

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593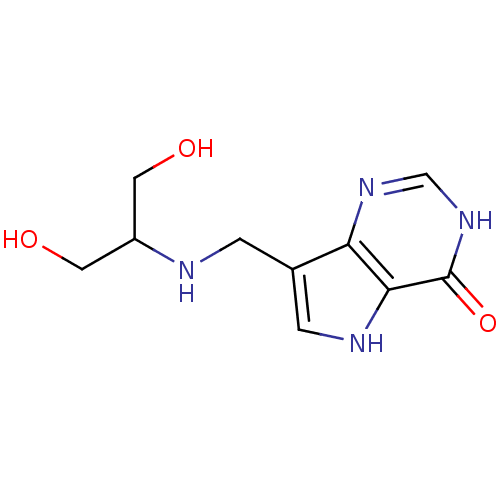 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711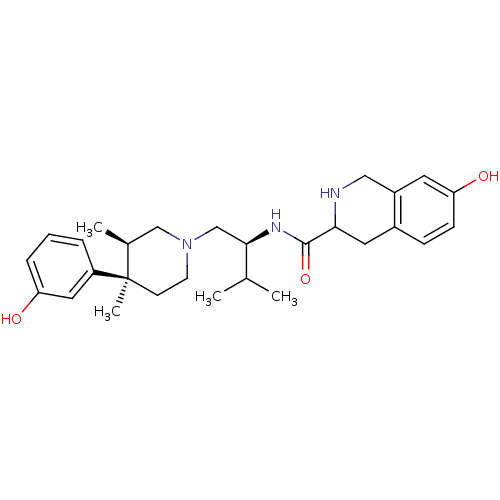 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246590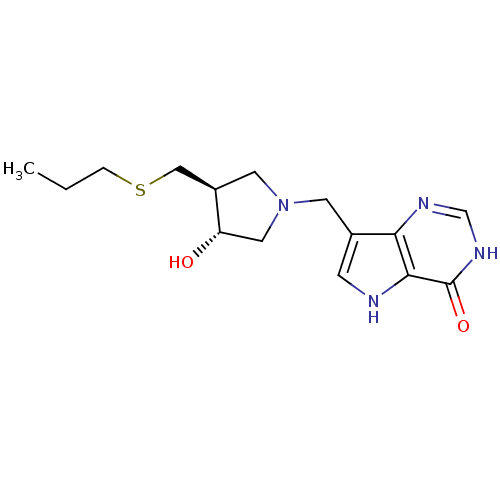 (7-(((3R,4S)-3-hydroxy-4-(propylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109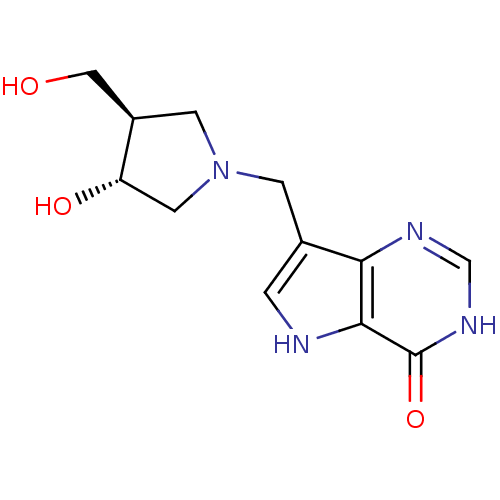 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151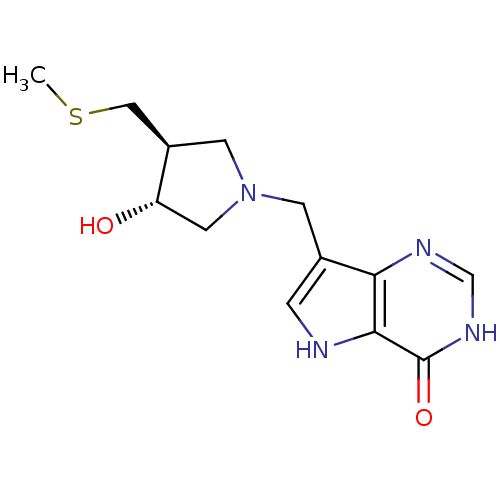 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391716 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 1 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391716 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391716 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 1 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391727 (N-[(2R)-1-({(2S)-3-{1-[2- | US10300056, Example 12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391727 (N-[(2R)-1-({(2S)-3-{1-[2- | US10300056, Example 12...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50539955 (CHEMBL4638938) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064518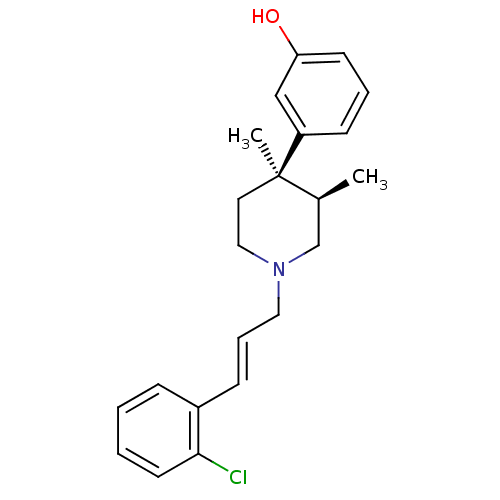 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726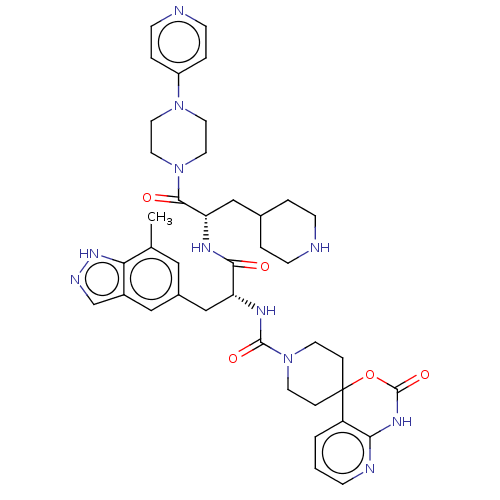 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50184069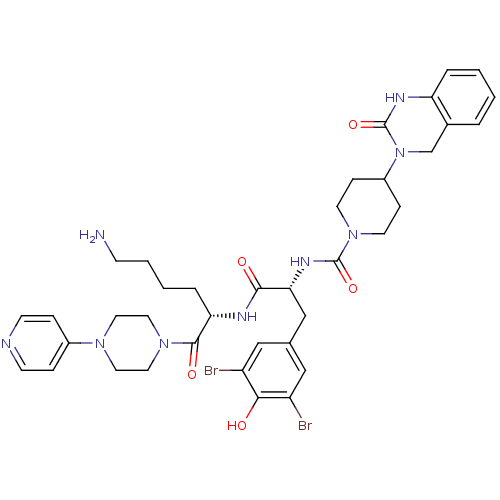 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064521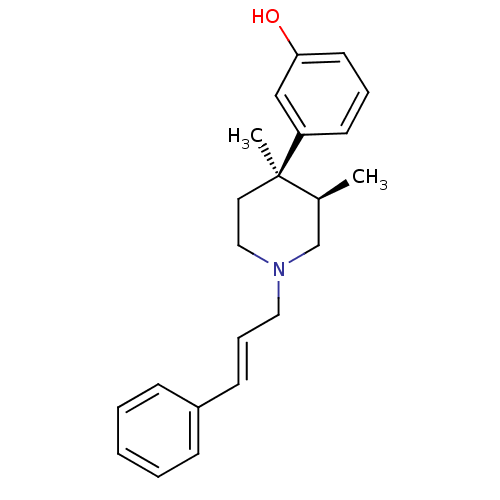 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970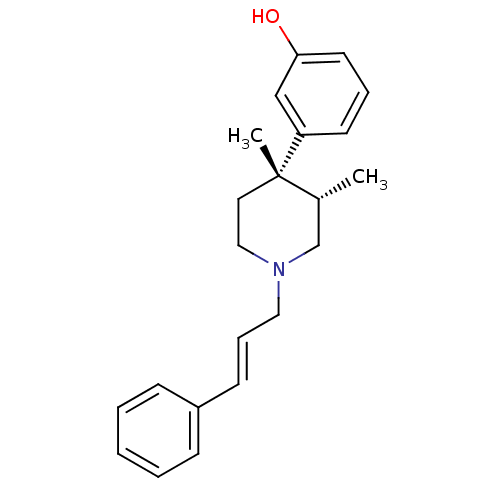 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391723 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 8...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391723 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391723 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391722 (N-[(2R)-1-({(2S)-3-(1- | US10300056, Example 7 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391719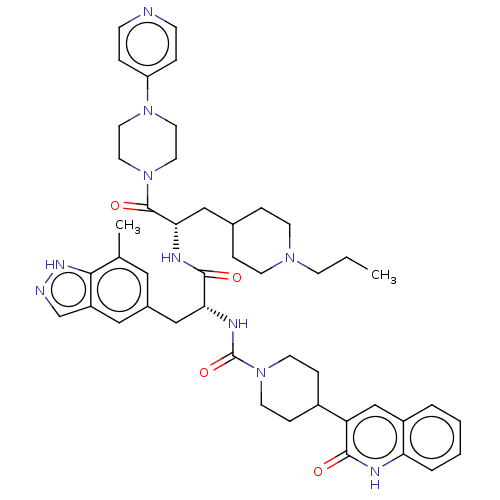 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 4 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391719 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 4 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391722 (N-[(2R)-1-({(2S)-3-(1- | US10300056, Example 7 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50184183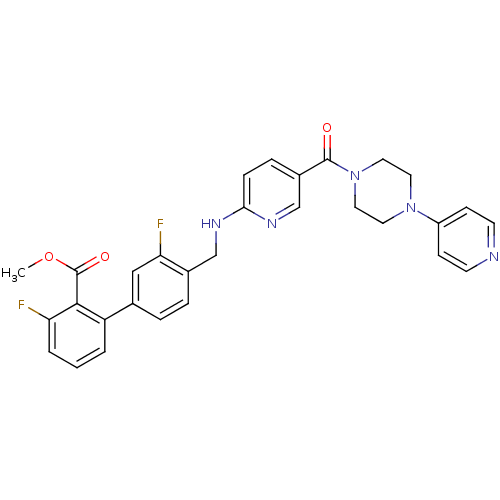 (3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited Curated by ChEMBL | Assay Description Antagonist activity at human bradykinin B1 receptor expressed in human HEK293 cells assessed as calcium flux by FLIPR assay | J Med Chem 54: 4283-311 (2011) Article DOI: 10.1021/jm200371q BindingDB Entry DOI: 10.7270/Q23N23RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080514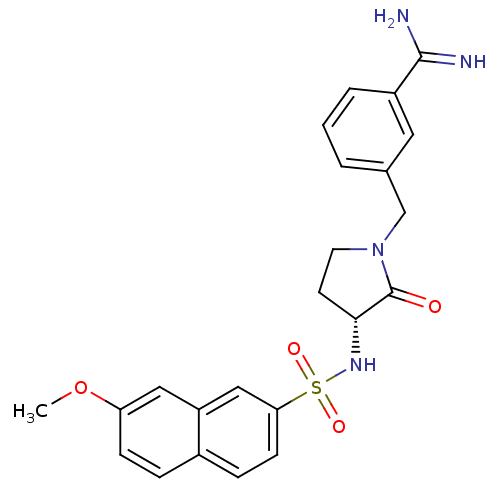 (3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide 1 (Homo sapiens (Human)) | BDBM311886 (N-[(2R)-1-[4-(1H-imidazol-2-yl)-1,4'-bipiperidin-1...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Human CGRP receptors (consisting of CRLR and RAMP1) expressed in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | US Patent US10166226 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide 1 (Homo sapiens (Human)) | BDBM312053 (5-{1'-[(2R)-3-(7-methyl-1H-indazol-5-yl)-2-({[4-(2...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Human CGRP receptors (consisting of CRLR and RAMP1) expressed in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | US Patent US10166226 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614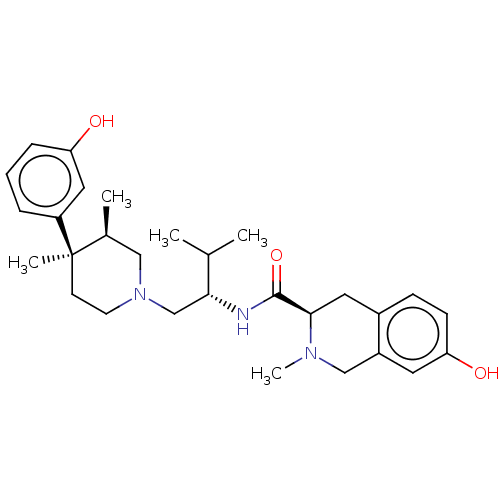 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0579 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine of Yeshiva University Curated by ChEMBL | Assay Description Equilibrium binding affinity to wild type human PNP | Bioorg Med Chem Lett 18: 5900-3 (2008) Article DOI: 10.1016/j.bmcl.2008.08.047 BindingDB Entry DOI: 10.7270/Q2SX6F4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide 1 (Homo sapiens (Human)) | BDBM312050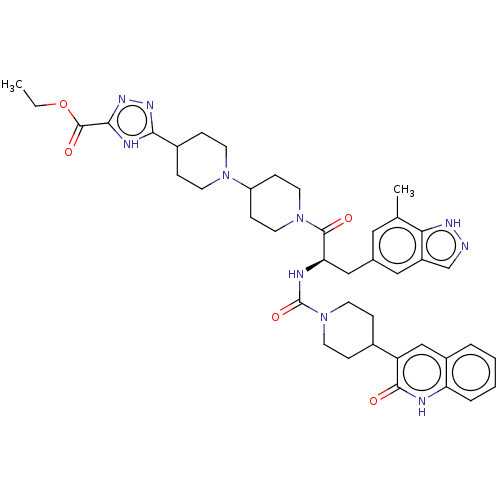 (US10166226, Example 6 | ethyl 5-{1'-[(2R)-3-(7-met...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Human CGRP receptors (consisting of CRLR and RAMP1) expressed in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | US Patent US10166226 (2019) BindingDB Entry DOI: 10.7270/Q28C9ZBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391725 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 10 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391725 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 10 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391720 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391720 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391725 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 10 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865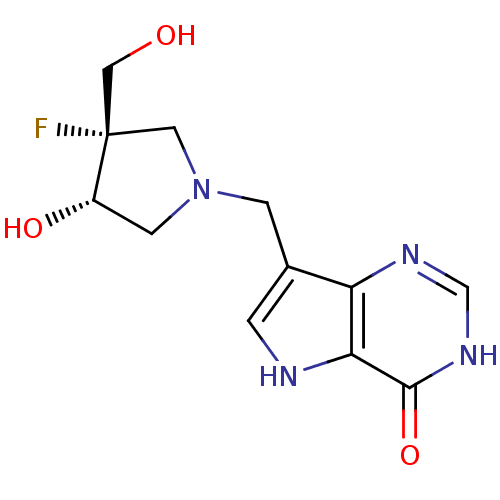 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183152 (CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description [3H]-Cl-DPDPE competition binding assay using human cloned opioid receptor delta 1 | J Med Chem 47: 281-4 (2004) Article DOI: 10.1021/jm030419a BindingDB Entry DOI: 10.7270/Q20K2990 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50183152 (CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT | Bioorg Med Chem Lett 16: 2714-8 (2006) Article DOI: 10.1016/j.bmcl.2006.02.008 BindingDB Entry DOI: 10.7270/Q2SF2VR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391718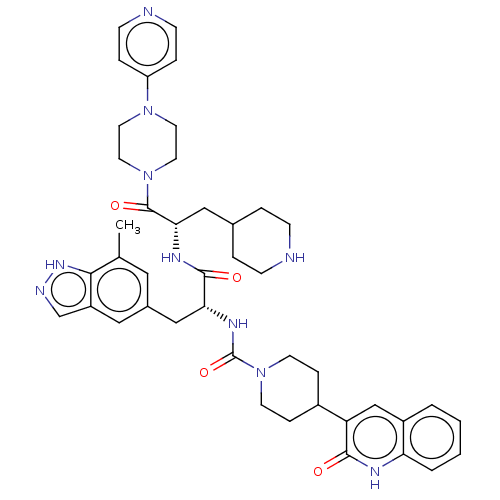 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 3 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391724 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) BindingDB Entry DOI: 10.7270/Q28S4T1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16904 total ) | Next | Last >> |