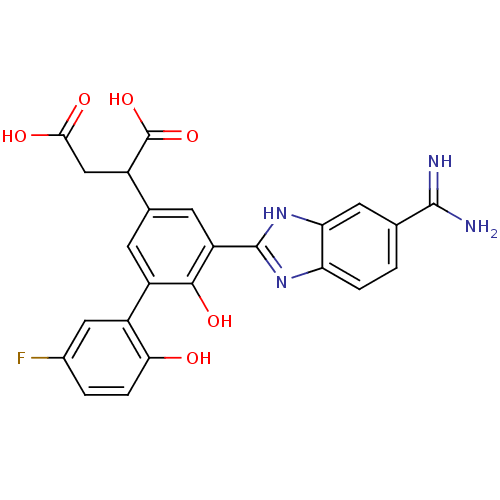
(2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(F)ccc1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C24H19FN4O6/c25-12-2-4-19(30)14(8-12)15-5-11(13(24(34)35)9-20(31)32)6-16(21(15)33)23-28-17-3-1-10(22(26)27)7-18(17)29-23/h1-8,13,30,33H,9H2,(H3,26,27)(H,28,29)(H,31,32)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180405
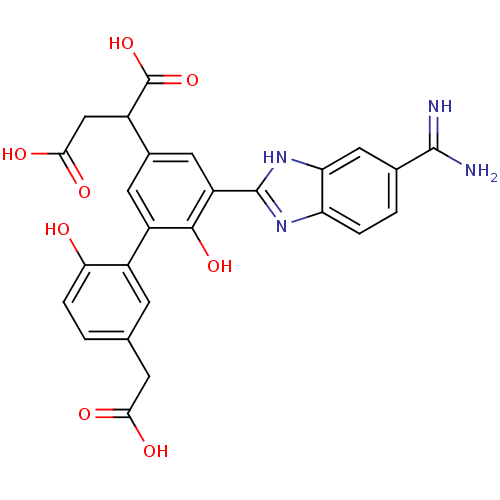
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-ca...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(CC(O)=O)ccc1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H22N4O8/c27-24(28)12-2-3-18-19(9-12)30-25(29-18)17-8-13(14(26(37)38)10-22(34)35)7-16(23(17)36)15-5-11(6-21(32)33)1-4-20(15)31/h1-5,7-9,14,31,36H,6,10H2,(H3,27,28)(H,29,30)(H,32,33)(H,34,35)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180403

(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-ca...)Show SMILES NC(=O)Cc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C26H23N5O7/c27-21(33)6-11-1-4-20(32)15(5-11)16-7-13(14(26(37)38)10-22(34)35)8-17(23(16)36)25-30-18-3-2-12(24(28)29)9-19(18)31-25/h1-5,7-9,14,32,36H,6,10H2,(H2,27,33)(H3,28,29)(H,30,31)(H,34,35)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180404

(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-(2...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(CCC(O)=O)ccc1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C27H24N4O8/c28-25(29)13-3-4-19-20(10-13)31-26(30-19)18-9-14(15(27(38)39)11-23(35)36)8-17(24(18)37)16-7-12(1-5-21(16)32)2-6-22(33)34/h1,3-5,7-10,15,32,37H,2,6,11H2,(H3,28,29)(H,30,31)(H,33,34)(H,35,36)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180401
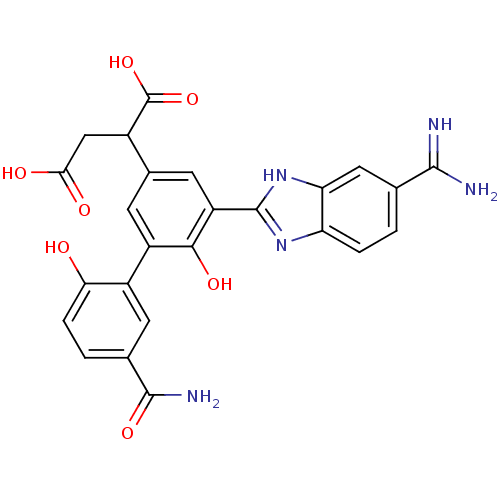
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-ca...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(ccc1O)C(N)=O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H21N5O7/c26-22(27)10-1-3-17-18(8-10)30-24(29-17)16-7-12(13(25(36)37)9-20(32)33)6-15(21(16)34)14-5-11(23(28)35)2-4-19(14)31/h1-8,13,31,34H,9H2,(H3,26,27)(H2,28,35)(H,29,30)(H,32,33)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180406
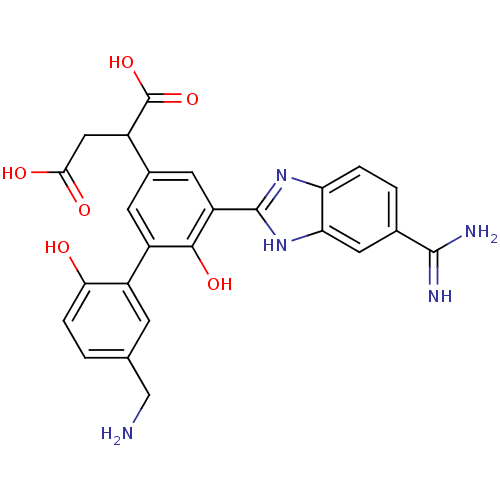
(2-[5'-aminomethyl-5-(5-carbamimidoyl-1H-benzoimida...)Show SMILES NCc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H23N5O6/c26-10-11-1-4-20(31)15(5-11)16-6-13(14(25(35)36)9-21(32)33)7-17(22(16)34)24-29-18-3-2-12(23(27)28)8-19(18)30-24/h1-8,14,31,34H,9-10,26H2,(H3,27,28)(H,29,30)(H,32,33)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180407

(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-5'-ca...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(cc(c1O)-c1cc(ccc1O)C(O)=O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H20N4O8/c26-22(27)10-1-3-17-18(8-10)29-23(28-17)16-7-12(13(25(36)37)9-20(31)32)6-15(21(16)33)14-5-11(24(34)35)2-4-19(14)30/h1-8,13,30,33H,9H2,(H3,26,27)(H,28,29)(H,31,32)(H,34,35)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50180402
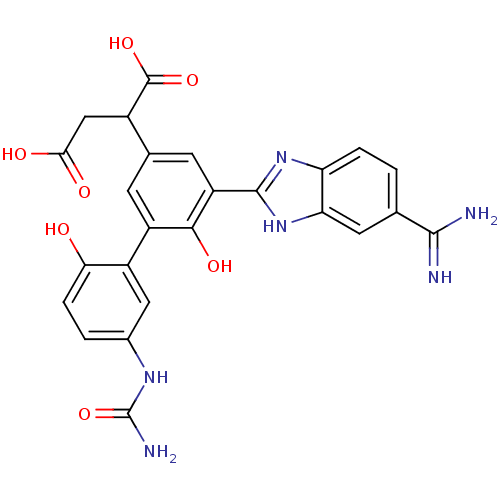
(2-[5-(5-carbamimidoyl-1H-benzoimidazol-2-yl)-6,2'-...)Show SMILES NC(=O)Nc1ccc(O)c(c1)-c1cc(cc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O)C(CC(O)=O)C(O)=O Show InChI InChI=1S/C25H22N6O7/c26-22(27)10-1-3-17-18(7-10)31-23(30-17)16-6-11(13(24(36)37)9-20(33)34)5-15(21(16)35)14-8-12(29-25(28)38)2-4-19(14)32/h1-8,13,32,35H,9H2,(H3,26,27)(H,30,31)(H,33,34)(H,36,37)(H3,28,29,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to F7a/TF complex |
Bioorg Med Chem Lett 16: 2037-41 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.059
BindingDB Entry DOI: 10.7270/Q2639PBP |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 87 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 114 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.40E+3 | >-30.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.50E+3 | >-30.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.90E+3 | >-30.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM14714

((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.00E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data